Abstract
Familial renal glucosuria is a rare co-dominantly inherited benign phenotype characterized by the presence of glucose in the urine. It is caused by mutations in the SLC5A2 gene that encodes SGLT2, the Na+-glucose cotransporter responsible for the reabsorption of the bulk of glucose in the proximal tubule. We report a case of FRG displaying both severe glucosuria and renal hypouricaemia. We hypothesize that glucosuria can disrupt urate reabsorption in the proximal tubule, directly causing hyperuricosuria.
Keywords: glucose, kidney, SGLT2, urate
Background
Familial renal glucosuria (FRG) is characterized by the presence of glucose in the urine in the absence of diabetes mellitus or generalized proximal tubular dysfunction. Mutations in the SLC5A2 gene, encoding SGLT2, are responsible for the large majority of FRG pedigrees [1, 2].
Recently, SGLT2 was targeted as an innovative strategy for the treatment of hyperglycaemia in type 2 diabetes mellitus (T2DM). One interesting and consistent observation with SGLT2 inhibitors is the almost dose-proportional decrease in serum uric acid levels [3, 4], which is paradoxical in light of the associated diuretic effect of these compounds.
In the current report, we describe an FRG individual with increased urate renal excretion and hypouricaemia, and discuss renal hypouricaemia in the setting of severe glucosuria.
Case report
This study was part of the medical evaluation for glucosuria in a 35-year-old female individual. She presented with a urinary glucose excretion (UGE) of 487.8 mmol (87.8 g)/1.73 m2/24 h.
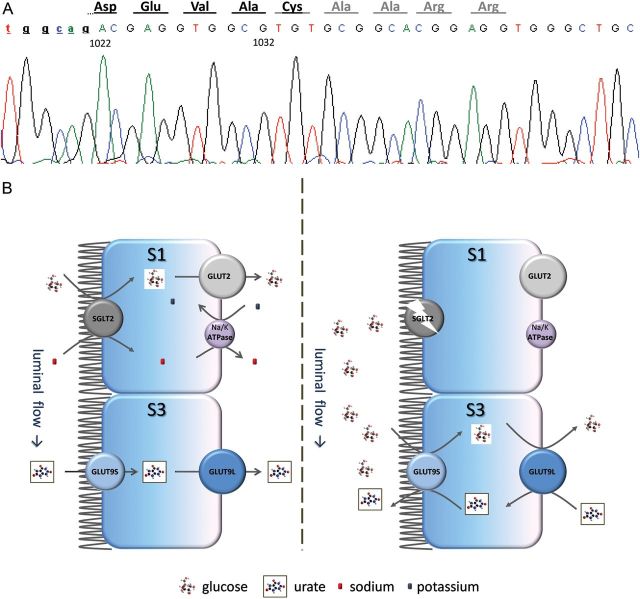
The clinical data are detailed in Table 1. She took no medications. Mutation analysis was performed as previously reported [6]. The novel c.1033–1060del; p.V346AfsX17 frameshift mutation was identified in homozygosity (Figure 1A).
Table 1.
Phenotype evaluation of case
| Age | Country of origin | Weight (kg) | Height (cm) | Glucose excretion mmol/1.73 m2/24 h (g/1.73 m2/24 h) | Serum glucose mmol/L (mg/dL) | HbA1c (%) | Serum creatinine μmol/L (mg/dL) | Serum uric acid μmol/L (mg/dL) | Urinary urate mmol/1.73 m2/24 h Ref: 1.5–4.4 (mg/1.73 m2/24 h) | mmol/kg a0.04 ± 0.01 (mg/kg) | Fractional excretion a10% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | Portugal | 65 | 167 | 487.8 (87.8) | 4.1 (74) | 5.4 | 53 (0.6) | 113.01 (1.9) | 7.33 (1242) | 0.13 (21.5) | 20 |
Ref, reference range for urinary urate.
aValues detail average (and standard deviation when specified) in an adult population [5].
Fig. 1.
(A) The c.1033–1060del mutation. The splice acceptor site for intron 8 is included (small caps). Nucleotides are numbered according to the SLC5A2 cDNA accession number NM_003041. The wild-type amino acid sequence is represented in black; in light gray, the new ORF. (B) Suggested mechanism for the renal hypouricaemia associated with defects of SGLT2. In the presence of normally functioning SGLT2 (left side) all the glucose is reabsorbed in the early segment (S1) of the proximal tubule, while in the late S3 segment GLUT9 is normally reabsorbing urate. If SGLT2 is disrupted (right side), either by genetic defects or pharmacological inhibition, the presence of significant amounts of glucose in the late segments of the proximal tubule reverses the direction of urate transport, with GLUT9 exchanging luminal glucose for intracellular urate and actively promoting urate secretion.
Owing to persistent low serum uric acid levels [113.01 μmol/L (1.9 mg/dL)], further evaluation was performed in order to exclude Fanconi syndrome. Urinary urate values were found to be raised, with an excretion of 7.33 mmol (1242 mg)/1.73 m2/24 h or 0.13 mmol (21.5 mg)/kg of body weight and a fractional excretion of 20%.
Phosphorus (urinary and serum), bicarbonate (plasma) and immunoglobulin light chains (urine) were all within normal range (data not shown). By high-performance liquid chromatography, cystine was the only amino acid over-excreted: 639.3 μmol (153.3 mg)/24 h (reference range: 0–158.5 μmol/24 h). This latter finding is characteristic of a type non-I heterozygous cystinuric individual [7]. Accordingly, the over-excretion of cystine was considered an incidental finding and unrelated to the glucosuric phenotype.
On follow-up the patient got pregnant. At gestational week 24, she was hyperfiltrating with a serum creatinine of 35.4 μmol/L (0.4 mg/dL), but there were no further decreases in urate serum levels [130.8 μmol/L (2.2 mg/dL)], compared with her earlier non-pregnant condition. Fractional excretion for urate was now 12%.
Discussion
Under physiological conditions, SGLT2 is responsible for reabsorbing the majority of the filtered glucose [8]. The p.V346AfsX17 frameshift mutation in our patient likely leads to a truncated protein between SGLT2 transmembrane domains 8 and 9 and, therefore, fully accounts for the severe glucosuria. However, the hyperuricosuria with hypouricaemia points out to an associated renal hypouricaemia.
The handling of urate by the kidney is complex, involving both secretion (inhibited by pyrazinamide) and reabsorption (inhibited by probenecid) [9]. The pregnancy of our patient precluded additional testing with any of these compounds. Pregnancy induces an increase in both the urate glomerular filtration and in its tubular reabsorption, with proportionally greater increments observed with the former and, hence, the lowering of serum urate usually observed in the first 24 gestational weeks [10]. Since our case did not display such a decrease in serum levels (and even improved its fractional excretion) with pregnancy, we can assume the urinary urate over-excretion seen with glucosuria to be a consequence of enhanced secretion rather than impaired reabsorption. Two solute carriers that reabsorb urate are known to be expressed in the proximal tubule, URAT1 (SLC22A2), the target of probenecid, and GLUT9 (SLC2A9), mutations of which are responsible for an isolated renal hypouricaemic phenotype [11, 12]. Interestingly, GLUT9 was also shown to exchange extracellular glucose for intracellular urate [13]. Two GLUT9 isoforms, GLUT9S (expressed at the apical side) and GLUT9L (at the basolateral side), act concertedly to reabsorb uric acid from the glomerular filtrate. We can hypothesize that in the presence of a high glucose concentration in the late proximal tubular lumen, the direction of urate transport could be reversed, with glucose being exchanged for urate and leading to excessive urinary excretion and secondary hypouricaemia (Figure 1B).
To our knowledge, only one report addressed the urinary urate excretion in FRG [14]. All six individuals were homozygous for the p.K321R mutation, had a UGE of 460.7–938.1 mmol (83–169 g)/1.73 m2/24 h and displayed generalized aminoaciduria. In one case, uricosuria was within the upper limit of the reference range. Although we have not pharmacologically detailed the involved pathway or screened our patient for mutations in URAT1 and GLUT9, our findings are in line with previous reports describing renal hypouricaemia in cases of hyperglycaemia induced glucosuria. Not only in T2DM [15], but also in maturity-onset diabetes of the young (MODY) [16], in particular, in HNF1α-mutated cases (MODY3), who are characterized by a paradoxically low renal threshold for glucose and, therefore, exceptional glucosuria [17].
This characterization of renal hypouricaemia as an incompletely penetrant feature of FRG needs confirmation by similar observations in other patients with severe forms of FRG. If replicated, this will argue for glucosuria being directly responsible for the disruption of urate transport in the proximal tubule and renal hypouricaemia in FRG as well with pharmacological inhibition of SGLT2.
Acknowledgments
This work was supported by AstraZeneca Produtos Farmacêuticos and Bristol-Meyers Squibb, Portugal, and APENE – Associação Portuguesa para o Estudo das Nefropatias.
Conflict of interest statement. None declared.
References
- 1.Santer R, Kinner M, Lassen CL, et al. Molecular analysis of the SGLT2 gene in patients with renal glycosuria. J Am Soc Nephrol. 2003;14:2873–2882. doi: 10.1097/01.asn.0000092790.89332.d2. doi:10.1097/01.ASN.0000092790.89332.D2. [DOI] [PubMed] [Google Scholar]
- 2.Calado J, Loeffler J, Sakallioglu O, et al. Familial renal glycosuria: SLC5A2 mutation analysis and evidence of salt-wasting. Kidney Int. 2006;69:852–855. doi: 10.1038/sj.ki.5000194. doi:10.1038/sj.ki.5000194. [DOI] [PubMed] [Google Scholar]
- 3.List JF, Woo V, Morales E, et al. Sodium-Glucose co-transport inhibition with dapagliflozin in type 2 diabetes mellitus. Diabetes Care. 2009;32:650–657. doi: 10.2337/dc08-1863. doi:10.2337/dc08-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, double blind, placebo–controlled trial. Lancet. 2010;375:2223–2233. doi: 10.1016/S0140-6736(10)60407-2. doi:10.1016/S0140-6736(10)60407-2. [DOI] [PubMed] [Google Scholar]
- 5.Baldree LA, Stapleton FB. Uric acid metabolism in children. Pediatr Clin North Am. 1990;37:391–418. doi: 10.1016/s0031-3955(16)36876-6. [DOI] [PubMed] [Google Scholar]
- 6.Calado J, Soto K, Clemente C, et al. Novel compound heterozygous mutations in SLC5A2 are responsible for autosomal recessive renal glucosuria. Hum Genet. 2004;114:314–316. doi: 10.1007/s00439-003-1054-x. doi:10.1007/s00439-003-1054-x. [DOI] [PubMed] [Google Scholar]
- 7.Dello Strologo L, Pras E, Pontesilli C, et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002;13:2547–2553. doi: 10.1097/01.asn.0000029586.17680.e5. doi:10.1097/01.ASN.0000029586.17680.E5. [DOI] [PubMed] [Google Scholar]
- 8.Vallon V, Platt KA, Cunard R, et al. SGLT2 Mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246. doi:10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamond HS, Paolino JS. Evidence for a postsecretory reabsorptive site for uric acid in man. J Clin Invest. 1973;52:1491–1499. doi: 10.1172/JCI107323. doi:10.1172/JCI107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindheimer MD, Conrad KP, Umans JG. The normal and diseased kidney in pregnancy. In: Schrier RW, editor. Diseases of the Kidney and Urinary Tract. Philadelphia, USA: Lippincott; 2007. pp. 1909–1940. [Google Scholar]
- 11.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 12.Matsuo H, Chiba T, Nagamori S, et al. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83:744–781. doi: 10.1016/j.ajhg.2008.11.001. doi:10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caulfield MJ, Munroe PB, O'Neill D, et al. SLC2A9 Is a high-capacity urate transporter in humans. Pub Lib of Science. 2008;5:e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magen D, Sprecher E, Zelikovic I, et al. A novel missense mutation in SLC5A2 encoding SGLT2 underlies autosomal-recessive renal glucosuria and aminoaciduria. Kidney Int. 2005;67:34–41. doi: 10.1111/j.1523-1755.2005.00053.x. doi:10.1111/j.1523-1755.2005.00053.x. [DOI] [PubMed] [Google Scholar]
- 15.Gotfredsen A, McNair P, Christiansen C, et al. Renal hypouricaemia in insulin treated diabetes mellitus. Clin Chim Acta. 1982;120:355–361. doi: 10.1016/0009-8981(82)90376-x. doi:10.1016/0009-8981(82)90376-X. [DOI] [PubMed] [Google Scholar]
- 16.Shichiri M, Iwamoto H, Shiigai T. Diabetic renal hypouricemia. Arch Intern Med. 1987;147:225–228. doi:10.1001/archinte.1987.00370020045033. [PubMed] [Google Scholar]
- 17.Pontoglio M, Prié D, Cheret C, et al. HNF1alpha Controls renal glucose reabsorption in mouse and man. EMBO Rep. 2000;1:359–365. doi: 10.1093/embo-reports/kvd071. doi:10.1093/embo-reports/kvd071. [DOI] [PMC free article] [PubMed] [Google Scholar]