Abstract
Extramedullary hematopoiesis (EMH), defined as the presence of hematopoietic elements outside of the medullary cavity of bone, has been reported in patients with various hematopoietic neoplasms including myelofibrosis. EMH commonly occurs in the liver and spleen (resulting in hepatosplenomegaly) and uncommonly involves the kidney. EMH involving the allograft kidney has not been reported in English literature. Herein, we report the first case of EMH in allograft kidney in a patient with myelofibrosis. The clinical and pathological findings are described. Through comparison of the medullary neoplastic infiltrate with the renal allograft infiltrate, we postulate the neoplastic nature of the infiltrate in the allograft kidney.
Keywords: allograft, biopsy, extramedullary hematopoiesis, myelofibrosis, transplant
Introduction
Allograft kidney biopsies are commonly performed to evaluate the possibility of rejection in patients with elevated creatinine. Other common causes of graft dysfunction include calcineurin inhibitor toxicity, chronic rejection, infections and recurrence of the patients' original kidney diseases. Less frequently, the elevation in serum creatinine is attributed to de novo diseases or neoplastic processes, though the rarity of the latter can hinder prompt diagnosis. Extramedullary hematopoiesis (EMH) can be one such example. EMH in native kidneys has been rarely reported in patients with hematopoietic neoplasms and in primary myelofibrosis [1–8]. Morphologically, EMH in the kidney can present as a mass-like lesion or as a diffuse infiltrate, the latter often offering a diagnostic challenge due to the morphological similarities with interstitial inflammation. Accurate diagnosis of EMH, however, is critical in guiding proper treatment. EMH in the allograft kidney has not been reported. Herein, we report a rare case of EMH that occurred in an allograft kidney in a patient with myelofibrosis. The clinical presentation, biopsy findings and differential diagnoses are discussed.
Case presentation
A 62-year-old Caucasian man with a history of end-stage renal disease secondary to autosomal-dominant polycystic kidney disease had undergone a deceased donor renal transplantation, three-antigen match, alemtusumab (Campath) induction; maintenance immunosuppression was mycophenolate mofetil and tacrolimus. Following transplantation, his baseline serum creatinine level was 106.08 µmol/L (1.2 mg/dL), and throughout 4 years of post-transplantation follow-up he had no episodes of acute rejection. He was admitted for a renal biopsy due to a rise in serum creatinine to 150.28 µmol/L (1.7 mg/dL) over the past 6 months. On admission, he was found to have a new-onset nephrotic range proteinuria (random urine protein–creatinine ratio: 3467 mg/g of creatinine) of 2 months duration. His urinalysis was otherwise unremarkable. Other laboratory data were as follows: hemoglobin 94 g/L (9.4 g/dL), white blood cell count 28.8 × 109/L, platelet count 333×109/L, sodium 141 mmol/L, total CO2 19 mmol/L, potassium 4.5 mmol/L, chloride 108 mmol/L, BUN 75.63 mmol/L (27 mg/dL), albumin 25 g/L (2.5 g/dL), total bilirubin 30.78 µmol/L (1.8 mg/dL), alkaline phosphatase 357 U/L and normal aspartate aminotransferase and alanine aminotransferase. Physical examination showed moderate ascites, hepatomegaly, spleenomegaly (8 cm below the costal margin) and trace bilateral lower extremity edema. Other positive past medical history included hypertension, post-transplant type 2 diabetes mellitus, hyperuricemia, gout, hypercholesterolemia, severe pulmonary hypertension, Stage I diastolic dysfunction of the left ventricle and erectile dysfunction. One year prior to admission—due to persistent mild leukocytosis, anemia, thrombocytosis, elevated liver enzymes and hepatosplenomegaly—a bone marrow biopsy was performed and established a diagnosis of myelofibrosis. He had received no treatment for myelofibrosis.
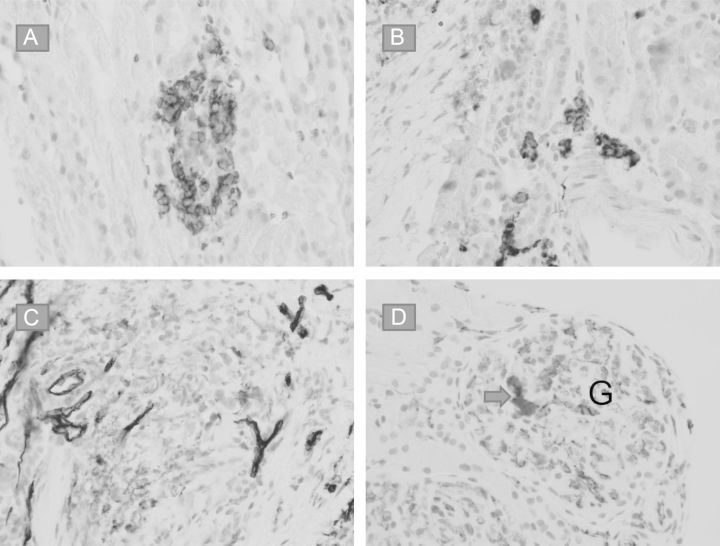
Kidney allograft needle biopsy
Out of a total of 25 glomeruli, 2 were globally sclerotic. Among non-globally sclerotic glomeruli, there was focal basement membrane duplication with mesangial interposition consistent with transplant glomerulopathy. The interstitium was expanded by a cellular infiltrate composed of nucleated red blood cells (RBCs), atypical megakaryocytes and immature granulocyte progenitor cells in a patchy distribution (Figure 1). Under higher magnification, the myeloid progenitor cells show large nuclei with open chromatin and a moderate amount of cytoplasm (Figure 2A). They frequently infiltrated interstitium and tubules mimicking interstitial inflammation and tubulitis, respectively. Subcapsular involvement was also noted (Figure 2B). Some of the megakaryocytes showed hyperchromatic nuclei with a clumping ‘cloudy’ chromatin pattern characteristic of myelofibrosis. The nucleated RBCs, confirmed by CD235 immunostain (Figure 3A), showed no morphologic atypia. The immature granulocyte progenitor cells labeled by myeloperoxidase (MPO) lacked CD34 immunoreactivity, indicating a degree of maturation (Figure 3B and C). The hematopoietic cells frequently involved glomeruli mimicking glomerulitis (Figure 3D). This cellular infiltrate shared the same morphology and immunophenotype with the cellular component of the patient's myelofibrosis in the bone marrow. Furthermore, CD3 immunostain (not shown) confirmed the lack of T-cell infiltrate in the tubules, thus no tubulitis was present. Focal tubular epithelial cell cytoplasmic vacuolization and arteriolar hyaline change (suspicious for calcineurin inhibitor effect) were also identified. Tubular atrophy was not evident. The interlobular arteries showed moderate intimal fibrosis and were free of vasculitis. No viral inclusions were seen; immunohistochemical stains for BK virus and cytomegalovirus were both negative. There was ∼5–10% interstitial fibrosis. A diagnosis of EMH in myelofibrosis was made, and the patient was referred to the Division of Hematology/Oncology service for further management.
Fig. 1.
Cellular infiltrate composed of immature erythroid cells (black arrow), myeloid progenitor cells (thick arrow) and megakaryocytes (asterisk). The myeloid progenitor cells have large nuclei with open chromatin, indicative of immaturity. Megakaryocytes show ‘cloudy’ nuclei with clumped chromatin, features characteristic of myelofibrosis. H&E ×400.
Fig. 2.
(A) Dense infiltrate of the hematopoietic cells in the interstitium and tubules resulting in focal destruction of tubular structures. The immature nature of the myeloid progenitor cells (double black arrow), characterized by open chromatin and large nuclei, is illustrated. PAS ×600. (B) Infiltration of subcapsular parenchyma. The megakaryocytes (arrow) demonstrate the characteristic ‘cloudy’ nuclear features seen in bone marrow biopsies of myelofibrosis. PAS ×600.
Fig. 3.
(A) Glycophorin (CD235) immunostain highlights erythroid precursors. (B and C)Myeloid progenitor cells show immunoreactivity to MPO (B) but not to CD34 (C), indicating certain degree of maturation. (D) A megakaryocyte in a glomerular capillary loop is highlighted by CD31 immunostain (arrow). G = glomerulus.
Patient follow-up
The patient was started on lisinopril and spironolactone for his proteinuria and lower extremity edema. His proteinuria initially decreased to 1393 mg/g of creatinine (random urine protein–creatinine ratio); however, his serum creatinine worsened to 212.6 µmol/L (2.4 mg/dL) in the subsequent 2 months. A repeat bone marrow biopsy showed progression of myelofibrosis. At this stage, the patient was started on ruloxitinib (Jakafi®, Incyte Corp.) by the oncology service. Over the course of the next 5 months, his serum creatinine level improved to 123.76 µmol/L (1.4 mg/dL) along with a decrease in proteinuria to 600 mg/g of creatinine by random protein–creatinine ratio.
Discussion
Allograft kidney biopsies are commonly performed in patients with elevated serum to evaluate the possibility of acute cellular or antibody-mediated rejection, chronic cellular or antibody-mediated rejection, acute tubular injury, acute interstitial nephritis (allergic or infectious), polyomavirus nephritis, calcineurin inhibitor toxicity and chronic allograft nephropathy. The clinical presentation of our patient was that of a recent renal insufficiency and proteinuria. The clinical impression prior to biopsy was acute rejection and possible transplant glomerulopathy. To our surprise, we found an atypical cellular infiltrate composed of nucleated RBCs, peculiar megakaryocytes and maturing granulocyte progenitor cells that established a diagnosis of EMH.
EMH has been reported in patients with various hematologic disorders, including chronic myeloid leukemia, essential thrombocytosis, myelofibrosis, sickle cell anemia, thrombocytopenia and polycythamia vera [1–8]. The most frequent site of occurrence is the reticuloendothelial system, i.e. the liver, spleen and lymph nodes. It has been described in other sites including the pleura, pericardium, adrenal glands, spinal cord, breast, thyroid and kidney [9]. Ahuja et al. [1] reported a case of EMH presenting as a solitary renal mass in the native kidney of a patient with hemolytic anemia of unknown cause and no history of kidney disease. In their report, they included five additional cases from the literature that EMH had presented as renal mass, one of which was in a patient with myelofibrosis [2]. Since the first report by Moskovitz et al., six additional cases of EMH in the native kidney have been reported in a patient with myelofibrosis, most of whom presented with acute renal failure [2–8]. To our knowledge, no case of EMH has been reported in kidney allograft in the English literature. This is the first case of EMH occurring in kidney allograft, presenting as acute renal insufficiency and proteinuria. While proteinuria is a common presentation for transplant glomerulopathy, the role of EMH in our patient's proteinuria is unclear.
EMH can either be reactive (as the physiological compensation for myelopthesis) or reflect a neoplastic clonal proliferation of atypical hematopoietic stem cells; therefore, it is of paramount importance to characterize the nature of the EMH. However, very few discussions are available on this matter. Kreuziger et al. [5] postulated renal EMH as nonphysiologic, but did not go so far as to categorize it as neoplastic. Mechanistically, Schnuelle et al., suggested that hematopoietic growth factors played a key role in the pathogenesis of this condition, supported by the demonstration of M-CSF, GM-CSF, IL-1b and PDGF expression [6]. With regard to renal localization, Ricci et al. speculated two possibilities: residual niche for hematopoietic stem cell differentiation in the kidney or excess erythropoietin-driven stem cell migration and proliferation [8]. The morphology, immunoprofile and, occasionally, cytogenetic abnormalities have provided evidence that the cells infiltrating the kidney and other organs are the same cell population as the patient's bone marrow disease (as in our patient) suggestive of the neoplastic nature of the infiltrate. It is possible that the allograft kidney provides a suitable milieu for the proliferation and uncontrolled growth of the neoplastic hematopoietic cell originating from the patients' bone marrow. Whether EMH is an appropriate name for such an involvement is debatable. The prognosis and treatment are largely dependent on the underlying etiology.
The cellular infiltrate mainly involves the interstitium with focal spread into glomerular and tubular compartments. It resembles interstitial nephritis in the native kidney. However, in an allograft kidney, it may be confused with acute tubulointerstitial (cellular) rejection. Although, at high magnification, the cells in EMH are heterogeneous and are composed of nucleated RBCs, atypical megakaryocytes, maturing myeloid cells and immature myeloid cells that could be easily mistaken for lymphocytes (especially activated lymphocytes) as would typify acute cellular rejection. Furthermore, acute cellular rejection is by far more common in kidney allograft biopsies than EMH. Therefore, the presence of EMH can be overlooked, especially when the pertinent clinical history is not available at the time of biopsy. Since the management is entirely different, the differentiation between the two processes is essential.
Conflict of interest statement
None declared.
References
- 1.Ahuja S, Grover G, Jha AK, et al. Extramedullary hematopoiesis presented as solitary renal mass: a case report with review of literature. Diagn Cytopathol. 2011;39:435–437. doi: 10.1002/dc.21452. [DOI] [PubMed] [Google Scholar]
- 2.Moskovitz B, Malberger E, Brenner B. Renal extramedullary hematopoiesis simulating hypernephroma. Eur Urol. 1991;19:343–345. doi: 10.1159/000473658. [DOI] [PubMed] [Google Scholar]
- 3.Gibbins J, Pankhurst T, Murray J, et al. Extramedullary haematopoiesis in the kidney: a case report and review of literature. Clin Lab Haematol. 2005;27:391–394. doi: 10.1111/j.1365-2257.2005.00724.x. [DOI] [PubMed] [Google Scholar]
- 4.Woodward N, Ancliffe P, Griffiths MH, et al. Renal myelofibrosis: an usual case of renal impairment. Nephrol Dial Transplant. 2000;15:257–258. doi: 10.1093/ndt/15.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Kreuziger LB, Carlson M, Mesa H, et al. Perinephric extramedullary haematopoiesis in primary myelofibrosis. Br J Haematol. 2012;157:157. doi: 10.1111/j.1365-2141.2012.09053.x. [DOI] [PubMed] [Google Scholar]
- 6.Schnuelle P, Waldherr R, Lehmann KJ, et al. Idiopathic myelofibrosis with extramedullary haematopoiesis in the kidneys. Clinical Nephrol. 1999;52:256–262. [PubMed] [Google Scholar]
- 7.Holt S, Field P, Carmichael P, et al. Extramedullary haematopoiesis in the renal parenchyma as a cause of acute renal failure in myelofibrosis. Nephrol Dial Transplant. 1995;10:1438–1440. [PubMed] [Google Scholar]
- 8.Ricci D, Mandreoli M, Valentino M, et al. Extramedullary haematopoiesis in the kidney. Clin Kidney J. 2012;5:143–145. doi: 10.1093/ckj/sfs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein IM. Idiopathic myelofibrosis: historical review, diagnosis and management. Blood Rev. 1991;5:98–104. doi: 10.1016/0268-960x(91)90041-a. [DOI] [PubMed] [Google Scholar]