Abstract
Background:
Cognitive deficits are a core symptom of schizophrenia, yet they remain particularly resistant to treatment. The model provided by repeatedly exposing adult nonhuman primates to phencyclidine has generated important insights into the neurobiology of these deficits, but it remains possible that administration of this psychotomimetic agent during the pre-adult period, when the dorsolateral prefrontal cortex in human and nonhuman primates is still undergoing significant maturation, may provide a greater understanding of schizophrenia-related cognitive deficits.
Methods:
The effects of repeated phencyclidine treatment on spine synapse number, dopamine turnover and BDNF expression in dorsolateral prefrontal cortex, and working memory accuracy were examined in pre-adult monkeys.
Results:
One week following phencyclidine treatment, juvenile and adolescent male monkeys demonstrated a greater loss of spine synapses in dorsolateral prefrontal cortex than adult male monkeys. Further studies indicated that in juvenile males, a cognitive deficit existed at 4 weeks following phencyclidine treatment, and this impairment was associated with decreased dopamine turnover, decreased brain derived neurotrophic factor messenger RNA, and a loss of dendritic spine synapses in dorsolateral prefrontal cortex. In contrast, female juvenile monkeys displayed no cognitive deficit at 4 weeks after phencyclidine treatment and no alteration in dopamine turnover or brain derived neurotrophic factor messenger RNA or spine synapse number in dorsolateral prefrontal cortex. In the combined group of male and female juvenile monkeys, significant linear correlations were detected between dopamine turnover, spine synapse number, and cognitive performance.
Conclusions:
As the incidence of schizophrenia is greater in males than females, these findings support the validity of the juvenile primate phencyclidine model and highlight its potential usefulness in understanding the deficits in dorsolateral prefrontal cortex in schizophrenia and developing novel treatments for the cognitive deficits associated with schizophrenia.
Keywords: brain derived neurotrophic factor, cognition, dopamine, phencyclidine, sex, spine synapse
Introduction
Cognitive deficits are considered to be a core symptom of schizophrenia (Elvevag and Goldberg, 2000; Heinrichs, 2005), yet they remain particularly resistant to treatment (Sharma et al., 2003). In fact, cognitive dysfunction is often present long before onset of the full clinical syndrome (Lencz et al., 2006), and the neurodevelopmental hypothesis of schizophrenia posits that cognitive deficits are, in these cases, a proximal manifestation of aberrant brain maturation (Marenco and Weinberger, 2000; Lewis and Levitt, 2002). An animal model with face, construct, and predictive validity for the cognitive signs of schizophrenia would be of great value in both understanding the neurobiology of these deficits and discovering new treatment options.
One such model, created by repeatedly administering the psychomimetic N-methyl-d-aspartate (NMDA) receptor antagonist phencyclidine (PCP) to adult nonhuman primates (Jentsch and Roth, 1999), leads to schizophrenia-like prefrontal cortex-dependent cognitive dysfunction, such as deficits in working memory and response inhibition (Jentsch et al., 1997; Barch and Ceaser, 2012; Elsworth et al., 2012), indicating face validity. The neurochemical and morphological alterations in the prefrontal cortex of monkeys treated repeatedly with PCP also resemble the alterations found in this brain region of patients with schizophrenia. For example, 1 to 2 weeks following PCP exposure in monkeys, there is downregulation of the mesocortical dopamine (DA) system (Jentsch et al., 1999), fewer parvalbumin-containing GABA interneurons (Morrow et al., 2007a), and loss of excitatory spine synapses on pyramidal neurons (Elsworth et al., 2011) compared with the same measures in control subjects. Thus, the primate repeated exposure PCP model has construct validity with both the glutamate hypothesis (Tamminga, 1998; Moghaddam and Javitt, 2012) and the hypo-DA hypothesis of schizophrenia (Davis et al., 1991; Knable and Weinberger, 1997; Akil et al., 1999) and also presents with loss of parvalbumin interneurons (Rotaru et al., 2012) and decreased dendritic spine density on pyramidal neurons that are observed in the dorsolateral prefrontal cortex (DLPFC) in schizophrenia (Glantz and Lewis, 2000; Kolluri et al., 2005). Furthermore, predictive validity is demonstrated by reversal of the cognitive deficits induced by repeated PCP exposure in monkeys by agents (Jentsch et al., 1997; Elsworth et al., 2012) that are clinically effective (Sharma et al., 2003; Cortese et al., 2013).
During the juvenile and adolescent periods of development, the PFC in primates is still undergoing significant maturation, both morphologically and biochemically (Gogtay et al., 2004; Hill et al., 2010). This is the time in development when individual symptoms coalesce, leading to a diagnosis of schizophrenia (Gogtay et al., 2011), and also is the age at which the vulnerability to the consequences of psychoactive drugs such as PCP is enhanced (Crews et al., 2007). Administration of PCP during this developmental window of risk may more comprehensively or accurately model the neurobiology and cognitive deficits in schizophrenia, so we examined the effects of repeated PCP treatment in monkeys of this age. Although previous studies in rodents have studied the consequences of early life administration of PCP (Deutsch et al., 1998; Broberg et al., 2008; Wang et al., 2008), the primate model offers the opportunity to study a model organism with a protracted adolescent period much more similar to that of humans. Additionally, because the incidence of schizophrenia is significantly higher in males than females (McGrath, 2006), possibly due to the neuroprotective properties of estradiol (Markham, 2012), we extended the current study to include a comparison of the effects observed following repeated PCP treatment in male and female monkeys.
Our recent data suggest that DA availability in the DLPFC regulates spine synapse number and prefrontal cortex function. Thus, in both the primate PCP model and primate 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model, reduced DA transmission in DLPFC is associated with loss of spine synapses and cognitive deficits (Elsworth et al., 2011, 2013a). The present study also provided us with an opportunity to more rigorously test the relationship between DA signaling in the prefrontal cortex, spine synapse number, and prefrontal cortex-dependent cognition.
We hypothesized that pre-adult monkeys would exhibit a greater impact of PCP treatment than adult monkeys, that males would show enhanced PCP-induced deficits compared with females, and that DA turnover, spine synapse number, and working memory performance will change in unison following PCP treatment.
Methods
Animals and PCP Treatment
All monkeys (Chlorocebus sabaeus, commonly known as vervet monkeys) were born and raised in social housing groups at the St. Kitts Biomedical Research Foundation in the West Indies, a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Monkeys were fed Harlan Teklad New Iberia Primate Diet (no. 8773) supplemented with seasonal local fruits. Water was constantly available. Protocols were reviewed and approved in advance by the Institutional Animal Care and Use Committees of both Yale University and St. Kitts Biomedical Research Facility; all studies were conducted in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1: Effect of PCP on DLPFC Spine Synapses during Development
Male monkeys of different ages were treated with either PCP or saline for 2 weeks and euthanized 1 week later. There were 4 controls and 4 PCP-treated monkeys in each of 3 age groups; these groups comprised either peri-adolescent (referred to as juvenile) (Terasawa and Fernandez, 2001), late adolescent (referred to as adolescent), or adult monkeys. The treatment schedule was the same as we have used before in all our primate studies on the effects of repeated PCP exposure (eg, Jentsch et al., 1999). This involved 2 intramuscular injections each day for 14 consecutive days of either PCP (0.3mg/kg) or an equal volume of saline (0.1mL/kg). There was no significant difference in weight or age between saline-treated controls and PCP-treated animals for any age group (mean values ± standard deviation for juveniles: 2.7±0.3kg, 2.0±0.4 years; adolescents: 4.9±0.8kg, 3.7±0.2 years; adults: 6.6±0.6kg, 6.1±0.4 years). Animals were euthanized by injection of an overdose of pentobarbital. Brains were perfused in situ with saline, followed by a fixation solution of 4% formaldehyde containing 0.1% glutaraldehyde in 0.1M phosphate buffer, pH 7.4, at room temperature. Formaldehyde solution was prepared from paraformaldehyde no longer than 1 week before use, as recommended (Helander, 2000). Glutaraldehyde (from a sealed ampoule of 50% concentration, Electron Microscopy Sciences, Hatfield, PA) was added to the fixative solution on the day of the perfusion. After removal, brains were stored at 4°C overnight in 4% buffered formaldehyde solution before transfer to 0.1M phosphate buffer containing 0.1% sodium azide at 4°C. Brains were shipped with ice packs by overnight courier to Yale University for electron microscope (EM) analysis. analysis to determine the number of asymmetric spine synapses in DLPFC (described below).
Experiment 2: Effect of PCP on Spine Synapses, DA Turnover, and Cognition in Male and Female Juvenile Monkeys
An equal number (8) of male and female juvenile (peri-adolescent, see Experiment 1) monkeys was entered into the study. After all monkeys were responding on the cognitive task with at least 80% accuracy (see below for details of behavior), 4 monkeys of each sex were treated with PCP and 4 of each sex were treated with saline, as described above. There was no significant difference in weight or age between PCP-treated and saline-treated monkeys for either sex (mean values ± standard deviation for males: 2.4±0.3kg, 2.1±0.3 years; females: 2.3±0.2kg, 2.1±0.4 years).
Behavior
Working memory was assessed in monkeys using a 2-choice spatial delayed response task (James et al., 2007). A custom-designed Wisconsin General Test apparatus was fitted directly onto the cage, where an opaque screen separated the monkey from 2 equally spaced wells that could be covered to conceal rewards (ie, a small piece of banana, grape, or apple). A trial began when the screen was raised and the monkey watched the experimenter bait 1 of the 2 wells. The reward was concealed from view (using 2 identical objects placed directly over each well) and the screen lowered and immediately raised (an approximately 0-second delay). Monkeys made a response by displacing 1 of the objects and, when a correct response was made, were allowed to retrieve the reward. The next trial began following a 20-second inter-trial interval. Monkeys completed 30 trials per day where delays and rewarded well were counterbalanced and randomized across trials on each testing day. Once monkeys were achieving >80% accuracy in the 0-second delay condition (chance performance in this 2 choice task is 50%), they were exposed to a variable delay condition. In this condition, one-third of trials involved an approximately 0-second delay, one-third of trials involved a short delay, and one-third of trials involved a longer delay. One week and 4 weeks after completing the PCP (or saline) injections, monkeys were tested again with variable delay conditions.
Tissue Collection
Four weeks after PCP or saline treatment, animals were euthanized by an injected overdose of sodium pentobarbital. Brains were perfused in situ with cold saline, then removed and sectioned into 4-mm slabs using a brain mold. Several samples of DLPFC were dissected from the slabs on a refrigerated surface (2°C), placed in labeled cryotubes, and immediately frozen in liquid nitrogen. A slab containing DLPFC was immersion-fixed in formaldehyde solution containing glutaraldehyde for EM, as previously described in detail (Elsworth et al., 2013a). Frozen samples were transported to Yale University in a liquid nitrogen tank, where samples were stored in a −70°C freezer. Fixed samples were transported as described above (Experiment 1).
Biochemistry
DA and its main metabolite in primate brain, homovanillic acid (HVA) (Bacopoulos et al., 1978), were extracted from DLPFC samples and electrochemically detected after reverse-phase isocratic high-performance liquid chromatograph (HPLC) separation, as previously described (Elsworth et al., 2000; Morrow et al., 2007b). Quantification was achieved by reference to internal and external standards. The protein content of the centrifuged tissue pellet was measured using the Lowry method, and concentrations of DA and HVA were expressed as nanogram per milligram protein.
Electron Microscopy
The number of asymmetric (excitatory) spine synapses in layers 2/3 of Walker’s area 46 of DLPFC was calculated as previously published (Elsworth et al., 2013b). Layers 2/3 of DLPFC were examined, because pyramidal neurons in these layers send collaterals to associational cortical regions (Hoftman and Lewis, 2011), and asymmetric spine density in these layers has been correlated with working memory performance in primates (Peters et al., 2008). In addition, in primates there is a relatively dense DA innervation of layers 2/3 in DLPFC (Kroner et al., 2007), which appears to regulate asymmetric spine synapse number (Elsworth et al., 2013a). Asymmetric spine synapses were counted according to the rules of the disector technique (MacLusky et al., 2006) within an unbiased counting frame superimposed onto each electron micrograph. Asymmetric spine synapses were distinguished from symmetric synapses by prominent postsynaptic densities and wider synaptic junctions, with the presence of fewer mitochondria, as well as by uniformly round synaptic vesicles.
Estradiol Level
Quantification of plasma estradiol levels was achieved using a sensitive competitive binding enzyme-linked immunosorbent assay (ELISA) (Kit EIA4399, DRG International, Springfield, NJ). Each plasma sample was extracted prior to analysis for 3 reasons: (1) to increase the reliability of measurements in samples with low levels of estradiol as recommended (Ankarberg-Lindgren and Norjavaara, 2008), (2) to obviate interferences from hemolyzed plasma samples, and (3) to allow the sample to be concentrated by dissolving the extracted residue in a smaller volume than the initial sample. Extraction was achieved by vortexing 0.5mL plasma with 10 volumes of hexane and ethyl acetate (3:2) for 30 seconds in silanized glass tubes (Dighe and Sluss, 2004). After centrifugation, the tube was placed in dry ice and the supernatant poured off the frozen aqueous layer. The organic phase was dried under nitrogen in a silanized glass tube, and the residue was reconstituted in 0.25mL steroid-free serum. A separate experiment showed that recovery of estradiol with this procedure was 95%. Duplicate 100-µL samples were entered in the ELISA assay along with standards and blanks.
Brain Derived Neurotrophic Factor (BDNF) messenger RNA (mRNA)
Total RNA was extracted by using a QIAGEN RNAeasy kit (QIAGEN). One µg of total RNA was subjected to complementary DNA (cDNA) synthesis using a High Capacity cDNA Reverse transcription Kit (Applied Biosystems, Carlsbad, CA). Real-time PCR with 10-fold diluted cDNA was performed using the LightCycler 480 (Roche) and Taqman Gene Expression Master mix (Applied Biosystems) in a 20-μL reaction volume in triplicate. BDNF and Beta-actin (both Applied Biosystems) primers were used in this study. Beta-actin was used as a reference gene. The calculations of average crossing point values, standard deviations, and resulting expression ratios for BDNF gene were performed using the Roche LightCycler 480 software.
BDNF Levels
The BDNF concentration in DLPFC of each monkey was measured using a sandwich ELISA system (BDNF Emax Immunoassay system, catalog G7610, Promega Corporation, Madison, WI). Samples were processed according to the protocol, except that individual peptidase inhibitors (phenylmethylsulfonyl fluoride (PMSF), aprotinin, leupeptin) in the lysis buffer were replaced with a commercial cocktail of peptidase inhibitors (Complete Protease Inhibitor Tablets, EDTA-free, Roche Diagnostics Corporation, Indianapolis, IN).
Statistics
Statistical significance of the data was determined using either Prism (software version 6.0d; GraphPad Software, La Jolla, CA) or SPSS (software version 21; SPSS Inc, Chicago, IL). The Results section contains the specifics of each analysis. In all cases, statistical significance was set as P<.05.
Results
Experiment 1
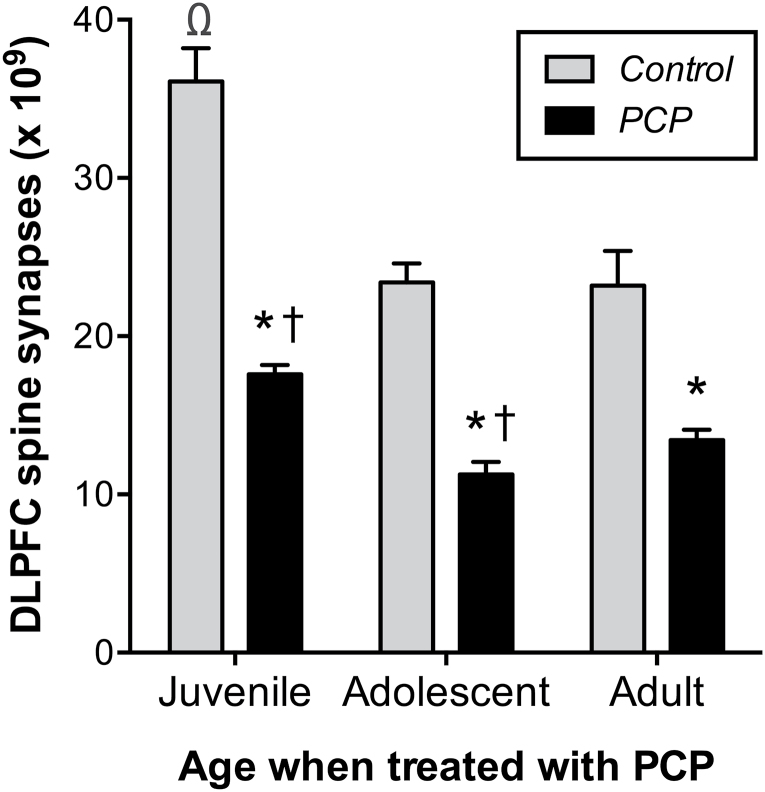
The first study was conducted to assess whether repeated PCP treatment induced a greater effect on spine synapses in the DLPFC of pre-adult monkeys compared with adult monkeys. Because of the possible neuroprotective effect of estradiol, only male monkeys were used so that circulating estradiol levels would vary little within each age group. These animals were euthanized 1 week after the final PCP or saline injection. The endpoint measure was the number of spine synapses in DLPFC. We have found this to be the most sensitive index of the impact of PCP on DLPFC in terms of variability between animals and percent change from control levels. The data were analyzed by 2-way analysis of variance (ANOVA), which indicated significant effects of treatment [F(1,18)=135, P<.0001], age [F(2,18)=27.2, P<.0001], and an interaction between these factors [F(2,18)=5.1, P<.05], which is shown in Figure 1. In addition, there was a difference in number of asymmetric spine synapses in DLPFC among the control groups of differently aged monkeys [ANOVA, F(2,9)=70.2, P<.0001], with juvenile monkeys possessing a greater number (36.1±1.0x109) than either adolescent (23.4±0.6x109) or adult (23.3±1.0x109) groups [Tukey’s multiple comparison test, P<.0001].
Figure 1.
Impact of phencyclidine (PCP) on asymmetric spine synapses in dorsolateral prefrontal cortex (DLPFC) is dependent on age of the monkey. The effect of PCP on the mean number of spine synapses (±SEM) is shown at 1 week after treatment. * indicates a significant difference from age-matched control value. Both juvenile and adolescent male monkeys had greater loss of synapses compared with adult male monkeys, as indicated by †. The number of spine synapses in juvenile control monkeys was greater than in control monkeys of adolescents and adults, indicated by Ω.
Other analysis of the data revealed that there was significant difference between the 3 groups of monkeys (juvenile, adolescent, and adult) in terms of the proportion of spine synapses lost at 1 week following PCP (Kruskal-Wallis statistic=7.5, P=.01). Subsequent testing showed that juvenile and adolescent monkeys each exhibited a greater difference between the number of spine synapses in PCP-exposed compared with controls than did the adult group (2-tailed Mann-Whitney test, P<.05).
Experiment 2
Based on the result of Experiment 1, the next study (Experiment 2) used juvenile monkeys to compare the response to PCP treatment in male and female monkeys and to examine the relationship between spine synapses in DLPFC, DA turnover in DLPFC, and performance on the spatial delayed response task that is dependent on the DLPFC (Bauer and Fuster, 1976). It became possible for us to make measurements of DA turnover and spine synapses in the same monkey when we established a modified immersion fixation technique (Elsworth et al., 2013a).
Behavior
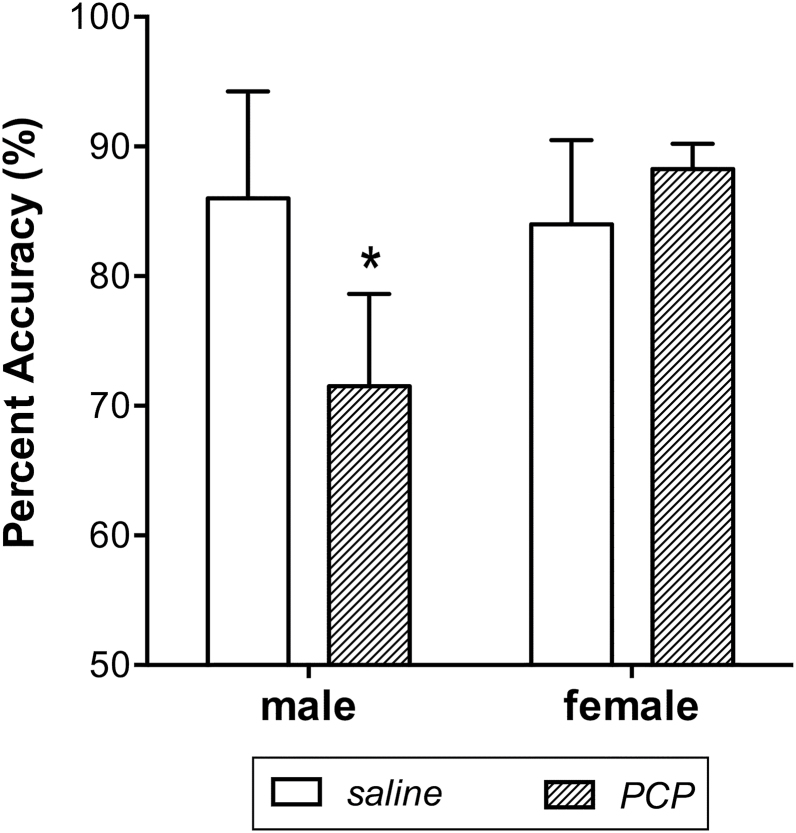
Performance in the 2-choice spatial delay response task was examined using a repeated-measures multivariate analysis of variance (MANOVA) with the average accuracy before, 1 week after, and 4 weeks after PCP (or saline) injections at the middle and longest delays entered as repeated measures and drug and sex as between-subject factors. The effect of drug on accuracy in the spatial delay response task revealed a significant drug by sex by time point interaction [F(4,46)=2.75; P=.04] with no significant drug by time point [F(4,46)=0.91; P=.47] or sex by time point [F(4,46)=1.39; P=.25] interactions detected. Univariate analyses revealed that the 3-way interaction was strongest for accuracy in the middle delay [F(2,24)=5.44; P=.01]. To determine if sex was a significant factor in the PCP or saline groups, similar to our biochemical findings, an omnibus MANOVA was conducted independently for each drug group using the same accuracy measures and with sex as the between-subjects factor. In the saline group, the sex by time point interaction was not significant [F(4,22)=0.18; P=.94]; however, in the PCP group, there was a significant sex by time point interaction [F(4,22)=3.57; P=.02], which was significant for the middle delay [middle delay: F(2,12)=8.88; P=.004; longest delay: F(2,12)=0.26; P=.78]. Post-hoc 1-tailed t tests, comparing the accuracy of PCP-exposed males to that of PCP-exposed females, indicated that the accuracy of PCP-exposed male monkeys was lower than that of PCP-exposed female monkeys at the 4-week time point [t(6)=−2.25; P=.03] (Figure 2) but not at the behavioral assessment 1 week following the PCP injections [t(6)=0.16; P=.88].
Figure 2.
Sex difference in cognitive performance 4 weeks following phencyclidine (PCP) treatment in juvenile monkeys. Mean accuracy (±SEM) was significantly lower in male monkeys exposed to PCP compared with female monkeys exposed to PCP. Accuracy did not differ between male and female monkeys exposed to saline. * indicates a significant difference from female PCP monkeys.
Biochemistry
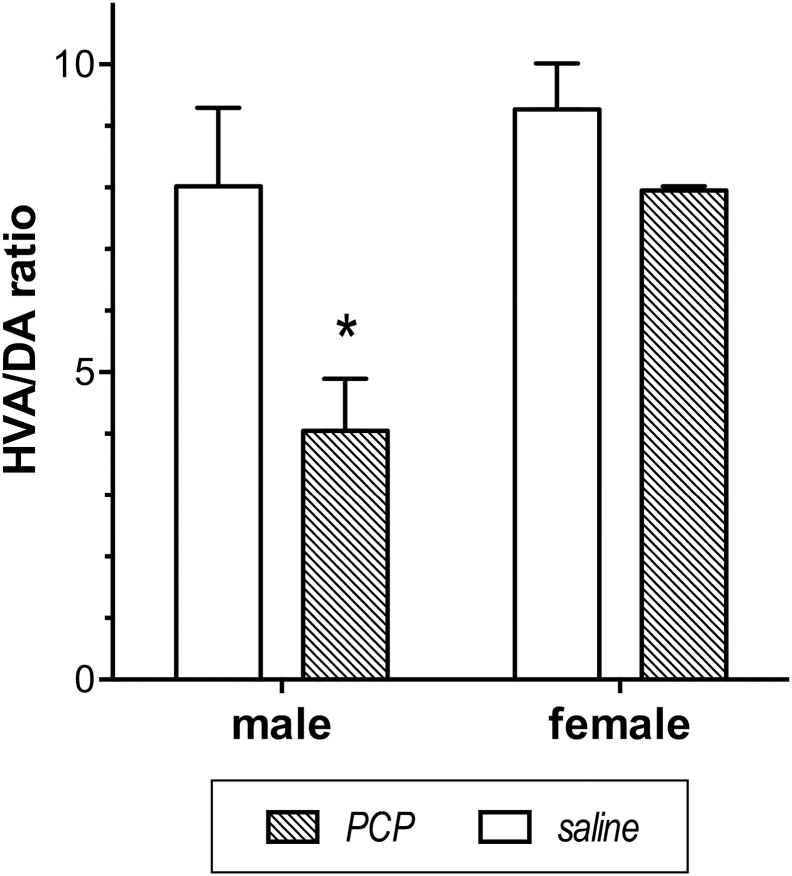
Analysis of the biochemical data revealed that at 4 weeks after PCP treatment, the HVA/DA ratio in the DLPFC was lower in male, but not female, monkeys compared with sex- and age-matched controls (Figure 3). Thus, there was a significant interaction between drug treatment and sex in a 2-way ANOVA [F(1,12)=8.2, P=.01], and a subsequent 2-tailed unpaired t-test confirmed a lower HVA/DA ratio in the male PCP-treated monkeys than in male saline-treated monkeys [t(6)=2.6, P<.05] along with no change in the female monkeys. A separate 2-way ANOVA showed that there was no treatment or sex difference in DA tissue concentration in DLPFC, supporting the validity of HVA/DA ratio as an index of DA turnover.
Figure 3.
Sex difference in the dopamine (DA) turnover in dorsolateral prefrontal cortex (DLPFC) 4 weeks following phencyclidine (PCP) in juvenile monkeys. DA turnover, assessed by mean HVA/DA ratio (±SEM), was significantly lowered in male monkeys exposed to PCP unlike in female monkeys exposed to PCP. There was no difference in the HVA/DA ratio between male and female monkeys exposed to saline. * indicates a significant difference from female PCP monkeys.
Electron Microscopy
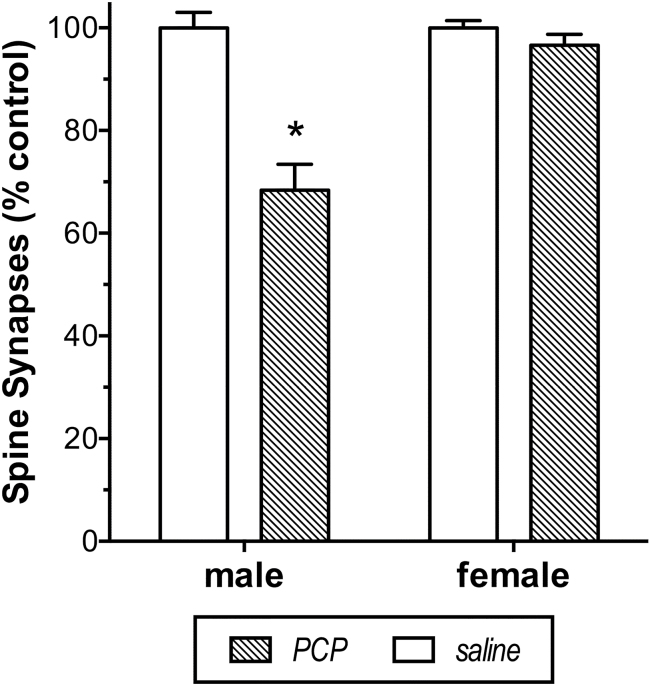
The number of asymmetric spine synapses was entered into a 2-way ANOVA, and this reported a significant interaction between the treatment and sex [F(1,12)=19.0, P<.001], which is shown in Figure 4. A subsequent 2-tailed unpaired t test indicated a significantly lower number of spine synapses in male [t(6)=5.4, P<.005] but not female PCP-treated monkeys compared with appropriate controls.
Figure 4.
Sex difference in the number of spine synapses in dorsolateral prefrontal cortex (DLPFC) 4 weeks following phencyclidine (PCP) in juvenile monkeys. Mean number of asymmetric spine synapses (±SEM) was significantly lowered in layer 2/3 of DLPFC in male monkeys exposed to PCP unlike in female monkeys exposed to PCP. There was no difference in number of spine synapses between male and female monkeys exposed to saline. Mean number of asymmetric spine synapses in control males was 21.7x109 and 22.7x109 in control females. * indicates a significant difference from female PCP monkeys.
Correlations
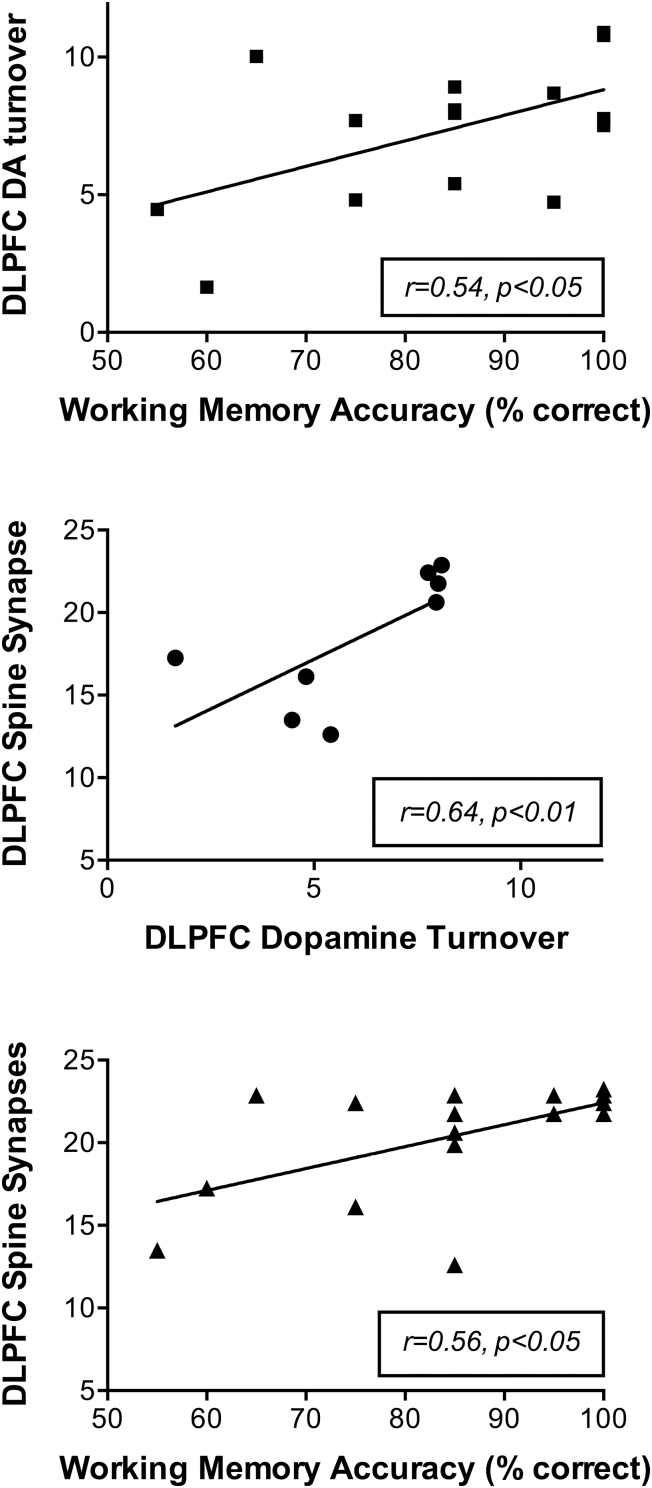
The Pearson correlation coefficient was derived to test the strength of linear relationships between HVA/DA ratio in DLPFC, number of spine synapses in DLPFC, and working memory accuracy, although it is recognized that a nonlinear model may actually describe these associations better. As shown in Figure 5, the 2-tailed Pearson’s correlation coefficient between HVA/DA ratio in DLPFC and number of spine synapses in DLPFC was statistically significant [r(14)=0.64, p<.01]. In addition, the 2-tailed Pearson’s correlation coefficient between the accuracy on the cognitive test and HVA/DA ratio in DLPFC among all monkeys was significant [r(14)=0.54, P<.05], as is depicted in Figure 5. Furthermore, the Pearson’s correlation coefficient between the accuracy on the cognitive test and number of spine synapses in DLPFC among all monkeys (Figure 5) was significant [r(14)=0.56, P<.05]. The correlations are only meaningful if there is a reasonable spread of values for both variables. Inspection of the distribution of individual points on the graphs in Figure 5 shows that this appears to be so, although the values for the male PCP-treated animals tended to be clustered closer together relative to values from other groups.
Figure 5.
Correlations between dopamine (DA) turnover in dorsolateral prefrontal cortex (DLPFC), accuracy on a working memory task, and number of asymmetric spine synapses in DLPFC. Significant correlations existed between each pair of these 3 outcome measures following phencyclidine (PCP) in male and female juvenile monkeys.
Estradiol Levels
A Mann-Whitney test confirmed that plasma estradiol levels were significantly higher in female monkeys compared with male monkeys receiving PCP (median values 19.0 vs 5.0 pg/mL; P<.03).
BDNF mRNA
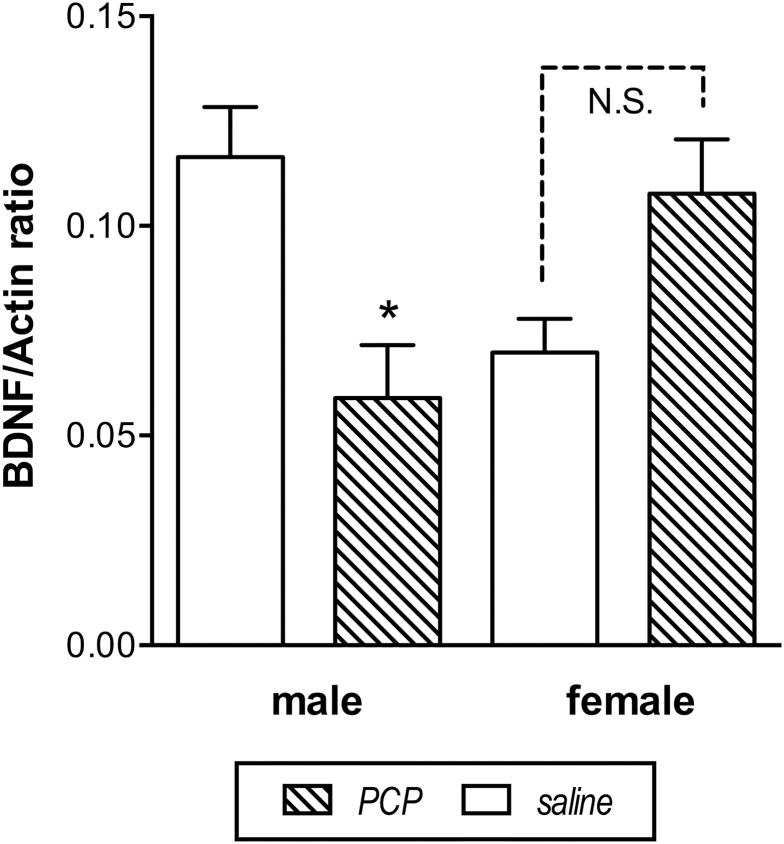
A 2-way ANOVA with BDNF mRNA as the dependent variable identified a significant interaction between treatment and sex [F(1,12)=7.7, P<.05], and subsequent analysis by Tukey’s multiple comparison test revealed a significant difference only between saline and PCP-treated males (P<.05), as shown in Figure 6.
Figure 6.
Lower BDNF mRNA in male phencyclidine (PCP)-exposed monkeys compared with saline-treated controls. Data (mean ±SEM) are adjusted for expression of actin mRNA. * indicates significant difference from controls.
BDNF Levels
Although statistical analysis did not detect an effect of PCP on BDNF levels, DLPFC from all male monkeys (regardless of treatment) contained a lower BDNF concentration [t test, P<.05] than in all female monkeys (2.8±0.3 vs 4.4±0.6 pg/mg protein).
Discussion
The current studies produced several new findings. The first was that juvenile and adolescent male monkeys demonstrated a greater loss of spine synapses in DLPFC than adult male monkeys when treated with our standard regimen of PCP. Subsequent studies conducted in male juvenile monkeys revealed that working memory impairments existed at 4 weeks following PCP treatment and were associated with both lower DA turnover in DLPFC and fewer dendritic spine synapses in DLPFC. In contrast, female juvenile monkeys displayed no working memory deficits at 4 weeks after PCP treatment, and in addition, DA turnover and spine synapse number in DLPFC of female monkeys exposed to PCP were not significantly different from those of controls. In the combined group of male and female monkeys, significant linear correlations were detected between DA turnover, synapse number, and cognitive performance. Together, these results enhance the validity of the primate PCP model of cognitive deficits in schizophrenia and strengthen the argument that DA levels in DLPFC regulate spine synapse number and working memory performance.
Greater Impact of PCP in Juvenile Monkeys
During the juvenile and adolescent phase of development, the prefrontal cortex exhibits marked changes in structure and neurochemistry, which is reflected in altered behavior and pharmacological responses compared with adults (Sturman and Moghaddam, 2011). Indeed, PCP is known to exert more potent effects in developing rodent brain compared with the mature brain (Olney, 2002; Mouri et al., 2007). There is no indication that altered metabolism of PCP accounts for the greater impact of PCP on spine synapses observed in juvenile monkeys compared with adults in the current study. Thus, the observed difference may be connected to the existence of supernumerary dendritic spines in the cerebral cortex during the pre-adult period in primates. During puberty in primates, selective elimination of the initially overproduced number of synaptic spine in cerebral cortex occurs, and this synaptic pruning continues into early adulthood (Bourgeois et al., 1994; Petanjek et al., 2011). Other maturation processes that occur prior to adulthood include changes to transmitter systems that subserve the actions of PCP in the prefrontal cortex, namely the DA, glutamate, and GABA systems (Sturman and Moghaddam, 2011), in addition to PCP-binding sites (Saransaari and Oja, 1993). Such adaptations within the primate PFC probably contribute to the exaggerated response to PCP noted in juvenile monkeys in the present study.
Relationship between Cognition, DA, and Spine Synapses
We have previously postulated that DA availability is an important regulator of the number of asymmetric spine synapses in DLPFC. This was based on the marked loss of spine synapses in DLPFC following either a decrease in DA turnover or a loss of DA innervation (Elsworth et al., 2011, 2013a). However, data from the present study are the strongest evidence so far in favor of this interpretation. Here, we demonstrated that at 4 weeks after PCP treatment, males had working memory deficits together with a decrease in DA turnover and loss of spine synapses in DLPFC. In contrast, PCP-treated females did not display working memory impairments and also did not have altered DA turnover or loss of spine synapses in DLPFC. Indeed, in the combined group of males and females, there was a significant correlation between DA turnover and number of spine synapses in layer 2/3 of DLPFC, between DA turnover and cognitive performance, and between number of spine synapses in layer 2/3 of DLPFC and cognition. We previously noted a significant correlation between DA turnover in DLPFC and cognitive performance in PCP-treated monkeys performing a different PFC-dependent cognitive test, the object retrieval/detour task (Jentsch et al., 1999). In addition, another group (Peters et al., 2008) observed a loss in the number of spine synapses in layer 2/3 of DLPFC of aged monkeys that correlated with cognitive performance on a delayed nonmatching to sample task. Thus, the current study provides strong support for the hypothesis that DA availability regulates spine synapse number in DLPFC.
This supposition raises the question of how a decrease in dopaminergic signaling leads to a loss of asymmetric spine synapses. As in schizophrenia, the mechanism underlying the abnormal PFC DA system in the PCP model is not clear. PCP’s primary pharmacological property is blockade of neurotransmission at NMDA-type glutamate receptors, so other biochemical changes, such as alteration in DA system, are generally assumed to be secondary to this action. As the glutamate hypothesis of schizophrenia is largely based on the similarity between behavioral effects of PCP and symptoms of schizophrenia, explanations of perturbation in the DA system in schizophrenia have also been attributed as secondary to a hypofunction of the glutamate system in the PFC (Laruelle et al., 2003; Volk and Lewis, 2010; Gao, 2011; Moghaddam and Javitt, 2012). DA tone is known to regulate NMDA receptor subunit trafficking in pyramidal neurons. In fact, dendritic spines are enriched with D1 and NMDA receptors, and D1 signals have been shown to enhance NMDA receptor-mediated current on dendritic spines in PFC (Li et al., 2010). Thus, it seems likely that the strong correlation between DA turnover and spine synapse number is due to DA regulation of spine synapse number rather than vice versa. Additional support for this contention derives from studies in the MPTP model of parkinsonian cognitive deficits in monkeys (Elsworth et al., 2013a). The pharmacological actions of MPTP are quite specific for the DA system, and in cognitively impaired MPTP-treated monkeys with no overt motor deficits, there is a loss of DA concentration in the DLPFC together with a decrease in number of spine synapses. Thus, the commonalty in the PCP and MPTP models is reduction in DA transmission and marked loss of spine synapses in DLPFC.
Excitatory synapses on dendritic spines of pyramidal neurons are critical components that regulate the outflow of information from the DLPFC and the function of the DLPFC. In fact, the loss of asymmetric spine synapses in DLPFC of aged monkeys compared with younger monkeys is associated with a decrease in the frequency of spontaneous excitatory postsynaptic current (Peters et al., 2008). The present data suggest that in the PCP model (and maybe in schizophrenia), strategies that directly or indirectly reinstate appropriate DA transmission may be necessary to restore the synapse or spine loss and function of the PFC.
Recovery in Female Monkeys
It is not yet known why juvenile male, but not female, monkeys exhibited PCP-induced behavioral, biochemical, and synaptic changes at the end of this study. There is no indication that pharmacokinetic factors are responsible; in fact, studies in rodents demonstrated that after the same dose of PCP, the concentration of drug in the female rat brain was higher than in the male rat brain and that the half-life of PCP in the brain and plasma was longer in female rats than in male rats. These differences were attributed to a decreased ability of females to metabolize the drug (Nabeshima et al., 1984; Shelnutt et al., 1999). However, it is noteworthy that sex differences in metabolism of PCP have not been found in humans (Owens, 1997). We used our standard dose regimen of PCP administration in the current studies, so we do not yet know at what dose of PCP female juvenile monkeys might exhibit the changes observed here in juvenile males. The most straightforward explanation for the sex-dependent effects of PCP in this study is based on the protective and reparative effects that estradiol and progesterone can exert in the CNS (Azcoitia et al., 2011; Singh and Su, 2013). Although the level of estradiol is substantially lower in juveniles than in adults, circulating estradiol concentration was higher in females than males, which is consistent with a role of peripherally derived hormone in the recovery. However, the contribution of locally synthesized estradiol or progesterone in the brain cannot be discounted (McEwen and Alves, 1999; Schumacher et al., 2004; Roselli et al., 2009).
The existence of an estrogen response element-like motif in the BDNF gene indicates that estradiol regulates BDNF expression levels (Sohrabji et al., 1995), and this interaction provides a plausible explanation for the relatively rapid recovery of spine synapse number in female PCP-treated monkeys. This is because mRNA for BDNF, which has an established critical role in regulating spine plasticity and spine synapse number (reviewed by Luine and Frankfurt, 2013; Srivastava et al., 2013) was reduced in male, but not female, PCP-treated monkeys compared with controls. Analysis of BDNF levels by ELISA did not reveal the same profile of changes as BDNF mRNA levels by quantitative PCR, although mean BDNF level in DLPFC in all females (regardless of treatment) was higher than in all males, which is still consistent with a sex-dependent protective effect of BDNF on spine synapses and working memory in this model. Interestingly, BDNF is reduced in DLPFC from patients with schizophrenia (Weickert et al., 2003; Hashimoto et al., 2005). In addition, there is evidence that estradiol controls expression of TrkB, the protein kinase receptor for BDNF, which provides for an additional possible level of estradiol-mediated regulation of BDNF signaling (Brito et al., 2004). In view of the apparent role of estradiol in maintaining TH-expression in monkey DLPFC (Kritzer and Kohama, 1998), it is also possible that estradiol plays a role in the restoration of DA turnover in PCP-treated female monkeys. Furthermore, DA itself in some models has been reported to activate BDNF expression (Xing et al., 2012). So, it is possible to construct a model in which BDNF expression is relatively higher in female monkeys as a result of direct and indirect actions of estradiol in DLPFC and that BDNF signaling induces spine synapse recovery, which in turn accounts for improved working memory performance compared with male PCP-treated monkeys. However, it is recognized that estradiol is certainly not the only determinant of BDNF expression in either the PCP model or schizophrenia.
The parallel between the sex-dependent effect observed in the primate PCP model of schizophrenia and the lower incidence of schizophrenia in females is intriguing. Evidence for the latter includes meta-analyses that have reported men having an approximately 40% greater chance of developing schizophrenia than women (Aleman et al., 2003; McGrath, 2006). In addition, the onset of schizophrenia is earlier and the rate of progression steeper for males than females, and particularly relevant is the observation that women have a second peak of incidence following menopause (Markham, 2012). Although all the biochemical mechanisms underlying the sex-dependent effects in the PCP model and in schizophrenia are not yet known, this apparent similarity extends the correspondence between the model and the disorder.
The current findings strengthen the validity of the juvenile primate PCP model and highlight its potential usefulness in developing novel treatments for the cognitive deficits associated with schizophrenia. Such progress will be dependent on understanding the molecular underpinnings of the synaptic changes in the primate PCP model and identifying pharmacological strategies to ameliorate the maladaptive remodeling.
Statement of Interest
None
Acknowledgments
This work was supported by NIH grants MH57483 and NS064129. We thank Feng-Pei Chen Klara Szigeti-Buck and Dorothy Cameron for their excellent technical work at Yale University. We are grateful to the staff of the St. Kitts Biomedical Research Foundation staff for their expertise in treating and caring for the animals in this study.
References
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. (1999). Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156:1580–1589. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP. (2003). Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry 60:565–571. [DOI] [PubMed] [Google Scholar]
- Ankarberg-Lindgren C, Norjavaara E. (2008). A purification step prior to commercial sensitive immunoassay is necessary to achieve clinical usefulness when quantifying serum 17beta-estradiol in prepubertal children. Eur J Endocrinol 158:117–124. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. (2011). Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab 22:467–473. [DOI] [PubMed] [Google Scholar]
- Bacopoulos NG, Maas JW, Hattox SE, Roth RH. (1978). Regional distribution of dopamine metabolites in human and primate brain. Commun Psychopharmacol 2:281–286. [PubMed] [Google Scholar]
- Barch DM, Ceaser A. (2012). Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. (1976). Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol 90:293–302. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. (1994). Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4:78–96. [DOI] [PubMed] [Google Scholar]
- Brito VI, Carrer HF, Cambiasso MJ. (2004). Inhibition of tyrosine kinase receptor type B synthesis blocks axogenic effect of estradiol on rat hypothalamic neurones in vitro. Eur J Neurosci 20:331–337. [DOI] [PubMed] [Google Scholar]
- Broberg BV, Dias R, Glenthoj BY, Olsen CK. (2008). Evaluation of a neurodevelopmental model of schizophrenia--early postnatal PCP treatment in attentional set-shifting. Behav Brain Res 190:160–163. [DOI] [PubMed] [Google Scholar]
- Cortese L, Bressan RA, Castle DJ, Mosolov SN. (2013). Management of schizophrenia: clinical experience with asenapine. J Psychopharmacol 27:14–22. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. (2007). Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 148:1474–1486. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Mastropaolo J, Rosse RB. (1998). Neurodevelopmental consequences of early exposure to phencyclidine and related drugs. Clin Neuropharmacol 21:320–332. [PubMed] [Google Scholar]
- Dighe AS, Sluss PM. (2004). Improved detection of serum estradiol after sample extraction procedure. Clin Chem 50:764–766. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Taylor JR, Sladek JR, Jr, Collier TJ, Redmond DE, Jr, Roth RH. (2000). Striatal dopaminergic correlates of stable parkinsonism and degree of recovery in old-world primates one year after MPTP treatment. Neuroscience 95:399–408. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Hajszan T, Leranth C, Roth RH. (2011). Loss of asymmetric spine synapses in dorsolateral prefrontal cortex of cognitively impaired phencyclidine-treated monkeys. Int J Neuropsychopharmacol 14:1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Groman SM, Jentsch JD, Valles R, Shahid M, Wong E, Marston H, Roth RH. (2012). Asenapine effects on cognitive and monoamine dysfunction elicited by subchronic phencyclidine administration. Neuropharmacology 62:1442–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Leranth C, Redmond DE, Jr, Roth RH. (2013a) Loss of asymmetric spine synapses in prefrontal cortex of motor-asymptomatic, dopamine-depleted, cognitively impaired MPTP-treated monkeys. Int J Neuropsychopharmacol 16:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, Jr DE, Leranth C. (2013b) Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology 35:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. (2000). Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol 14:1–21. [PubMed] [Google Scholar]
- Gao WJ. (2011). Dopaminergic and Glutamatergic dysfunctions in the neuropathophysiology of schizophrenia. In: Dopamine: Functions, regulation and health effects (Kudo E, Fujii Y, eds). Hauppauge, NY: Nova Science Publishers. [Google Scholar]
- Glantz LA, Lewis DA. (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57:65–73. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. (2011). Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull 37:504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bergen SE, Nguyen QL, Xu B, Monteggia LM, Pierri JN, Sun Z, Sampson AR, Lewis DA. (2005). Relationship of brain-derived neurotrophic factor and its receptor TrkB to altered inhibitory prefrontal circuitry in schizophrenia. J Neurosci 25:372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW. (2005). The primacy of cognition in schizophrenia. Am Psychol 60:229–242. [DOI] [PubMed] [Google Scholar]
- Helander KG. (2000). Formaldehyde prepared from paraformaldehyde is stable. Biotech Histochem 75:19–22. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. (2010). Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A 107:13135–13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. (2011). Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull 37:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. (2007). Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci 27:14358–14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20:201–225. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. (1997). Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science 277:953–955. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Elsworth JD, Redmond DE, Jr, Roth RH. (1999). Altered frontal cortical dopaminergic transmission in monkeys after subchronic phencyclidine exposure: involvement in frontostriatal cognitive deficits. Neuroscience 90:823–832. [DOI] [PubMed] [Google Scholar]
- Knable MB, Weinberger DR. (1997). Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 11:123–131. [DOI] [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. (2005). Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry 162:1200–1202. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. (1998). Ovarian hormones influence the morphology, distribution, and density of tyrosine hydroxylase immunoreactive axons in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol 395:1–17. [PubMed] [Google Scholar]
- Kroner S, Krimer LS, Lewis DA, Barrionuevo G. (2007). Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex 17:1020–1032. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A. (2003). Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci 1003:138–158. [DOI] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. (2006). Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry 59:863–871. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. (2002). Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25:409–432. [DOI] [PubMed] [Google Scholar]
- Li YC, Liu G, Hu JL, Gao WJ, Huang YQ. (2010). Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. J Neurochem 114:62–73. [DOI] [PubMed] [Google Scholar]
- Luine V, Frankfurt M. (2013). Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Johansen JA, Jordan CL, Leranth C. (2006). Androgen effects on hippocampal CA1 spine synapse numbers are retained in Tfm male rats with defective androgen receptors. Endocrinology 147:2392–2398. [DOI] [PubMed] [Google Scholar]
- Marenco S, Weinberger DR. (2000). The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol 12:501–527. [DOI] [PubMed] [Google Scholar]
- Markham JA. (2012). Sex steroids and schizophrenia. Rev Endocr Metab Disord 13:187–207. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. (1999). Estrogen actions in the central nervous system. Endocr Rev 20:279–307. [DOI] [PubMed] [Google Scholar]
- McGrath JJ. (2006). Variations in the incidence of schizophrenia: data versus dogma. Schizophr Bull 32:195–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. (2007a) Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 192:283–290. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Roth RH, Redmond DE, Jr, Sladek JR, Jr, Elsworth JD. (2007b) Apoptotic natural cell death in developing primate dopamine midbrain neurons occurs during a restricted period in the second trimester of gestation. Exp Neurol 204:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. (2007). Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int 51:173–184. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T. (1984). Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur J Pharmacol 97:217–227. [DOI] [PubMed] [Google Scholar]
- Olney JW. (2002). New insights and new issues in developmental neurotoxicology. Neurotoxicology 23:659–668. [DOI] [PubMed] [Google Scholar]
- Owens SM. (1997). Antibodies as pharmacokinetic and metabolic modifiers of neurotoxicity. NIDA Res Monogr 173:259–272. [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. (2011). Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. (2008). Synapses are lost during aging in the primate prefrontal cortex. Neuroscience 152:970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD. (2009). Brain aromatization: classic roles and new perspectives. Semin Reprod Med 27:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Lewis DA, Gonzalez-Burgos G. (2012). The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci 23:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. (1993). Phencyclidine-binding sites in mouse cerebral cortex during development and ageing: effects of inhibitory amino acids. Mech Ageing Dev 68:125–136. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. (2004). Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res 14 Suppl A:S18–33. [DOI] [PubMed] [Google Scholar]
- Sharma T, Hughes C, Soni W, Kumari V. (2003). Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl) 169:398–403. [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Gunnell M, Owens SM. (1999). Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J Pharmacol Exp Ther 290:1292–1298. [PubMed] [Google Scholar]
- Singh M, Su C. (2013). Progesterone and neuroprotection. Horm Behav 63:284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Miranda RC, Toran-Allerand CD. (1995). Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. Proc Natl Acad Sci U S A 92:11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD. (2013). Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience 239:17–33. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B. (2011). The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev 35:1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA. (1998). Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol 12:21–36. [DOI] [PubMed] [Google Scholar]
- Terasawa E, Fernandez DL. (2001). Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev 22:111–151. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. (2010). Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci 4:485–508. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. (2008). Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology 33:2442–2455. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. (2003). Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 8:592–610. [DOI] [PubMed] [Google Scholar]
- Xing B, Guo J, Meng X, Wei SG, Li SB. (2012). The dopamine D1 but not D3 receptor plays a fundamental role in spatial working memory and BDNF expression in prefrontal cortex of mice. Behav Brain Res 235:36–41. [DOI] [PubMed] [Google Scholar]