Abstract
Determining the prognosis of cirrhotic patients is not an easy task. Prognostic scores, like Child-Pugh and Model of End-stage Liver Disease scores, are commonly used by hepatologists, but do not always reflect superimposed events that may strongly influence the prognosis. Among them, bacterial intestinal translocation is a key phenomenon for the development of cirrhosis-related complications. Several biological variables (C-reactive protein, serum free cortisol, copeptin, von Willebrand factor antigen) are surrogates of “inflammatory stress” and have recently been identified as potential prognostic markers in cirrhotic patients. Most of these above mentioned markers were investigated in pilot studies with sometimes a modest sample size but allow us to catch a glimpse of the pathophysiological mechanisms leading to the worsening of cirrhosis. These new data should generate further well-designed studies to better assess the benefit for liver function of preventing intestinal bacterial translocation and microvascular thrombosis. The control of infection is vital and among all actors of immunity, vitamin D also appears to act as an anti-infective agent and therefore has probably a prognostic value.
Keywords: Cirrhosis, C-reactive protein, Copeptin, Vitamin D, Serum free cortisol, Von Willebrand factor antigen
Core tip: This review provides new insights on the prognosis of cirrhotic patients. Several biological markers account for events that strongly impact on prognosis but are not taken into account by common prognosis scores such as Child-Pugh or Model of End-stage Liver Disease. The rationale for the use of these markers is discussed on the basis of the most recent available data.
INTRODUCTION
The prognosis of patients with cirrhosis depends on several factors such as the etiology and severity of liver disease, the presence of associated complications and comorbidities. Several prognostic scores have been developed to estimate the survival of patients in a simple and reliable manner, thus allowing to better adapting their management. Child-Pugh and Model of End-stage Liver Disease (MELD) scores, commonly used in clinical practice, mainly reflect the degree of liver failure but do not account for events superimposed to liver failure that are strongly involved in the onset of extrahepatic organ failures. Therefore, beyond a certain severity of liver failure, MELD and Child-Pugh scores appear limited for accurately predict short-term death. In critically ill cirrhotic patients admitted to intensive care unit (ICU), for instance, general ICU scores such as Acute Physiology and Chronic Health Evaluation (APACHE) II, Sequential Organ Failure Assessment (SOFA), its derivative from the Chronic Liver Failure Consortium of the European Association for the Study of the Liver and simplified acute physiology score (SAPS) II scores provide better prediction of short-term death than liver specific scores[1]. The purpose of this review is to introduce readers to some new recently published biomarkers that may provide additional prognostic information to that given by the usual prognostic scores (Child-Pugh and MELD) and their derivatives (MELD-Na, iMELD, MESO index) even out of the context of multiorgan failure. Such variables, combined with the MELD score should be useful to better sort patients awaiting a liver transplant. The list of these variables of interest discussed in this article is probably not exhaustive.
C-REACTIVE PROTEIN
C-reactive protein (CRP) is a protein of the acute phase of inflammation. Its hepatic synthesis is primarily stimulated by interleukin-6 (IL-6), a proinflammatory cytokine, and maintained even in the context of advanced liver failure[2,3]. The value of CRP reflects the degree of systemic inflammation, regardless of its cause. The physiological role of CRP is to bind to apoptotic cells and microorganisms through the recognition of different molecular patterns. This binding results in activating the complement system and stimulating phagocytosis. Recent studies also suggest a role of CRP in the induction of endothelial dysfunction[4] (Figure 1). Serum CRP increases in the event of systemic inflammatory response syndrome (SIRS) (even in the absence of overt bacterial infection). It was able to predict the risk of death in different populations of non-cirrhotic patients. In cirrhotic patients, SIRS is associated with the occurrence of complications such as hepatic encephalopathy, kidney failure and death[5]. However, the diagnostic criteria of SIRS may be modified in the context of cirrhosis, making the interpretation difficult. Hypersplenism can hide leukocytosis or increase leukopenia; subclinical hepatic encephalopathy can increase the respiratory rate and favor hypocapnia; hyperkinetic syndrome can increase heart rate in cirrhotic patients whereas beta-blockers may mask tachycardia. This is why the measurement of CRP could be both easier and more reliable than the SIRS criteria to identify a systemic inflammation that may adverse the prognosis of cirrhotic patients. We investigated the prognostic value of CRP in a prospective series of 148 cirrhotic patients hospitalized for clinical decompensation (Child Pugh ≥ 8), without hepatocellular carcinoma. The CRP levels were not correlated to the MELD score and had a strong prognostic significance[6]. High CRP levels were indeed associated with an independent risk of mortality at 6 mo, especially when considered the subgroup of 32 patients in whom the value of CRP remained above 29 mg/L during the first 15 d of hospitalization despite the resolution of any overt bacterial infection initially documented. The prognostic value of CRP remained significant when the analysis was restricted to patients without bacterial infection or alcoholic hepatitis at baseline. Taking into account the MELD, the existence of comorbidities, and the variation of CRP levels during the first 15 d, it was possible to build a prognostic model that was able to predict the mortality at 6 mo with a performance of 0.80 (AUROC) vs 0.67 for the MELD alone. Recently, the CANONIC study including 1343 cirrhotic patients from 29 European centers showed that the risk of organ failure and death was significantly associated with the value of CRP, even when the analyzes were restricted to uninfected patients[7]. When we analyzed the subgroup of 583 patients from the CANONIC study in which serial measures of CRP were available, our prognostic model was still relevant[8].
Figure 1.
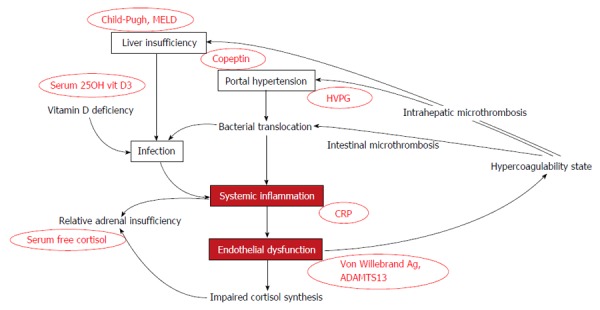
Mechanisms involved in the poor prognosis of cirrhosis (favoring the occurrence of multiorgan failures) and their related markers. Systemic inflammation and endothelial dysfunction appear as high relevant factors not reflected by Child-Pugh, MELD score, or measure of the HVPG. They worsen liver function, increase portal hypertension, and self-sustain the processes. HVPG: Hepatic venous pressure gradient; CRP: C-reactive protein; ADAMTS13: A metalloprotease with decreased activity.
SERUM FREE CORTISOL
The existence of a cortisol deficiency in cirrhosis remains uncertain and the reported prevalence of adrenal insufficiency (AI) (approximately one third of patients “hemodynamically stable” and up to 77% of septic patients) was overestimated by the measure of total serum cortisol concentrations that closely depends on its two main carrier proteins (CBG and albumin) synthesized by the liver and generally lowered in cirrhotic patient. Hence, total serum cortisol is low in case of decreased serum concentrations of CBG and albumin, whereas the free fraction of total cortisol that corresponds to the biologically active hormone is increased[9]. In addition, the lack of specificity of symptoms (fatigue, malaise, reduced muscle strength) renders the clinical diagnosis of AI difficult, especially in the setting of cirrhosis, in which malnutrition is common. Conventionally, the diagnosis of AI is made when the plasma cortisol measured at 8:00 AM is < 83 nmol/L and/or when adrenal stimulation exhibits poor adrenal reactivity (cortisol < 500 nmol/L 30 or 60 min after the intravenous injection of 250 mcg adrenocorticotropic hormone (Synacthen). In patients under stress (especially with septic shock), adrenal dysfunction is defined by a delta cortisol < 250 nmol/L or a random total cortisol < 276 nmol/L[10]. In cirrhotic patients, the mechanisms involved in the onset of AI are not well understood but may involve an exhaustion of the adrenal gland by lack of substrate (high density lipoprotein-cholesterol) for the synthesis of cortisol, or a corticoresistance induced by pro-inflammatory cytokines.
In cirrhotic patients, the study of adrenal function remains difficult because the definition of AI is lacking. Due to changes in cortisol binding proteins concentrations related to liver impairment, the “normal” levels of total serum cortisol are not determined, and measuring serum free cortisol (SFC) is challenging and not routinely feasible. In a recent study we conducted, however, the determination of SFC provided unexpected results regarding its prognosis implications[11]. When the 1 mcg synacthen test was performed in 95 hemodynamically stable cirrhotic patients, a poor prognosis was associated with high levels of SFC (deaths occurred in 26.2% of patients with SFC ≥ 79 nmol/L vs 3.4% of patients with SFC < 79 nmol/L, P = 0.027 by log-rank test). By adjusting on the severity of cirrhosis (MELD) and on serum albumin levels, the risk of death of patients with SFC ≥ 79 nmol/L was increased by 5 times but the relationship was no longer significant probably due to the small sample size of this pilot study. Another interesting finding of this study was that concentrations of SFC were positively correlated with CRP levels, suggesting that SFC increases in the context of systemic inflammation. Further studies with sufficient numbers and serial determinations of cortisol are warranted to clarify the prognostic role of SFC by distinguishing different categories of cirrhotic patients (compensated, decompensated without sepsis, and septic). From our results, the concept of “hepatoadrenal syndrome”[12] mimicking the hepatorenal syndrome (i.e., AI occurring in end-stage liver disease and impacting on mortality) is probably wrong or at least largely overestimated. Recent experimental data using deuterated cortisol-tracer technique show that the cortisol synthesis is increased by 83% in critically ill patients and that the conversion of cortisol to cortisone is reduced in order to maintain high levels of serum cortisol[13]. The levels of pro-inflammatory cytokines (IL-6 and tumor necrosis factor alpha) were positively correlated with cortisol production, and patients with a SIRS had 90% higher cortisol production than those who did not have SIRS. These new data allow us to better understand the discrepancies between the two landmark studies which evaluated the impact of hydrocortisone administration on survival in patients with septic shock[14,15]. Conversely to these findings, a rationale for a true “hepatoadrenal syndrome” in end-staged cirrhotic patients still persists. It has been shown that inflammation may cause endothelial dysfunction, leading to impaired cortisol synthesis[16] (Figure 1). Thus, in patients with compensated cirrhosis, although AI may be a latent condition, the proinflammatory cytokines appropriately stimulate the hypothalamic-pituitary adrenal axis leading to an increase of cortisol. When the stress becomes more intense (and associated with an excessive production of pro-inflammatory cytokines), a steroid-resistance may occur leading to an insufficient cortisol production regarding the clinical situation. This concept of relative AI is probably of high relevance in cirrhotic patients, particularly in the event of septic shock. It was a rationale to conduct a randomized controlled trial to assess the effect of hydrocortisone on survival in 75 cirrhotic patients with septic shock. Despite initial hemodynamic improvement, an interim analysis found no difference in 28-d mortality between the two groups, and the trial was thus interrupted[17].
VITAMIN D
Vitamin D (calciferol) is essential to the maintenance of phosphate homeostasis of the body. It comes from food and skin. Under the action of sunlight (UV-B), skin 7-dehydrocholesterol is converted to previtamin D3 then vitamin D3 and is subsequently transported to the liver where hydroxylation to 25-OH vitamin D3 occurs. Then, 25-OH D3 undergoes a second hydroxylation in the kidney thereby resulting in 1,25-(OH)2-vitamin D3 (calcitriol), which is the biologically active hormonal form of vitamin D3 (rarely measured in clinical practice). Serum 25-OH vitamin D3 (the storage form of the vitamin) concentration is nearly 1000 times higher than that of serum 1,25-(OH)2 vitamin D3. The measure of serum 25-OH vitamin D3 is thus appropriate for the diagnosis of vitamin D deficiency[18]. In cirrhotic patients, however, because of low albumin and vitamin D binding protein levels, recent reports highlighted the overestimation of vitamin D deficiency and suggested that the measure of free 25-OH D3 would be more appropriate[19].
The role of vitamin D is not confined to its skeletal actions. Vitamin D also regulates immune defenses and is able to modulate the differentiation and proliferation of certain cell types. Its deficiency has been associated with an increased risk of cancer, cardiovascular disease, autoimmune diseases and infectious diseases[18]. The impact of vitamin D on immune system may be relevant for the prognosis of cirrhotic patients. Indeed, vitamin D it is commonly deficient in this population and it’s easy to hypothesize that it favors bacterial infections, which increase by four-fold the risk of death in cirrhotic patients[20] (Figure 1). Because of the antiproliferation, pro-differentiation, pro-apoptosis, anti-inflammation, and immune regulation properties of vitamin D, Vitamin D deficiency may also contribute to the development of hepatocellular carcinoma. Although the epidemiologic evidence regarding the association of vitamin D and hepatocellular carcinoma is still inconclusive, biochemical evidence clearly indicates that hepatocellular carcinoma cells are responsive to the inhibitory effect of vitamin D and its analogs[21] and genetic determinants have been identified for the role of vitamin D to modulate the development of hepatitis C associated hepatocellular carcinoma (HCC)[22]. Unfortunately, the therapeutic use of vitamin D analogs for HCC provided disappointing results to date.
The reasons why vitamin D is deficient in patients with cirrhosis are multiple and include solar underexposure of these patients, malnutrition, malabsorption of vitamin D by a lack of bile acids and/or impairment of the hepatic hydroxylation[23]. In a recent study, low vitamin D levels (< 6 ng/mL) were associated with increased mortality (OR = 6.3, P = 0.024) in cirrhotic patients regardless of their MELD score, and sepsis were the cause of death in the majority of these patients[24]. Similar findings were observed in 75 cirrhotic patients from Austria, in which negative correlations were observed between 25-OH vitamin D concentrations and MELD scores (r = -0.34, P = 0.003)[25]. By distributing vitamin D levels into tertiles, the authors observed an increased risk (OR = 6.37, P = 0.005) of hepatic decompensation (ascites, encephalopathy, gastrointestinal bleeding, hepatorenal syndrome) in patients with the lowest values of vitamin D (1st tertile) compared to those with the highest values (3rd tertile). Patients of the 1st tertile had also a higher risk of death compared with patients of the 3rd tertile in multivariate analysis adjusted for age and sex (HR = 4.31, P = 0.012). However, the adjustment on the Child-Pugh score and/or MELD score no longer allowed retaining vitamin D as a significant prognostic marker per se. A more recent study conducted in 324 patients with alcoholic liver disease showed that low vitamin D levels (< 10 ng/mL) were associated with: (1) cirrhosis; (2) high hepatic venous pressure gradients; (3) high Child-Pugh and MELD scores; (4) occurrence of portal hypertension-related complications; and (5) one-year mortality[26]. Vitamin D deficiency may contribute to deterioration of liver functions by increasing liver inflammation and fibrosis. Indeed, experimental models have shown a reduction in inflammatory and profibrotic activity of hepatic stellate cells after vitamin D[27]. An unresolved issue is whether vitamin D supplementation could improve liver function and survival of cirrhotic patients. Anyway, screening and treatment of vitamin D deficiency in patients with cirrhosis is already justified given the high prevalence (12%-86%) of osteoporosis and fracture risk (5%-20%) in these patients. Further studies should investigate the free faction of vitamin D and may determine more in depth the true spectrum of vitamin D deficiency in cirrhosis and its clinical implications.
COPEPTIN
In cirrhotic patients, intestinal bacterial translocation is responsible for overproduction of nitric oxyde (NO) via activation of monocytes and lymphocytes and increase in circulating levels of proinflammatory cytokines. NO increases splanchnic vasodilation that stimulates compensatory systems to restore adequate blood volume: sympathetic nervous system, renin-angiotensin-aldosterone system and arginine vasopressin (AVP, also called antidiuretic hormone). It has been shown that AVP concentrations increase with deterioration of liver function and this biological marker may thus have a prognostic value. However, its measurement is difficult and not routinely available. Copeptin, the pre-pro-AVP C terminal fragment, is released into the serum in equimolar quantities than AVP. Hence, copeptin concentrations closely reflect the production of AVP, either in healthy subjects or in stressful situations such as sepsis[28]. The main interest of copeptin is its serum stability, conversely to AVP. Copeptin is thus easy to measure. Moreover, its concentration increases much more than cortisol in the event of stress. The prognostic value of copeptin was recently mentioned in several diseases: high concentrations of copeptin were associated with unfavorable outcomes in patients with chronic heart failure, pulmonary infections, and in patients with transient ischemic stroke[28]. We studied this marker in a cohort of 125 cirrhotic patients including 34 Child-Pugh A, 29 Child-Pugh B, 32 Child-Pugh C and 30 infected patients with Child-Pugh score > B8[29]. Copeptin concentrations were higher in infected patients (18.81 pmol/L vs 6.64 pmol/L in patients without infection, P = 0.0007), patients with ascites (13.27 pmol/L vs 6.06 pmol/L in others, P < 0.0001) and patients with renal impairment (44.67 pmol/L vs 8.40 pmol/L in patients with normal renal function, P = 0.0018). Copeptin concentrations were positively correlated with Child-Pugh, MELD scores (r = 0.43, P < 0.0001) and CRP levels (r = 0.49, P < 0.0001). After a median follow-up of 12 mo, 8 patients were transplanted and 28 (24%) patients had died. In univariate analysis, patients who died or were transplanted had higher baseline copeptin concentrations compared with others (15.02 pmol/L vs 6.68 pmol/L; P = 0.0006), and nearly three quarters of patients who had died belonged to the two highest quintiles regarding copeptin concentrations. Survival analysis showed excess mortality in patients with copeptin values > 13 pmol/L. In multivariate analysis, high value (> 13 pmol/L) of copeptin kept its detrimental impact on prognosis after adjustment on CRP and MELD score. This study suggests that copeptin could be a good marker of stress during cirrhosis. Its impact on survival warrants confirmation by larger studies.
THE VON WILLEBRAND FACTOR ANTIGEN
The vascular endothelium plays a critical role in the regulation of vascular tone through its ability to release vasoactive substances, including NO and prostacyclin (vasodilators) and endothelin and thromboxane A2 (vasoconstrictors). In cirrhotic patients, an endothelial dysfunction is responsible for abnormal vascular reactivity which is involved in portal hypertension both by increasing intrahepatic vascular resistance (due to intrahepatic vasoconstriction) and portal flow (by increase in cardiac output to compensate for systemic arteriolar vasodilatation). The measurement of hepatic venous pressure gradient (HVPG) identifies cirrhotic patients at high risk of complications (mainly esophageal varices) and death when HVPG exceeds 10 mmHg but this measure is not feasible in routine. The Von Willebrand antigen (VWF Ag) is a glycoprotein synthesized by activated endothelial cells and can be used as a marker of endothelial dysfunction. Endotoxemia activates endothelial cells and is well correlated with the levels of serum VWF Ag[30]. In 42 cirrhotic patients with severe portal hypertension (HVPG ≥ 12 mmHg), the team from Barcelona has shown a positive correlation between serum VWF Ag and MELD score (r = 0.34, P = 0.032), and between serum VWF Ag and HVPG (r = 0.47, P < 0.001)[31]. However, given the small sample size and the inclusion of patients with HVPG ≥ 12 mmHg, the prognostic value of VWF Ag could not be determined convincingly. A more recent study[32] has come to fill this gap by including 286 patients with cirrhosis at different stages of severity (Child-Pugh 148 A, 104 B and 34 C). These authors confirmed the previous results and showed a good correlation (r = 0.68, P < 0.001) between VWF Ag and HVPG[32]. A nice consequence for clinical practice was that levels of VWF Ag > 241% accurately predicted the risk of variceal bleeding, as well as HVPG ≥ 12 mmHg did. In addition, VWF Ag > 315 % was able to predict death (HR = 2.92, P < 0.001) regardless of the HVPG, the Child-Pugh, the MELD, and the presence of hepatocellular carcinoma. This spectacular result suggests that marked endothelial dysfunction, as assessed by high levels of VWF Ag, has a strong detrimental prognostic influence per se. During cirrhosis, VWF Ag may remain high due to the presence of circulating endotoxins but also due to a decreased activity of a metalloprotease (ADAMTS13), which cleaves the multimers of Willebrand factor. A recent study suggests that a low enzymatic activity of ADAMTS13 predicts the risk of death at 1 year and 2 years, as well as Child-Pugh and MELD scores do[33]. The mechanisms involved in the pejorative influence of high levels of VWF Ag are unknown. VWF Ag plays a key role in both primary hemostasis (mediator of platelet adhesion to sub-endothelium) and coagulation (plasma factor VIII carrier). It is likely that elevated serum VWF Ag levels participate to a hypercoagulable state that is often underestimated in severe cirrhotic patients. This hypercoagulable state may contribute to deterioration of liver functions by inducing thrombosis in the hepatic microcirculation, as well as it may increase portal hypertension as hypothesized by Wanless twenty years ago[34] (Figure 1). It is also possible that these thrombotic events, when located in the intestinal microcirculation, favor enterocyte ischemia and intestinal bacterial translocation subsequently. This self-perpetuates the phenomenon and causes systemic inflammation.
CONCLUSION
Risk assessments of cirrhotic patients is sometimes tricky but remains crucial for optimal identifications of liver transplant candidates. Even commonly used for a long time in clinical practice, Child-Pugh and MELD scores lack finesse, whereas general scores (such as SAPS II, APACHE or SOFA), are inappropriate outside of the context of intensive care. New biological variables (CRP, serum free cortisol, copeptin, vitamin D, or Willebrand antigen) account for well identified event which impact on prognosis of cirrhotic patients and deserve further research to build a new prognostic score, easy to use but more powerful than Child-Pugh, MELD score, or its derivatives created to date.
Footnotes
P- Reviewer: Bruha R, Khattab MA, Liu QD S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: All authors have nothing to disclose regarding the present review.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 2, 2014
First decision: January 8, 2015
Article in press: February 12, 2015
References
- 1.Levesque E, Hoti E, Azoulay D, Ichaï P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2012;56:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 2.Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med. 2005;146:347–351. doi: 10.1016/j.lab.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Park WB, Lee KD, Lee CS, Jang HC, Kim HB, Lee HS, Oh MD, Choe KW. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn Microbiol Infect Dis. 2005;51:227–230. doi: 10.1016/j.diagmicrobio.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Devaraj S, Kumaresan PR, Jialal I. C-reactive protein induces release of both endothelial microparticles and circulating endothelial cells in vitro and in vivo: further evidence of endothelial dysfunction. Clin Chem. 2011;57:1757–1761. doi: 10.1373/clinchem.2011.169839. [DOI] [PubMed] [Google Scholar]
- 5.Thabut D, Massard J, Gangloff A, Carbonell N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T, Moreau R. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–1882. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 6.Cervoni JP, Thévenot T, Weil D, Muel E, Barbot O, Sheppard F, Monnet E, Di Martino V. C-reactive protein predicts short-term mortality in patients with cirrhosis. J Hepatol. 2012;56:1299–1304. doi: 10.1016/j.jhep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437, 1437.e1-9. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Cervoni JP, Amoros A, Moreau R, Arroyo V, Jalan R, Saliba F. Prognostic value of C-reactive protein in patients with cirrhosis: external validation from the CANONIC cohort. Hepatology. 2014;60(Suppl):495A. doi: 10.1097/MEG.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 9.Thevenot T, Borot S, Remy-Martin A, Sapin R, Cervoni JP, Richou C, Vanlemmens C, Cleau D, Muel E, Minello A, et al. Assessment of adrenal function in cirrhotic patients using concentration of serum-free and salivary cortisol. Liver Int. 2011;31:425–433. doi: 10.1111/j.1478-3231.2010.02431.x. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med. 2008;36:1937–1949. doi: 10.1097/CCM.0b013e31817603ba. [DOI] [PubMed] [Google Scholar]
- 11.Thevenot T, Dorin R, Monnet E, Qualls CR, Sapin R, Grandclement E, Borot S, Sheppard F, Weil D, Degand T, et al. High serum levels of free cortisol indicate severity of cirrhosis in hemodynamically stable patients. J Gastroenterol Hepatol. 2012;27:1596–1601. doi: 10.1111/j.1440-1746.2012.07188.x. [DOI] [PubMed] [Google Scholar]
- 12.Fede G, Spadaro L, Tomaselli T, Privitera G, Germani G, Tsochatzis E, Thomas M, Bouloux PM, Burroughs AK, Purrello F. Adrenocortical dysfunction in liver disease: a systematic review. Hepatology. 2012;55:1282–1291. doi: 10.1002/hep.25573. [DOI] [PubMed] [Google Scholar]
- 13.Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Vanwijngaerden YM, Spriet I, Wouters PJ, Vander Perre S, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–1488. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 15.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 16.Kanczkowski W, Chatzigeorgiou A, Grossklaus S, Sprott D, Bornstein SR, Chavakis T. Role of the endothelial-derived endogenous anti-inflammatory factor Del-1 in inflammation-mediated adrenal gland dysfunction. Endocrinology. 2013;154:1181–1189. doi: 10.1210/en.2012-1617. [DOI] [PubMed] [Google Scholar]
- 17.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 19.Lai JC, Terrault N, Bikle D, Lizaola BL, Schwartz JB. Total 25(OH) Vitamin D is Not an Accurate Marker of Vitamin D Status in Patients with Cirrhosis and Synthetic Dysfunction. Hepatology. 2014;60(suppl):304A. [Google Scholar]
- 20.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol. 2011;26:1597–1603. doi: 10.1111/j.1440-1746.2011.06892.x. [DOI] [PubMed] [Google Scholar]
- 22.Lange CM, Miki D, Ochi H, Nischalke HD, Bojunga J, Bibert S, Morikawa K, Gouttenoire J, Cerny A, Dufour JF, et al. Genetic analyses reveal a role for vitamin D insufficiency in HCV-associated hepatocellular carcinoma development. PLoS One. 2013;8:e64053. doi: 10.1371/journal.pone.0064053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zúñiga S, Firrincieli D, Housset C, Chignard N. Vitamin D and the vitamin D receptor in liver pathophysiology. Clin Res Hepatol Gastroenterol. 2011;35:295–302. doi: 10.1016/j.clinre.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176–183. doi: 10.1111/eci.12205. [DOI] [PubMed] [Google Scholar]
- 25.Putz-Bankuti C, Pilz S, Stojakovic T, Scharnagl H, Pieber TR, Trauner M, Obermayer-Pietsch B, Stauber RE. Association of 25-hydroxyvitamin D levels with liver dysfunction and mortality in chronic liver disease. Liver Int. 2012;32:845–851. doi: 10.1111/j.1478-3231.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 26.Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, Gustot T, Degré D, Vercruysse V, Deltenre P, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344–350. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 27.Potter JJ, Liu X, Koteish A, Mezey E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human α1 (I) collagen expression and type I collagen formation. Liver Int. 2013;33:677–686. doi: 10.1111/liv.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgenthaler NG, Struck J, Jochberger S, Dünser MW. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Moreno JP, Grandclement E, Monnet E, Clerc B, Agin A, Cervoni JP, Richou C, Vanlemmens C, Dritsas S, Dumoulin G, et al. Plasma copeptin, a possible prognostic marker in cirrhosis. Liver Int. 2013;33:843–851. doi: 10.1111/liv.12175. [DOI] [PubMed] [Google Scholar]
- 30.Ferro D, Quintarelli C, Lattuada A, Leo R, Alessandroni M, Mannucci PM, Violi F. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: relationship to endotoxemia. Hepatology. 1996;23:1377–1383. doi: 10.1002/hep.510230613. [DOI] [PubMed] [Google Scholar]
- 31.La Mura V, Reverter JC, Flores-Arroyo A, Raffa S, Reverter E, Seijo S, Abraldes JG, Bosch J, García-Pagán JC. Von Willebrand factor levels predict clinical outcome in patients with cirrhosis and portal hypertension. Gut. 2011;60:1133–1138. doi: 10.1136/gut.2010.235689. [DOI] [PubMed] [Google Scholar]
- 32.Ferlitsch M, Reiberger T, Hoke M, Salzl P, Schwengerer B, Ulbrich G, Payer BA, Trauner M, Peck-Radosavljevic M, Ferlitsch A. von Willebrand factor as new noninvasive predictor of portal hypertension, decompensation and mortality in patients with liver cirrhosis. Hepatology. 2012;56:1439–1447. doi: 10.1002/hep.25806. [DOI] [PubMed] [Google Scholar]
- 33.Takaya H, Uemura M, Fujimura Y, Matsumoto M, Matsuyama T, Kato S, Morioka C, Ishizashi H, Hori Y, Fujimoto M, et al. ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child-Turcotte-Pugh score and the Model for End-Stage Liver Disease score. Hepatol Res. 2012;42:459–472. doi: 10.1111/j.1872-034X.2011.00950.x. [DOI] [PubMed] [Google Scholar]
- 34.Wanless IR, Wong F, Blendis LM, Greig P, Heathcote EJ, Levy G. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. [PubMed] [Google Scholar]


