Highlight
Abscisic acid specifically upregulates expression of the barley tonoplast intrinsic protein gene HvTIP3;1, which is necessary for ABA inhibition of coalescence of protein storage vacuoles in aleurone cells.
Key words: Abscisic acid, aleurone, cis-acting element, coalescence, protein storage vacuole, tonoplast intrinsic protein.
Abstract
Tonoplast intrinsic proteins (TIPs) are integral membrane proteins that are known to function in plants as aquaporins. Here, we propose another role for TIPs during the fusion of protein storage vacuoles (PSVs) in aleurone cells, a process that is promoted by gibberellic acid (GA) and prevented by abscisic acid (ABA). Studies of the expression of barley (Hordeum vulgare) TIP genes (HvTIP) showed that GA specifically decreased the abundance of HvTIP1;2 and HvTIP3;1 transcripts, while ABA strongly increased expression of HvTIP3;1. Increased or decreased expression of HvTIP3;1 interfered with the hormonal effects on vacuolation in aleurone protoplasts. HvTIP3;1 gain-of-function experiments delayed GA-induced vacuolation, whereas HvTIP3;1 loss-of-function experiments promoted vacuolation in ABA-treated aleurone cells. These results indicate that TIP plays a key role in preventing the coalescence of small PSVs in aleurone cells. Hormonal regulation of the HvTIP3;1 promoter is similar to the regulation of the endogenous gene, indicating that induction of the transcription of HvTIP3;1 by ABA is a critical factor in the prevention of PSV coalescence in response to ABA. Promoter analysis using deletions and site-directed mutagenesis of sequences identified three cis-acting elements that are responsible for ABA responsiveness in the HvTIP3;1 promoter. Promoter analysis also showed that ABA responsiveness of the HvTIP3;1 promoter is likely to occur via a unique regulatory system distinct from that involving the ABA-response promoter complexes.
Introduction
Aleurone cells from mature barley grain are characterized by the presence of many small spherical protein storage vacuoles (PSV) less than 5 μm in diameter. This organelle is easily visible by light microscopy because of the numerous inclusions in the vacuole lumen and the refractive tonoplast due to the presence of oleosomes that form between the inner and outer leaflets of the membrane. The PSV in barley aleurone cells stores proteins, carbohydrates, neutral lipids, and minerals (Jacobsen et al., 1971; Stewart et al., 1988; Yupsanis et al., 1990; Bewley and Black, 1994). During germination, the PSV undergoes metamorphosis from its role as a storage organelle to an acidic, lytic compartment, rapidly hydrolysing the stored polymers in its lumen, often with the use of pre-existing enzymes (Fincher, 1989; Bethke et al., 1997). This transition is a highly regulated process mediated by the phytohormones gibberellic acid (GA) and abscisic acid (ABA); GA promotes and ABA inhibits this process. In cereal aleurone cells, the hydrolysis of vacuolar storage proteins is required to supply the amino acids necessary for GA-induced de novo synthesis of secreted hydrolases (Filner and Varner, 1967). Neutral lipids stored in oleosomes have also been shown to be converted via gluconeogenesis to sugars (Eastmond and Jones, 2005) including a large pool of available ribose required for the synthesis of mRNAs for various hydrolases (Jones, 1972; Chrispeels et al., 1973; Bethke et al., 1998). The synthesis of various hydrolases and their secretion into endosperm are critical for the mobilization of reserves stored in dead starchy endosperm cells during cereal grain germination; the non-photosynthetic embryonic axis depends on sugars and amino acids mobilized from endospermal reserves in its growth until it becomes photoautotrophic. Stored mineral nutrients released provide additional nourishment for the embryo (Fincher, 1989; Jones and Jacobsen, 1991).
This mobilization of PSV polymers is accompanied by dramatic changes in the structure of this organelle (Jones, 1969; Ory and Henningsen, 1975); PSVs gradually increase in size but decrease in number as smaller PSVs coalesce with each other (Jones and Price, 1970). When synthesis of secreted hydrolases has ceased, a single large vacuole (approx. 40 μm in diameter) occupies almost the entire volume of the cell. Such structural change in PSVs appear to be closely associated with the glandular activity of the aleurone cell as a site of secretion of hydrolases (Haberlandt, 1884; Hwang et al., 2003). For example, acidification of the PSV has been known to be required for the activity of proteases and other hydrolytic enzymes such as phytase included in this organelle (Bethke et al., 1996; Kinoshita et al., 1999) to mobilize stored vacuolar polymers. When PSV acidification is inhibited, secretion of α-amylases is reduced and vacuolar coalescence is concomitantly prevented (Hwang et al., 2003).
Tonoplast intrinsic proteins (TIPs) are integral membrane proteins in the vacuolar membrane (Johnson et al., 1989) and are part of a large family of major intrinsic proteins, which are known to facilitate passive transport of small polar molecules across cellular membranes in organisms ranging from bacteria to fungi, animals, and plants (Maurel, 1997). TIPs have been suggested to function as aquaporins that regulate water transport by acting as channels for water and small, uncharged molecules (Chrispeels and Maurel, 1994; Maurel, 1997). All eight barley TIPs possess six transmembrane-spanning domains and two highly conserved Asn-Pro-Ala (NPA) motifs, typical of members of the aquaporin gene family. In addition to their roles as water channels, other roles have also been assigned to TIPs. For example, the large number of TIPs in vacuolar membranes appears to be in excess of what is required merely for physiological water transport, suggesting an additional structural function for this family of proteins (Higuchi et al., 1998). Despite their structural simplicity, vacuoles are multifunctional organelles (Wink, 1993), and different TIP isoforms appear to correlate with different types of vacuole, suggesting a functional link between TIPs and vacuolar function (Jauh et al., 1998, 1999).
Aleurone cells are an excellent model system to study vacuolation. Aleurone layers from mature barley grains consist of a homogeneous population of non-dividing cells filled with small PSVs that fuse to form one large central vacuole, a process that is tightly controlled by GA and ABA. Ever since the progressive vacuolation of rye aleurone cells was described by Haberlandt in 1884, hormonal control of vacuolation of aleurone cells has been characterized in various cereals, including barley (Jones and Price, 1970; Bush et al., 1986), wheat (Kuo et al., 1996), and wild oat (Hooley, 1982). In the current study, we elucidated hormonally altered expression of barley TIP genes in aleurone cells, revealing a new role for TIP in the process of PSV coalescence and central vacuole formation.
Materials and methods
Aleurone layer preparation
Embryoless half grains were prepared by removing the embryo of barley grains (Hordeum vulgare cv. Himalaya). Half grains were first surface sterilized by washing in 7% commercial bleach for 20min with shaking at 125rpm, and the bleach was completely removed by four washes with sterile ddH2O. Any remaining hypochlorite was neutralized by incubating the half grains in 0.01M HCl for 10min. Finally, the half grains were washed four times with ddH2O and placed in sterilized Petri dishes containing Whatman filter paper and 5–7ml of filter-sterilized l-arginine solution (50mM l-arginine/HCl, 20mM CaCl2). After a 4 d incubation at 25 °C, the aleurone layers were isolated by squeezing out the starchy endosperm.
Fluorescein diacetate (FDA) staining
After each treatment, aleurone layers were stained with 10mM FDA (Sigma) in 20mM CaCl2 for 10min, and excess staining solution was removed by washing the layers in 20mM CaCl2.
Plasmid construction
A pH-sensitive green fluorescent protein (GFP; Phluorin) optimized for plant expression (Moseyko and Feldman, 2001) was transferred to pLZUbi, an expression cassette containing the promoter and first intron from the maize (Zea mays) ubiquitin gene and a nopaline synthase terminator, using BamHI and SacI, resulting in pLZUbi-GFP. Genomic DNA containing the HvTIP3;1 gene was PCR amplified from genomic DNA of H. vulgare cv. Himalaya under the following conditions using a thermal controller (PTC-100; MJ Research, Waterdown, MA, USA): a 1min pre-denaturing step at 95 °C was followed by 35 cycles of a 30 s denaturing step at 95 °C, a 1min annealing step at 60 °C, and a 1min extension step at 72 °C. The final extension step was 5min at 72 °C. The PCR product containing HvTIP3;1 was cloned into a pMD20-T vector (Takara Bio, Otsu, Japan), and translationally fused to pLZUbi-GPF at the BamHI site, resulting in the HvTIP3;1–GFP construct used for subcellular localization studies.
The C-terminal coding region of HvTIP3;1, which is highly conserved in HvTIP genes, was amplified from genomic DNA of H. vulgare cv. Himalaya by PCR consisting of a pre-denaturation step of 95 °C 1min and 35 cycles at 95 °C for 10 s, annealing at 58 °C for 30 s, and 72 °C for 30 s, with a final elongation at 72 °C for 5min. The PCR product was first cloned into the pMD20-T vector and transferred to pBluescript containing the partial sequence of β-glucuronidase (GUS) coding region via XbaI and SacII sites, making the construct pSK-dGUS-TIP. Another copy of the PCR product from the pMD20-T vector was cloned into pSK-dGUS-TIP through the XhoI and HindIII sites in reverse orientation, yielding RNAi-TIP3;1. For constructing RNAi-TIP3;1 3′UTR, a 3′ untranslated region (UTR), unique to the HvTIP3;1 gene, was amplified by PCR from genomic DNA of cv. Himalaya barley using the same PCR amplification and cloning strategy as used for RNAi-TIP3;1.
A 2330bp segment of 5′-flanking region of the HvTIP3;1 gene was amplified from genomic DNA of cv. Himalaya barley using primer sets designed from the sequence of cv. Morex barley (http://barleygenome.org). After subcloning into the pMD20-T vector (pMD20-5′HvTIP3;1), this 5′ flanking region was excised with HindIII and PstI. The firefly luciferase gene (LUC+) was excised from pSP-LUC+ with XbaI and NheI and transferred to the XbaI site of pBluescript KS (pBS-KS), resulting in pBS-KS-LUC. The firefly luciferase gene was cut out again from pBS-KS-LUC by BamHI and SacI and ligated into a pLZUbi vector through the same restriction enzyme sites, yielding pLZUbi-LUC. The maize ubiquitin promoter in the pLZUbi-LUC construct was replaced by this HindIII and PstI HvTIP3;1 promoter fragment, finally yielding HvTIP3;1 prm::LUC.
5′-Flanking regions of HvTIP3;1 with different lengths were amplified from pMD20-5′HvTIP3;1 by PCR using an appropriate primer set and subcloned into the pMD20-T vector and transferred to the firefly luciferase-containing construct as described for the HvTIP3;1 prm::LUC construct. The sequences of all PCR-amplified products were confirmed by DNA sequencing.
To identify the specific promoter elements, mutations were introduced into specific regions of the HvTIP3;1 promoter using oligonucleotide-directed in vitro mutagenesis. To facilitate screening of putative mutants, the SacI site was included in the mutagenic sequences. All site-directed mutations (SDMs) were confirmed by DNA sequencing. Each mutagenized HvTIP3;1 promoter was transferred to the firefly luciferase construct as described for the HvTIP3;1 prm::LUC construct.
A 98bp segment of the 5′-flanking region of the cauliflower mosaic virus (CaMV) 35S gene was amplified from the pBI221 vector using the primers M35S FW and M35S RV. After subcloning into the pMD20-T vector (pMD20-5′ CaMV 35S), this 5′ flanking region was removed by digestion with HindIII and PstI. The maize ubiquitin promoter in the pLZUbi-LUC construct was replaced by this HindIII and PstI CaMV 35S promoter fragment, finally yielding M35S::LUC, the CaMV 35S minimal promoter-driven firefly luciferase construct.
The HvTIP3;1 promoter region between nt –89 and –169 was PCR amplified from pMD20-5′HvTIP3;1 using the primer set 1C FW and 1C RV. After subcloning into the pMD20-T vector, this 80bp of the promoter fragment was removed by digestion with HindIII and cloned into M35S::LUC through the HindIII site, resulting in 1C-M35S::LUC. The same 80bp region of the HvTIP3;1 promoter was reamplified by PCR from pMD20-5′HvTIP3;1, using the another set of primers, 2C FW and 2C RV, and subcloned into the pMD20-T vector. This region was removed by digestion with KpnI and transferred to 1C-M35S::LUC into the KpnI site, yielding 2C-M35S::LUC. 3C- and 4C- M35S::LUC.constructs were produced basically in the same way as described above, using the sets of 3C or 4C FW and RV primers via the NdeI site and NotI site, respectively. All primers used in this study are listed in Supplementary Table S1 at JXB online.
Transformation of barley aleurone protoplasts
H. vulgare cv. Himalaya aleurone layers were used to prepare aleurone protoplasts as described by Bethke and Jones (2001). Each flask of protoplasts was made from 40–50 quarter grains and the volume of arginine and cellulase solutions was 3ml. Freshly prepared protoplasts were released into 3–5ml of Gamborg’s medium (Gamborg’s B-5 salts with minimal organics; Sigma, St Louis) augmented with 5mM KNO3, 58mM sucrose, 10mM l-Arginine/HCl, 0.68M mannitol, 111mM glucose, and 10.9mM MES monohydrate, pH 5.4). The protoplast suspension from one to four flasks was filtered through one layer of 50mm nylon mesh in order to remove large debris, and then transferred to a sterile 12ml, round-bottomed culture tube and centrifuged at 60g for 3min at 4 °C in a Combi-514R centrifuge (Hanil Science Industrial, Incheon, Korea). The supernatant was discarded and the protoplast pellet was resuspended gently in 10ml of MW5 (200mM mannitol, 154mM NaCl, 125mM CaCl2, 5mM KCl, 5mM glucose, pH 5–6). The tube was kept on ice for 30–60min and centrifuged as before. The pellet was resuspended in MaMg (0.5M mannitol, 15mM MgCl2, 5mM MES, pH 5.6) at 0.5ml per flask of protoplasts. Protoplast density was at least 106 ml–1. For transformations, 0.3ml of protoplast suspension was transferred to a fresh culture tube and 45–50 μg of purified plasmid DNA was added. Plasmid DNA for transfection was isolated using GeneAll Midiprep Columns (GeneAll Biotechnology Co., Korea) prior to precipitating in isopropanol, washing in 75% ethanol, drying, and resuspending in sterile water to at least 1mg ml–1. After a 5min incubation, the protoplasts were swirled gently to resuspend, and 0.3ml of polyethylene glycol (PEG) solution [40% PEG 4000 or 6000, 0.4M mannitol, 0.1M Ca(NO3)2, pH 7–9] was added and mixed gently. The cells were incubated at room temperature for 30min. The PEG-containing solution was added to MW5 over approximately 10min by repeatedly (eight times) adding 1ml of MW5 and mixing. The protoplasts were centrifuged as before, resuspended in 1.5ml of Gamborg’s medium containing an additional 0.08M glucose, transferred to 25ml sterile flasks, and treated with hormones at the indicated concentration as necessary. Transient expression assays were repeated at least three times and a typical result is presented for PSV fusion experiments. To assay for promoter activity, the plasmid 35S::RUC served as the internal control and firefly luciferase activity from the specific promoter was normalized to Renilla luciferase activity from co-transfected protoplasts.
Fluorescence microscopy
GFP or FDA fluorescence from living barley aleurone protoplasts or aleurone layers was visualized using a Zeiss Axioskop fluorescence microscope (Zeiss, Shinjuku, Tokyo, Japan), with an X-Cite 120 fluorescence microscope illumination (Lumen Dynamics, Ontario, Canada). Digital images were captured with an AxioCam MRm Microscope Cameras (Carl Zeiss, Gottingen, Germany). Adjustments to image brightness and contrast were made using Adobe Photoshop (San Jose, CA, USA).
RNA analysis
Barley aleurone layers were ground to a fine powder with a mortar and pestle in liquid N2 and dissolved in mixed solution at a ratio 0.7:1:1 of TLE/SDS buffer, equilibrated with phenol (Bioneer, Daejeon, Korea) and chloroform, vortexed vigorously, and incubated with shaking for 10min. The aqueous phase was separated from the organic phase by a short centrifugation and transferred to a new tube. Total RNA was separated from DNA and other contaminants using TRI Reagent (Takara Bio, Otsu, Japan).
Quantitative real-time RT-PCR
Total RNA (1 μg) was used for synthesis of first-strand cDNA using an iScript™ cDNA Synthesis kit (Bio-Rad, Richmond, CA, USA) following the manufacturer’s instructions. Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed using a SYBR Premix Ex Taq™ kit (Takara Bio). First-strand cDNA (1 μg) was used as the template for PCR. PCR cycling conditions were 40 cycles after a 10min pre-denaturing step at 95 °C, with a 30 s denaturing step at 95 °C, a 1min annealing step at 60 °C, and a 1min extension step at 72 °C. The final extension step was 5min at 72 °C. The accumulation of fluorescent PCR products was monitored using a Thermal Cycler Dice Real Time System (Takara Shuzo, Japan). All procedures were performed according to the manufacturers’ instructions. The relative amplification of the barley ubiquitin gene was used as an internal control to normalize all data. Triplicates of a sample were examined to evaluate quantitative variation for each sample, and each experiment was repeated at least twice. The gene-specific primers used for quantitative PCR are listed in Supplementary Table S1.
Results
GA and ABA regulate PSV coalescence and HvTIP expression in barley aleurone cells
In freshly imbibed barley grains, aleurone cells are filled with numerous small PSVs, coalescence of which is hormonally controlled. Figure 1 shows the process of vacuolation in aleurone cells treated with GA, ABA, or no hormone up to 24h. To assess changes in PSVs, aleurone layers were stained with FDA, a marker for the cytosol. Since fluorescein is excluded from vacuoles, the vacuole appears dark against a bright background. The progression in PSV coalescence following treatment of aleurone layers with and without ABA and GA is shown in Fig. 1. Coalescence of PSVs was already discernible at 12h in GA-treated aleurone cells, and one large central vacuole was established by 24h. By contrast, PSV coalescence was much slower in ABA-treated cells, and many small PSVs remained even after 24h. Vacuolation of aleurone cells incubated without hormones was intermediate between GA- and ABA-treated cells (Fig. 1).
Fig. 1.
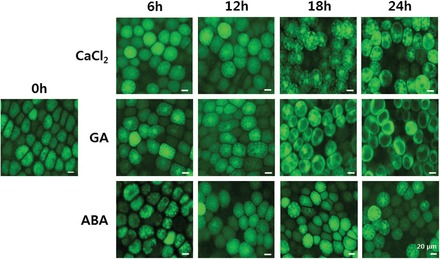
GA promotes and ABA prevents formation of the central vacuole in barley aleurone cells. Isolated barley aleurone layers were incubated in CaCl2 medium containing 5 μM GA, 10 μM ABA, or no hormone and examined for the progress of vacuolation at the indicated times up to 24h after FDA staining by fluorescence microscopy.
We used protoplasts isolated from aleurone layers to determine whether hormone treatments affected the volume of aleurone cells. Whereas there were differences in vacuolation between GA, ABA, and no-hormone treatments, there were no significant differences in protoplast size (Fig. 2). Because protoplast size did not change during PSV fusion, we inferred that there is a concomitant reduction in the surface area of the tonoplast as PSVs coalesce. Thus, the average size of PSVs in freshly isolated aleurone cells was about 4–5 μm whereas after 24h of GA treatment the central vacuole was ~40 μm, an 8- to 10-fold difference in tonoplast surface area. Since TIPs are known to be one of the most abundant integral membrane proteins in vacuoles, we hypothesized that the alteration in abundance of TIPs may accompany the 10-fold change in the amount of tonoplast membrane. We therefore examined the effect of GA or ABA on the expression of HvTIP genes.
Fig. 2.
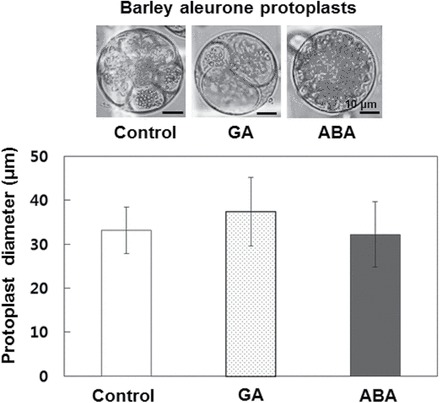
The volume of aleurone protoplasts is not affected by the presence or absence of ABA or GA. Barley aleurone protoplasts were prepared and incubated in protoplast culture medium containing 5 μM GA, 10 μM ABA, or no hormone for 24h and the diameters of the protoplasts were measured. Error bars indicate standard deviation.
Eight putative barley TIP genes have been identified from the contiguous sequences predicted from the expressed sequence tag database. These genes fall into five groups (HvTIP1–5) based on a phylogenetic tree analysis using amino acid alignment (Ligaba et al., 2011). We monitored expression of all eight barley TIP genes in aleurone cells treated with GA, ABA or no hormone during the period (24h) in which the progress of PSV coalescence was examined (Fig. 3). Our qRT-PCR analysis of relative transcript abundance suggested that four HvTIP members (HvTIP1;1, -1;2, -2;1, and -3;1) were the major TIP members expressed in aleurone cells, while expression of the others remained at quite low levels regardless of treatment (Fig. 3). For example, in freshly isolated aleurone cells, the most abundantly expressed gene was HvTIP1;2, which accounted for more than 61% of total HvTIP gene expression, followed by HvTIP3;1 (21%), -2;1 (8.8%), and -1;1 (7.6%). Expression of the remaining TIP members was less than 1.2% of total expression (data not shown). With the exception of HvTIP1;2 and HvTIP3;1, the majority of HvTIP multigenes showed no hormonal responsiveness in their levels of expression. Transcript levels of HvTIP1;2 and -3;1, the two most highly expressed HvTIP members, were specifically decreased by GA, whereas the expression of HvTIP3;1 was rapidly increased by up to 70-fold upon ABA treatment (Supplementary Fig. S1 at JXB online). HvTIP3;1 was the second most abundantly expressed TIP gene in freshly isolated aleurone layers and accounted for more than 90% of total TIP expression in ABA-treated aleurone cells.
Fig. 3.
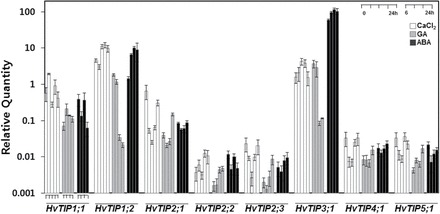
HvTIP genes are differentially expressed in aleurone cells treated with GA and ABA. Barely aleurone layers were isolated from freshly imbibed seeds and incubated in medium containing 5 μM GA, 10 μM ABA, or no hormone, and total RNA was extracted at the indicated times to measure expression of each HvTIP member by qRT-PCR. Expression of each member of the HvTIP multigene family was normalized by the expression level of the internal control barley ubiquitin gene. The relative ratio between the transcript abundance of the HvTIP gene and ubiquitin gene was plotted on the y-axis, revealing the relative abundance of each HvTIP transcript. Error bars represent standard deviation.
ABA inhibition of PSV coalescence is mediated by upregulation of HvTIP3;1 expression in aleurone cells
Since expression of HvTIP3;1 was specifically enhanced by ABA, our functional analysis of barley TIP genes focused on this member. Repetitive failure to amplify the full-length cDNA for the HvTIP3;1 isoform led us to use genomic DNA including the open reading frame of this gene to produce a translational fusion of HvTIP3;1 to the N-terminal region of the GFP gene (Fig. 4A). Subcellular localization studies of HvTIP3;1 using this construct shows that this protein is targeted to the vacuolar membrane, contrasting sharply with expression of GFP, which was cytoplasmically targeted (Fig. 4B). Some diffuse fluorescence was also detectable outside the edge of the vacuole, which may reflect the activity of the secretory pathway.
Fig. 4.
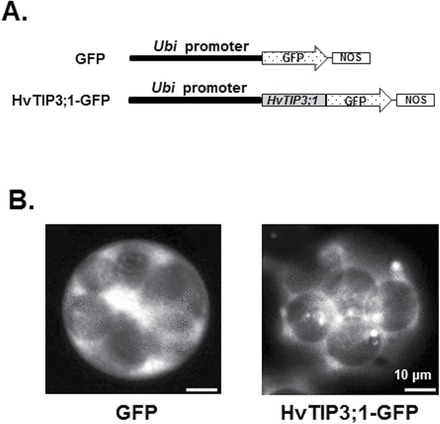
HvTIP3;1 is expressed on the tonoplast of barley aleurone cells. (A) Diagram of the constructs for subcellular localization studies. The genomic DNA fragment containing the open reading frame for HvTIP3;1 was PCR amplified and translationally fused to the N-terminal region of the GFP protein. (B) Subcellular localization of HvTIP3;1 in an aleurone cell. Barley aleurone protoplasts were transfected with HvTIP3;1–GFP and the subcellular localization of GFP was examined by fluorescence microscopy after 18h of incubation.
Since the pattern of hormonal regulation of two major TIP genes correlated strongly with the effects of ABA and GA on vacuolation, we investigated the potential role of TIP in PSV coalescence using an RNAi-TIP3;1 effector construct. This RNAi vector contained the C-terminal region of the HvTIP3;1 coding sequence, which is highly conserved throughout all HvTIP genes (Supplementary Fig. S2 at JXB online). Barley aleurone protoplasts were co-transfected with the plasmid containing a GFP expression cassette (Ubi::GFP) and either the RNAi-TIP3;1 effector construct or the empty vector (pLZUbi), as shown in Fig. 5A, and incubated in medium containing ABA. To provide a quantitative analysis, the extent of PSV coalescence of aleurone cells was determined based on the index of five stages of vacuolation as shown in Fig. 5B. The number of cells displaying the specific stage of vacuolation was monitored from 6 to 30h after transfection (Fig. 5C). Bright-field microscopy showed that most freshly isolated protoplasts represented stages 1 and 2 of vacuolation. Cells expressing GFP were detectable 6h after transfection, and a difference in the degree of vacuolation was already observed between cells of control and RNAi-TIP3;1 at this time. Scoring of vacuolated cells during incubation from 6 to 30h of ABA treatment showed clearly that PSV fusion was more advanced in RNAi-TIP3;1-transfected cells than in controls. For example, among cells co-transfected with the empty vector, about 40% of cells were at stage 2 of vacuolation after 30h of incubation. By contrast, in cells expressing the RNAi-TIP3;1 effector, more than 40% of cells represented stage 4, indicating that more extensive PSV fusion had occurred when TIP expression was repressed. Another RNAi construct was designed using 167bp of the 3′UTR of the HvTIP3;1 gene, the sequence of which is highly specific to this gene, as shown in Fig. 5A. This RNAi-TIP3;1 3′UTR construct was also effective in interfering with ABA-induced central vacuole formation. For example, among aleurone protoplasts co-transfected with RNAi-TIP3;1 3′UTR/Ubi::GFP, around 45% of cells were at stage 4 of vacuolation, while 50% of cells transfected with the empty vector/GFP control were at vacuolation stage 2 (Fig. 5D). All these results suggested that high-level expression of HvTIP3;1 is necessary for ABA action on PSV coalescence.
Fig. 5.
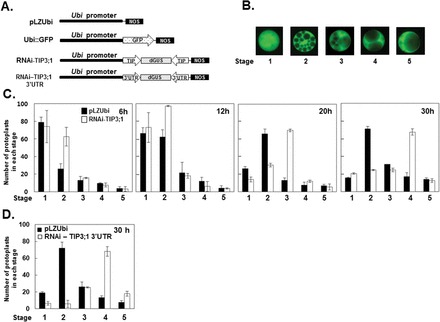
RNAi for the HvTIP3:1 gene interferes with ABA-mediated inhibition of PSV coalescence. (A) Diagram of the constructs used in transient expression assays. The C-terminal coding region or 167bp of the highly gene-specific 3’UTR of HvTIP3;1 was used to construct RNAi vectors to repress expression of HvTIP3;1 in aleurone cells. Expression of RNAi constructs and the GFP gene was under the control of the maize ubiquitin promoter, which works effectively as a constitutive and strong promoter in monocot grain. (B) Five stages of vacuolation in barley aleurone cells. The five stages indicated were used as an index of vacuolation in aleurone cells. (C) Transient expression assay of RNAi-TIP3;1 for PSV coalescence. Barley aleurone protoplasts were co-transfected with RNAi-TIP3;1/Ubi::GFP or empty vector/Ubi::GFP and incubated in medium containing ABA (10 μM) for up to 30h. At the indicated times, protoplasts from each transfection were examined for the status of vacuolation using the five categories indicated in (B). Bars indicate the number of protoplasts of each stage ± standard deviation after incubation with ABA for the indicated time. (D) Transient expression assay of RNAi-TIP3;1 3’UTR for PSV coalescence. The co-transfected protoplasts were assayed after 30h of incubation with ABA (10 μM) as described above.
Additional experiments using HvTIP3;1–GFP, which was used to study the subcellular localization of TIP, further confirmed the positive role of HvTIP3;1 in ABA inhibition of PSV coalescence (Fig. 6A). Aleurone protoplasts were transfected with either HvTIP3;1–GFP or empty vector and incubated in medium containing GA up to 30h. Our data showed that expression of HvTIP3;1–GFP delayed the vacuolation process in GA-treated aleurone cells compared with that of the control (Fig. 6B, C). For example, in cells overexpressing HvTIP3;1–GFP, more than 60% of GA-treated cells were at stage 3, while in controls more than 60% of cells were at stage 5 of vacuolation. Furthermore, when the RNAi-TIP3;1 effector construct was co-expressed with HvTIP–GFP, the inhibitory effect of HvTIP–GFP on PSV coalescence was alleviated, indicating that RNAi-TIP3;1 exerted its silencing effect through its effect on TIP gene expression (Fig. 6D). Taken together, these gain- and loss-of-function analyses confirm that ABA-enhanced expression of HvTIP3;1 contributes to ABA-mediated inhibition of PSV coalescence.
Fig. 6.
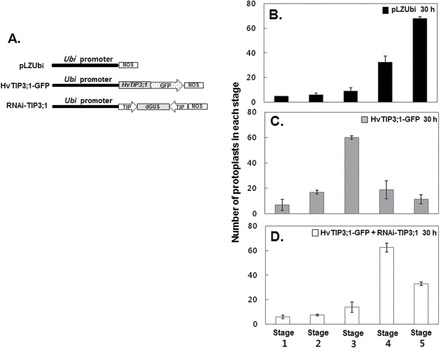
Expression of HvTIP3:1–GFP slows down the PSV coalescence process. (A) Diagram of the constructs used in the transient expression assay. The GFP vector used in the subcellular localization studies shown in Fig. 4A and the RNAi-TIP3;1 construct shown in Fig. 5A were used to examine the effect of HvTIP3;1 overexpression. (B) Transient expression assay for PSV coalescence. Barley aleurone protoplasts were co-transfected with empty vector/Ubi::GFP, HvTIP3;1–GFP/Ubi::GFP, or HvTIP3;1–GFP/Ubi::GFP plus RNAi-TIP3;1 and incubated in medium containing GA (10 μM) for 30h. After incubation, the protoplasts from each transfection were examined for the status of vacuolation using the five categories indicated in Fig. 4B. Bars indicate the number of protoplasts of each stage ± standard deviation after 30h of incubation with GA.
Hormonal regulation of HvTIP3;1 gene expression is largely mediated by transcriptional control
The antagonism between GA and ABA is a major factor regulating the developmental transition from seed formation to germination (Ho et al., 2003). Antagonism between GA and ABA is also observed in the process of PSV coalescence. For example, ABA suppression of vacuolation in aleurone cells could be partially reversed when high concentrations of GA (100 μM) were added at the same time as ABA, suggesting that hormonal regulation of PSV coalescence is also under an antagonistic relationship of GA and ABA (Fig. 7A). The expression of HvTIP3;1 by ABA and GA treatments were matched by the progression of vacuolation. For example, expression of HvTIP3;1 was increased significantly in ABA-treated cells, but its induction level was reduced by up to 25% when aleurone cells were co-incubated with 10 μM ABA and 100 μM GA (Fig. 7B).
Fig. 7.
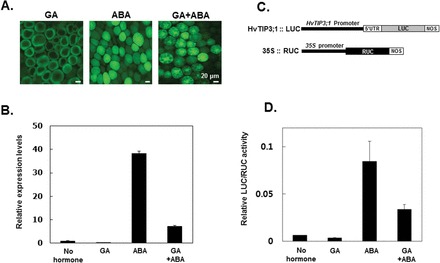
Expression of HvTIP3;1 is induced by ABA and repressed by GA at the level of transcription. (A) Antagonistic effects of GA and ABA on PSV coalescence. Barley aleurone layers were incubated in medium containing 5 μM GA, 10 μM ABA, or 100 μM GA plus 10 μM ABA for 24h. Vacuolation of aleurone cells was examined after staining with FDA. (B) HvTIP3;1 expression in aleurone cells incubated in medium containing 5 μM GA, 10 μM ABA, or 10 μM ABA plus 100 μM GA for 24h. Transcript abundance for HvTIP3;1 was examined by qRT-PCR. Error bars indicate standard deviation. (C) Diagram of the HvTIP3;1::LUC construct. The 5’-flanking region (2330bp) of HvTIP3;1 was PCR amplified from the genomic DNA of barley cv. Himalaya and transcriptionally fused to the firefly luciferase (LUC) reporter gene. The Renilla luciferase (RUC) gene under the control of the 35S promoter was used as an internal control for normalization of differential transfection efficiency. (D) Transient expression assay for responsiveness of HvTIP3;1 promoter activity to GA and ABA. Barley aleurone protoplasts were co-transfected with HvTIP3;1 promoter::LUC and 35S::RUC and incubated in a medium containing 5 μM GA, 10 μM ABA, or 10 μM ABA plus 100 μM GA for 24h. The LUC/RUC ratio represents the specific HvTIP3;1 promoter activity. Error bars indicate standard deviation. (This figure is available in colour at JXB online.)
The sequence of the HvTIP3;1 promoter was retrieved from the genome sequence of barley cv. Morex, available through the IPK Barley BLAST Server (http://webblast.ipk-gatersleben.de/barley/viroblast.php) of the International Barley Sequencing Consortium. The promoter region of HvTIP3;1 was PCR amplified from barley cv. Himalaya and analysed to characterize the molecular mechanism for ABA induction of HvTIP3;1 gene expression (Fig. 7C). As shown in Fig. 7D, the activity of the HvTIP3;1 promoter was regulated in the same way as the endogenous gene, indicating that hormonal regulation of HvTIP3;1 gene expression is most likely mediated at the transcriptional level.
Upregulation of the HvTIP3;1 promoter activity is not mediated by the ABA-response promoter complex (ABRC)
Since 2330bp of the 5′-flanking region of HvTIP3;1 including 83bp of the 5′UTR could confer ABA responsiveness, as observed for the endogenous gene as shown in Fig. 7C, the cis elements responsible for ABA regulation must be contained within this region. As shown in Fig. 8A, a series of 5′ deletions of the HvTIP3;1 promoter was produced by PCR, and the ABA responsiveness of each construct was examined by a transient expression assay (Fig. 8B). A deletion construct containing 417bp of the 5′-flanking region of HvTIP3;1 showed ABA induction (11.8-fold) that was comparable to that of the undeleted construct (9.5-fold). The deletion construct of nt –182 had about a 50% reduction in promoter activity, but still retained 6.6-fold of ABA induction capability, suggesting that the region between nt –417 and nt –182 contains promoter element(s) that support promoter strength. A further deletion to nt –117 abolished ABA responsiveness (1.7-fold) with a major loss of the promoter activity, indicating that critical promoter elements required for ABA induction are located between nt –182 and nt –117 of the 5′-flanking region of this gene.
Fig. 8.
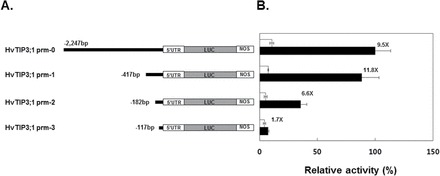
The region between nt –116 and –182 delivers ABA responsiveness to the HvTIP3;1 promoter. (A) Diagram of the deletion series of the HvTIP3;1 promoter. Successive 5’ promoter deletions were generated by PCR and transcriptionally fused to the firefly luciferase (LUC) reporter gene. (B) Transient expression assays for the responsiveness of the 5’ deletion series of the HvTIP3;1 promoter to ABA. Barley aleurone protoplasts were co-transfected with HvTIP3;1 promoter::LUC and 35S::RUC and incubated in medium with or without ABA (10 μM) for 24h. The specific activities of native and 5’ successively deleted HvTIP3;1 promoters were expressed as the LUC/RUC ratio and are presented relative to the LUC/RUC ratio of the undeleted HvTIP3;1 promoter from ABA-treated cells. The promoter activities from no-hormone and ABA-treated cells are indicated as open and closed bars, respectively. Error bars indicate standard deviation.
Figure 9B shows the effects of mutations of the HvTIP3;1 promoter spanning from nt –89 to nt –169 and additional mutation of the ACGT core at nt –252 on ABA responsiveness (Fig. 9A). Mutation of the distal ACGT core-containing sequence located at nt –252 did not affect the ABA responsiveness of this promoter because it showed an 8.3-fold induction compared with that of the control (9.79-fold). However, all mutations of the ACGT core-containing sequences located at the region between nt –89 and nt –169 significantly affected ABA induction of the promoter. For example, mutation SDM#2 decreased ABA responsiveness up to 42% relative to the non-mutated promoter. A mutation at location SDM#5 significantly reduced the ABA response to 2.6-fold relative to that of the control at 9.6-fold, and mutation SDM#7 also reduced ABA responsiveness as much as that of SDM#5 (Fig. 9B). When the two copies of the 80bp fragment from HvTIP3;1 promoter from nt –89 to nt –169 were fused to the 98bp minimal promoter from the CaMV 35S gene, the activities of the minimal promoter became ABA responsive. For example, in contrast to the minimal 35S promoter, the recombinant promoter containing two or four copies of the 80bp HvTIP3;1 promoter fragment showed at least 9.5- and 18-fold higher levels of promoter activity in response to ABA, respectively (Fig. 9C).
Fig. 9.
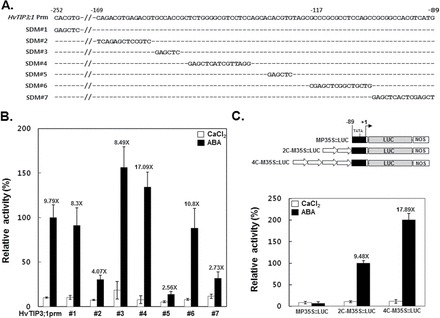
Three cis-acting elements are responsible for the ABA responsiveness of the HvTIP3;1 promoter. (A) Sequences of the native HvTIP3;1 promoter and SDMs in the promoter. Sequences matching the native promoter are indicated with ‘–’. (B) Transient expression assay for the activities of the native and mutated HvTIP3;1 promoters in response to ABA. Barley aleurone protoplasts were co-transfected with each of the mutated constructs of HvTIP3;1 promoter::LUC and 35S::RUC and incubated in medium with or without ABA (10 μM) for 24h. The specific activities of native and mutated promoters were expressed as the LUC/RUC ratio and are presented relative to the LUC/RUC ratio of the native HvTIP3;1 promoter from ABA-treated cells. The promoter activities from no-hormone and ABA-treated cells are indicated as open and closed bars, respectively. The number indicated above the bar represents the fold induction. Error bars indicate standard deviation. (C) Transient expression assay for the activities of the minimal 35S promoter containing two copies of an 80bp fragment of the HvTIP3;1 promoter in response to ABA. Expression of the luciferase reporter gene was driven by 98bp of the minimal CaMV 35S promoter (M35S::LUC) or by the minimal promoter containing two or four copies of the 80bp fragment of the HvTIP3;1 promoter from –89 to –169 nt (2C-M35S::LUC). Barley aleurone protoplasts were co-transfected with M35S::LUC/35S::RUC or 2C- or 4C-M35S::LUC/35S::RUC and incubated in medium with or without ABA (10 μM) for 24h. The specific promoter activities were expressed as described above.
Two barley transcription factors, HvABI5, a basic domain/leucine zipper (bZIP), and HvVP1 have been shown to be necessary for ABA induction of gene expression that is mediated by the ABRC, consisting of an ACGT core-containing element and a coupling element (CE) (Casaretto and Ho, 2003). Since the ACGT core-containing sequences appear to be critical for ABA induction of the HvTIP3;1 promoter activity, we investigated the effects of knockdown of HvVIP1 and HvABI5 using RNAi constructs against them (Fig. 10A). Knockdown of these ABA signalling components clearly interfered with the ABA inhibition of PSV coalescence, as indicated in the promotion of the vacuolation process of the protoplasts expressing RNAi for either of them (Fig. 10B). However, neither the native HvTIP3;1 promoter nor the ABA-responsive recombinant promoter, 4C-M35S was downregulated at all in their ABA responsiveness by knockdown of these ABA signalling components (Fig. 10C, D).
Fig. 10.
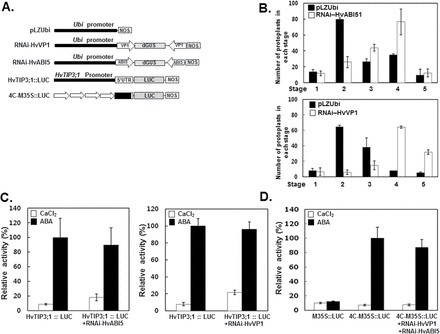
Activation of the HvTIP3;1 promoter is not mediated by HvVP1 and HvABI5. (A) Diagram of the RNAi constructs for HvVP1 and HvABI5. The coding regions of HvVP1 and HvABI5 were used to construct the RNAi vectors RNAi-HvVP1 and RNAi-HvABI5. (B) Transient expression assay for PSV coalescence. Barley aleurone protoplasts were co-transfected with empty vector/Ubi::GFP plus RNAi-HvABI5 or RNAi-HvVP1 and incubated in medium with or without ABA (10 μM) for 30h. (C, D) Transient expression assay for the effect of suppression of HvVP1 and HvABI5 expression on ABA activation of the native HvTIP3;1 promoter (C) or the ABA-responsive 4C-M35S promoter (D). Barley aleurone protoplasts were co-transfected by one of the firefly luciferase constructs (HvTIP3;1::LUC or 4C-M35S::LUC) and 35S::RUC with or without each effector RNAi construct and incubated in medium with or without ABA (10 μM) for 24h. The relative LUC/RUC ratio represents the specific activity of the HvTIP3;1 promoter. The relative LUC/RUC activities from control and ABA-treated cells are indicated as open and closed bars, respectively. Error bars indicate standard deviation.
Discussion
In the current study, we investigated the coalescence of PSV, a vacuolar structural change that is a highly co-integrated process, with the functional metamorphosis of PSV from a storage compartment to a lytic organelle during cereal seed germination. Here, we present evidence showing that hormonal control of the expression of TIP protein underlies the hormonal regulation of PSV coalescence in the aleurone cell. Expression of HvTIP3;1 was strongly induced and repressed by ABA and GA, respectively (Figs 3 and 7), and loss- or gain-of-function analysis for this TIP protein modulates hormonally controlled progression of vacuolation in aleurone cells (Figs 5 and 6), suggesting the positive role of a TIP in maintaining the architecture of small PSVs. The ABA signalling path leading to the transcriptional activation of HvTIP3;1 is different from that involving the ABRC1 complex, which is transactivated by HvVP1 and HvABI5 (Figs 8–10).
PSV coalescence and TIP gene expression are hormonally regulated in aleurone cells
TIP proteins are encoded by multiple genes in the genome of various plants such as maize (14 genes), rice (10 genes), and Arabidopsis (10 genes). Recent phylogenetic analysis using a full-length alignment of genomic DNAs classified TIP genes into five subfamilies, TIP1–5. Barley TIP genes belong to each of these five subfamilies and these were previously designated as γ (TIPs 1;1, 1;2, and 1;3), δ (TIPs 2;1, 2;2 and 2;3), α (TIP 3;1), β (TIP 3;2), ε (TIP 4;1), and ζ (TIP 5;1) (Johanson et al., 2001; Ligaba et al., 2011). TIPs are associated with many endomembranes in plants but α- and δ-TIPs have been shown to reside on the tonoplast of PSVs and to be useful markers for this organelle (Hoh et al., 1995; Paris et al., 1996; Jauh et al., 1999). The dramatic structural changes that accompany the functional transition from storage vacuole to an acidic, lytic organelle are also accompanied by changes in TIP isoforms. For example, storage vacuoles are marked by the presence of α- and δ-TIPs on their tonoplast, whereas lytic vacuoles, which are functionally equivalent to mammalian lysosomes, have γ-TIP.
α-TIP (HvTIP3;1) and γ-TIP (HvTIP1;2) are the most abundantly expressed TIPs in aleurone cells (Fig. 3). Interestingly, the δ -TIPs (HvTIP2;1, -2;2 and -2;3) of barley are expressed at low levels in aleurone cells and show no response to ABA or GA, emphasizing the role of α-TIP in maintenance of PSV as a storage vacuole. We showed that, in aleurone cells, GA specifically downregulates expression of HvTIP1;2 and -3;1 by as much as 100-fold, whereas ABA upregulates HvTIP3;1 expression by 70-fold (Supplementary Fig. S1). Because these are the major TIPs in aleurone cells, pronounced hormonal control of their expression by ABA and GA is likely to cause a significant alteration in the total amount of TIP proteins (Fig. 3). α-TIP (TIP 3:1) has been shown to be localized to the tonoplast of developing (Ibl et al., 2014) and mature (Jauh et al., 1999; Schuurink et al., 1996) aleurone cells and we have now shown that the TIP 3;1–GFP construct was localized to the PSV tonoplast in freshly isolated aleurone protoplasts (Fig. 4), an important observation establishing that turnover of the PSV tonoplast occurs in mature, freshly isolated aleurone protoplasts. The upregulation of HvTIP3;1 expression by ABA is also consistent with these data on tonoplast turnover, because ABA is required to maintain the integrity of small PSVs and to prevent formation of the large central vacuole.
GA-induced repression of the expression of the TIP 1;2 and TIP 3;1 genes is also consistent with the observation that the surface area of the PSV tonoplast was greatly reduced in GA-treated cells. When cells were treated with GA, there was an 8- to 10-fold reduction in the surface area of the tonoplast (Figs 1, 2 and 7) without a concomitant change in cell volume (Fig. 2). Taken together, these data show that PSV architecture is dynamically maintained through active expression of TIP proteins.
HvTIP3;1 and PSV coalescence in aleurone cells
Our gain- and loss-of-function experiments indicated that HvTIP3;1 plays a key role in regulating PSV fusion and the formation of the large central vacuole. An RNAi construct targeted to HvTIP3;1 allowed PSV fusion in the presence of ABA, showing that the effect of ABA on fusion requires an α-TIP (Fig. 5). Overexpression of HvTIP3;1, on the other hand, blocked GA-induced PSV fusion and formation of the large central vacuole (Fig. 6). These experiments emphasize the importance of α-TIP in vacuole function in aleurone cells and show that the effects of both ABA and GA can be blocked by the manipulation of this key tonoplast protein.
Little is currently known about the mechanism of PSV fusion and the link between TIP and this process. PSV coalescence is likely to involve SNAREs [soluble NSF attachment protein (SNAP) receptors], which are known to mediate all intracellular membrane fusion events (reviewed by Wickner and Haas, 2000). Previously, Hwang et al. (2003) showed that PSV fusion in barley aleurone cell is closely integrated with vacuolar acidification, and vacuole acidification has been shown to be a pre-requisite for trans-SNARE complex formation during docking in yeast (Ungermann et al., 1999). Barley has homologues of yeast proteins that interact with SNARES. HVA22 is the barley homologue of the yeast protein Yop1p, which physically interacts with Ypt7p, facilitating concentration of SNAREs and other proteins to activate the vacuole fusion machinery in vivo (Haas et al., 1995; Starai et al., 2007; Guo and Ho, 2008). Loss of function of HVA22 via transformation with HVA22 RNAi demonstrated that this protein inhibited GA-induced PSV coalescence in aleurone cells (Guo and Ho, 2008), indicating that the molecular machinery for barley is similar to that in yeast. One hypothetical role for α-TIP in regulating vacuolar fusion in aleurone cells could result from its high abundance in the vacuolar membrane, perhaps interfering with the accessibility of cognate SNAREs with each other, preventing efficient PSV fusion. Alternatively, it is worthwhile examining a possible link between TIP and the vacuolar acidifying process via overexpression or knockdown expression of TIP genes.
Upregulation of specific HvTIP genes via ABA signalling in aleurone cells
As is the case with many of the hormonal responses of the cereal aleurone to hormones, ABA and GA act antagonistically to regulate the transcription of HvTIP3;1. As shown in Fig. 7A and B, ABA greatly enhanced the expression of HvTIP3;1 and prevented vacuole fusion, whereas GA promoted the formation of the large central vacuole while suppressing HvTIP3;1. The effects of simultaneous application of ABA and GA were intermediate between the effects when these hormones were added separately. Transient expression assays using deletions from the HvTIP3;1 promoter showed that hormone-induced transcriptional control was confined to a 2330bp 5′-flanking region (2247bp of the promoter and 83bp of the 5′UTR). This region was necessary and sufficient to support transcriptional regulation in response to both ABA and GA (Fig. 7D). Promoter analyses via a 5′ deletion series and site-directed mutagenesis showed that the ABA responsiveness of the HvTIP3;1 promoter was mediated by three cis elements (SDM#2, -#5, and -#7). Each of these cis elements contained the ACGT core sequence. Since mutation in any one of these three core sequences led to a significant reduction in ABA responsiveness, they appear to work non-redundantly for delivering the ABA response to the promoter. The importance of these elements was further supported by the finding that the 98bp minimal 35S promoter could be responsive to ABA by fusion to the HvTIP3;1 upstream promoter fragment containing all of these three cis elements (Fig. 9C). Interestingly, the HvTIP3;1 promoter contained ABRC1 (ABA-response complex 1) located at nt –123 to nt –156, which was previously identified to be critical for the ABA responsiveness of the barley ABA-responsive gene HVA22 (Shen and Ho, 1995). The ABRC1 of the HVA22 promoter consists of an ABRE (ABA-response element; GCCACGTACA) and a CE1 element (TGCCACCGG) that are 22bp apart (Shen and Ho, 1995). Although the HvTIP3;1 promoter contains an almost identical CE-1 element (5′TGCCACCGC3′), which is 28bp away from the ABRE (SDM#5), it is unlikely that ABRC1-mediated signalling is involved in the regulation of this promoter because ABA responsiveness of the promoter was not affected by a mutation in the CE-1-like element (SDM#3). These data on cis-element analysis are consistent with our observation showing that ABA induction of the HvTIP3;1 promoter and 4C-M35S promoter was not affected when ABRC1 function was lost (via RNAi against HvABI5 and HvVP1) (Fig. 10C, D). HvABI5 and HvVP1, the barley orthologue of VP1, are required for the ABA activation of the promoter activity via ABRC1 (Casaretto and Ho, 2003). These results showed that the effects of ABA in the cereal aleurone can be mediated via several different signalling pathways.
In summary, our data establish the importance of TIPs located on the tonoplast of PSVs in the hormonal regulation of vacuolation in barley aleurone cells. Based on our data, we propose that ABA prevents the coalescence of PSVs by inducing α-TIP expression, while GA promotes central vacuole formation by reducing the abundance of α-TIP. To our knowledge, this is the first report on the specific function of a TIP protein that is related to vacuolar identity.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Table S1. The list of the primers used in the study.
Supplementary Fig. S1. Hormonal responsiveness of HvTIP members in aleurone cells.
Supplementary Fig. S2. The alignment of the nucleotide sequences of the highly conserved coding region throughout the barley TIP genes.
Acknowledgements
This work was supported by grants from the Next-Generation BioGreen 21Program (no. PJ008198022014), Rural Development Administration, Republic of Korea, and by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (no. 2012R1A1A2006267).
Glossary
Abbreviations:
- ABA
abscisic acid
- ABRC
ABA-response promoter complex
- CaMV
cauliflower mosaic virus
- GA
gibberellic acid
- GUS
β-glucuronidase
- FDA
fluorescein diacetate
- GFP
green fluorescent protein
- PSV
protein storage vacuole
- qRT-PCR
quantitative reverse transcription-PCR
- SDM
site-directed mutation
- TIP
tonoplast intrinsic protein
- UTR
untranslated region.
References
- Bethke PC, Hillmer S, Jones RL. 1996. Isolation of intact protein storage vacuoles from barley aleurone (identification of aspartic and cysteine proteases). Plant Physiology 110, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke PC, Jones RL. 2001. Cell death of barley aleurone protoplasts is mediated by reactive oxygen species. The Plant Journal 25, 19–29. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Schuurink RC, Jones RL. 1997. Hormonal signalling in cereal aleurone. Journal of Experimental Botany 48, 1337–1356. [Google Scholar]
- Bethke PC, Swanson SJ, Hillmer S, Jones RL. 1998. From storage compartment to lytic organelle: the metamorphosis of the aleurone protein storage vacuole. Annals of Botany 82, 399–412. [Google Scholar]
- Bewley J, Black M. 1994. Seeds: physiology of development and germination. New York: Plenum Press. [Google Scholar]
- Bush DS, Cornejo M-J, Huang C-N, Jones RL. 1986. Ca2+-stimulated secretion of α-amylase during development in barley aleurone protoplasts. Plant Physiology 82, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaretto J, Ho TH. 2003. The transcription factors HvABI5 and HvVP1 are required for the abscisic acid induction of gene expression in barley aleurone cells. Plant Cell 15, 271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M, Tenner A, Johnson K. 1973. Synthesis and release of sucrose by the aleurone layer of barley: regulation by gibberellic acid. Planta 113, 35–46. [DOI] [PubMed] [Google Scholar]
- Chrispeels MJ, Maurel C. 1994. Aquaporins: the molecular basis of hydraulic water movement between cells? Plant Physiology 105, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Jones RL. 2005. Hormonal regulation of gluconeogenesis in cereal aleurone is strongly cultivar-dependent and gibberellin action involves SLENDER1 but not GAMYB. The Plant Journal 44, 483–493. [DOI] [PubMed] [Google Scholar]
- Filner P, Varner JE. 1967. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley α-amylase induced by gibberellic acid. Proceedings of the National Academy of Sciences, USA 58, 1520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB. 1989. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Review of Plant Physiology and Plant Molecular Biology 40, 305–346. [Google Scholar]
- Guo WJ, Ho TH. 2008. An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant Physiology 147, 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar L, Gallwitz D, Wickner W. 1995. The GTPase Ypt7p of Saccharomyces cerevisiae is required on both partner vacuoles for the homotypic fusion step of vacuole inheritance. EMBO Journal 14, 5258–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberlandt G. 1884. Physiologisches Pflanzenanatomie. Leipzig, Germany: W. Engelman. [Google Scholar]
- Higuchi T, Suga S, Tsuchiya T, Hisada H, Morishima S, Okada Y, Maeshima M. 1998. Molecular cloning, water channel activity and tissue specific expression of two isoforms of radish vacuolar aquaporin. Plant and Cell Physiology 39, 905–913. [DOI] [PubMed] [Google Scholar]
- Ho TH, Gomez-Cadenas A, Zentella R, Casaretto J. 2003. Crosstalk between gibberellin and abscisic acid in cereal aleurone. Journal of Plant Growth Regulation 22, 185–194. [Google Scholar]
- Hoh B, Hinz G, Jeong B-K, Robinson DG. 1995. Protein storage vacuoles form de novo during pea cotyledon development. Journal of Cell Science 108, 299–310. [DOI] [PubMed] [Google Scholar]
- Hooley R. 1982. Protoplasts isolated from aleurone layers of wild oat (Avena fatua L.) exhibit the classic response to gibberellic acid. Planta 154, 29–40. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Bethke PC, Gubler F, Jones RL. 2003. cPrG-HCl a potential H+/Cl– symporter prevents acidification of storage vacuoles in aleurone cells and inhibits GA-dependent hydrolysis of storage protein and phytate. The Plant Journal 35, 154–163. [DOI] [PubMed] [Google Scholar]
- Ibl V, Kapusi E, Arcalis E, Kawagoe Y, Stoger E. 2014. Fusion, rupture, and degeneration: the fate of in vivo-labelled PSVs in developing barley endosperm. Journal of Experimental Botany 65, 3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JV, Knox RB, Pyliotis NA. 1971. The structure and composition of aleurone grains in the barley aleurone layer. Planta 101, 189–209. [DOI] [PubMed] [Google Scholar]
- Jauh GY, Fischer AM, Grimes HD, Ryan CA, Jr., Rogers JC. 1998. δ-Tonoplast intrinsic protein defines unique plant vacuole functions. Proceedings of the National Academy of Sciences, USA 95, 12995–12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauh GY, Phillips TE, Rogers JC. 1999. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11, 1867–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjovall S, Fraysse L, Weig AR, Kjellbom P. 2001. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology 126, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KD, Herman EM, Chrispeels MJ. 1989. An abundant, highly conserved tonoplast protein in seeds. Plant Physiology 91, 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL. 1969. The fine structure of barley aleurone cells. Planta 85, 359–374. [DOI] [PubMed] [Google Scholar]
- Jones RL. 1972. Fractionation of the enzymes of the barley aleurone layer: evidence for a soluble mode of enzyme release. Planta 103, 95–109. [DOI] [PubMed] [Google Scholar]
- Jones RL, Jacobsen JV. 1991. Regulation of synthesis and transport of secreted proteins in cereal aleurone. International Review of Cytology 126, 49–88. [DOI] [PubMed] [Google Scholar]
- Jones RL, Price JM. 1970. Gibberellic acid and the fine structure of barley aleurone cells. III. Vacuolation of the aleurone cell during the phase of ribonuclease release. Planta 94, 191–202. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Yamada K, Hiraiwa N, Kondo M, Nishimura M, Hara-Nishimura I. 1999. Vacuolar processing enzyme is up-regulated in the lytic vacuoles of vegetative tissues during senescence and under various stressed conditions. The Plant Journal 19 43–53. [DOI] [PubMed] [Google Scholar]
- Kuo A, Cappelluti S, Cervantes-Cervantes M, Rodriguez M, Bush DS. 1996. Okadaic acid, a protein phosphatase inhibitor, blocks calcium changes, gene expression, and cell death induced by gibberellin in wheat aleurone cells. Plant and Cell Physiology 8, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligaba A, Katsuhara M, Shibasaka M, Djira G. 2011. Abiotic stresses modulate expression of major intrinsic proteins in barley (Hordeum vulgare). Comptes Rendus Biologies 334, 127–139. [DOI] [PubMed] [Google Scholar]
- Maurel C. 1997. Aquaporins and water permeability of plant membranes. Annual Review of Plant Physiology and Plant Molecular Biology 48, 399–429. [DOI] [PubMed] [Google Scholar]
- Moseyko N, Feldman L. 2001. Expression of pH-sensitive green fluorescent protein in Arabidopsis thaliana . Plant, Cell & Environment 24, 557–563. [DOI] [PubMed] [Google Scholar]
- Ory RL, Henningsen KW. 1975. Changes in protein bodies during germination of barley seeds. Bios 6, 71–76. [Google Scholar]
- Paris N, Stanley CM, Jones RL, Rogers JC. 1996. Plant cells contain two functionally distinct vacuolar compartments. Cell 85, 563–572. [DOI] [PubMed] [Google Scholar]
- Schuurink RC, Chan PV, Jones RL. 1996. Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiology 111, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Ho TH. 1995. Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABA-responsive complexes each containing a G-box and a novel cis-acting element. Plant Cell 7, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Jun Y, Wickner W. 2007. Excess vacuolar SNAREs drive lysis and Rab bypass fusion. Proceedings of the National Academy of Sciences, USA 104, 13551–13558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Nield H, Lott JN. 1988. An investigation of the mineral content of barley grains and seedlings. Plant Physiology 86, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Wickner W, Xu Z. 1999. Vacuole acidification is required for trans-SNARE pairing, LMA1 release, and homotypic fusion. Proceedings of the National Academy of Sciences, USA 96, 11194–11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W, Haas A. 2000. Yeast homotypic vacuole fusion: a window on organelle trafficking mechanisms. Annual Review of Biochemistry 69, 247–275. [DOI] [PubMed] [Google Scholar]
- Wink M. 1993. The plant vacuole: A multifunctional compartment. Journal of Experimental Botany 44, 231–246. [Google Scholar]
- Yupsanis T, Burgess SR, Jackson PJ, Shewry PR. 1990. Characterization of the major protein component from aleurone cells of barley (Hordeum vulgare L.). Journal of Experimental Botany 41, 385–392. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


