Abstract
Background:
The hippocampus has been highly implicated in the pathophysiology of bipolar disorder (BD). Nevertheless, no study has longitudinally evaluated hippocampal metabolite levels in bipolar depression under treatment with lithium.
Methods:
Nineteen medication-free BD patients (78.9% treatment-naïve and 73.7% with BD type II) presenting an acute depressive episode and 17 healthy controls were studied. Patients were treated for 6 weeks with lithium in an open-label trial. N-acetyl aspartate (NAA), creatine, choline, myo-Inositol, and glutamate levels were assessed in the left hippocampus before (week 0) and after (week 6) lithium treatment using 3T proton magnetic resonance spectroscopy (1H-MRS). The metabolite concentrations were estimated using internal water as reference and voxel segmentation for partial volume correction.
Results:
At baseline, acutely depressed BD patients and healthy controls exhibited similar hippocampal metabolites concentrations, with no changes after 6 weeks of lithium monotherapy. A significant correlation between antidepressant efficacy and increases in NAA concentration over time was observed. Also, there was a significant positive correlation between the changes in glutamate concentrations over follow-up and plasma lithium levels at endpoint. Mixed effects model analysis revealed a bimodal effect of lithium plasma levels in hippocampal glutamate concentrations: levels of 0.2 to 0.49mmol/L (n=9) were associated with a decrease in glutamate concentrations, whereas the subgroup of BD subjects with “standard” lithium levels (≥0.50 mmol/L; n = 10) showed an overall increase in glutamate concentrations over time.
Conclusions:
These preliminary results suggest that lithium has a bimodal action in hippocampal glutamate concentration depending on the plasma levels.
Keywords: bipolar disorder, depression, glutamate, hippocampus, lithium, magnetic resonance spectroscopy
Introduction
The neurobiological basis of bipolar disorder (BD) and mechanisms of action of mood stabilizers are not fully elucidated (Machado-Vieira et al., 2009, 2012). Lithium is the mainstay of pharmacotherapy for both acute mood episodes and prophylactic treatment in BD, and it is still the gold standard proof-of-concept agent to perform neurobiological studies in the search of putative biomarkers in BD (Machado-Vieira et al., 2009; Machado-Vieira, Soeiro-de-Souza et al., 2014). Lithium has also been shown to improve synaptic strength, cellular resilience, and glial function, as well as to positively modulate neurotrophic factors, N-acetyl aspartate (NAA), and other proteins involved in cellular energy metabolism (Soeiro-de-Souza et al., 2012). Moreover, lithium’s action in the intracellular pathways implicated in cellular energy production and neuroplasticity may ultimately influence glutamatergic transmission (Du et al., 2010; Machado-Vieira et al., 2012).
A region important for the action of lithium in the brain is the hippocampus. Through its connections with the dorsal anterior cingulate and prefrontal cortices, the hippocampus is implicated in a wide range of cognitive functions and neurovegetative and behavioral changes important for both automatic and voluntary mood regulation (Phillips et al., 2008). Although volumetric magnetic resonance imaging (MRI) studies evaluating the hippocampus in BD have been conflicting (Phillips and Swartz, 2014), preclinical and post-mortem studies have provided consistent evidence for abnormalities of the hippocampus in BD. These include a reduced number of GABAergic interneurons (Konradi et al., 2011) and the decreased expression and altered binding profile of NMDA receptors (Beneyto et al., 2007; McCullumsmith et al., 2007). As hippocampal interneurons play an important role in modulating the activity of the glutamatergic (pyramidal) neurons, glutamatergic disinhibition in the hippocampus is a feature of BD (Konradi et al., 2011). Also, the hippocampus is particularly sensitive to the effects of stress and excessive glucocorticoid release (Colla et al., 2009; Teicher et al., 2012), which are known to be exacerbated during the depressive episodes in BD (Rybakowski and Twardowska, 1999; Watson et al., 2004) and might lead to a further increase in glutamate release in the hippocampus. The potential consequences of this imbalance in the hippocampal glutamatergic transmission are many and range from downstream effects upon excitatory neurotransmission, impaired synaptic and neuronal plasticity, altered neuron-glia homeostasis and even local toxic effects secondary to overexposure to high glutamate levels (Machado-Vieira et al., 2012).
Proton magnetic resonance spectroscopy (1H-MRS) assesses brain concentrations of NAA, creatine (Cr; composed of creatine and phosphocreatine), choline (Cho), myo-Inositol (mI), and glutamate (Glu) or glutamine (Gln) in specific brain areas. Thus, it allows us to indirectly estimate neuronal integrity and/or neuronal-glial homeostasis (NAA), membrane phospholipid turnover/ metabolism (Cho), cellular osmotic balance (mI), and glutamatergic transmission in vivo (Capizzano et al., 2007; Dager et al., 2008; Bustillo et al., 2013). Glu and Gln produce overlapping resonances and are often considered collectively as Glx; nevertheless, the Glu peak can be isolated at higher magnetic fields (≥3T; Dager et al., 2008). In the last decade, relatively few studies have assessed brain metabolites in the hippocampus of BD patients using 1H-MRS, with a great variability of findings across different investigations (Capizzano et al., 2007; Kraguljac et al., 2012; Howells et al., 2013). For instance, the largest study (58 BD type I patients versus 27 healthy controls) published so far found no significant differences with regard to NAA, Cr, mI, or Cho levels (Gigante et al., 2014). Also, only three studies have assessed Glu or Glx levels in the hippocampus of subjects with BD. Senaratne et al. (2009) and Gigante et al. (2014) did not find differences in Glx levels between BD patients and healthy controls. Colla et al. (2009) only found elevated Glu concentrations in euthymic BD subjects under lithium treatment compared to healthy controls. This heterogeneity in the results of 1H-MRS studies in BD is likely to reflect variability of technical approaches and study designs, including the recruitment of medicated patients at variable mood phases (Capizzano et al., 2007; Kraguljac et al., 2012). To date, there is no longitudinal study evaluating lithium’s effects on 1H-MRS hippocampal metabolites in bipolar depression.
Given the relevance of the hippocampus in stress regulation and glutamatergic metabolism in mood disorders, and also considering the key role of lithium at this brain area, the present study aimed to evaluate hippocampal metabolites in medication-free short-term BD patients during depressive episodes, using 3T 1H-MRS, before and after 6 weeks of lithium treatment. Most studies assessing the efficacy of lithium in the treatment of acute BD have adopted minimum lithium levels of 0.5–0.6 mmol/L (Amsterdam and Shults, 2008; Suppes et al., 2008; Young et al., 2010), with drop-out rates as high as 62.5% (Amsterdam and Shults, 2008). A recent investigation from our group using a flexible dosing prescription based on clinical improvement found that lower lithium plasma levels (0.2–0.49 mmol/L) were as effective as standard levels (≥0.5 mmol/L) in the treatment of bipolar depression, but with a smaller incidence of adverse effects (Machado-Vieira, Zanetti et al., 2014). Thus, it is relevant also to investigate the effects of different lithium plasma levels in the concentration of hippocampal metabolites.
Methods
Participants and Study Design
BD patients presenting with an acute depressive episode were included using the following criteria: (a) age between 18 and 45 years; (b) diagnosis of BD type I or II in a current depressive episode according to the Diagnostic and Statistical Manual for Mental Disorders, 4th edition (DSM-IV); (c) a score equal to or greater than 18 in the 21-item Hamilton Depression Rating Scale (HDRS; Hamilton, 1960); (d) no previous use of lithium (lifetime) and absence of use of any psychiatric medication for at least 6 weeks; and (e) illness duration of up to 5 years. Exclusion criteria specific for the patients included: (a) rapid cycling; (b) current substance (including alcohol) abuse or dependence, with the exception of tobacco use; and (c) previous treatment with electroconvulsive therapy.
A total of 59 individuals were pre-screened by phone interviews and were subsequently evaluated at the Institute of Psychiatry, University of Sao Paulo. A total of 26 subjects with BD fulfilled criteria for the present study and were initially enrolled.
Patients were initially evaluated with a clinical interview, blood tests (including complete blood count, electrolytes, and renal and thyroid function) and pregnancy testing (women). All patients were then started on treatment with oral lithium (450mg/day) and a systematic follow-up was carried out for 6 weeks. Visits for clinical assessment and lithium monitoring in the plasma were performed at weeks 1, 2, 4, and 6. Subsequent dosage adjustments were permitted in a flexible manner to a dose of ≤900mg/day, based on their clinical efficacy for individual patients and the level of lithium in the patient’s plasma (in order to ensure compliance and prevent risk of intoxication). 1H-MRS scanning was performed at baseline (week 0) and endpoint (week 6).
Seven subjects were excluded due to the following reasons: 2 patients dropped out before the completion of follow-up, and 5 had technical problems either in the baseline or follow-up 1H-MRS examination (poor spectra quality or Cramer-Rao lower bound >20%). Thus, our final sample comprised 19 medication-free subjects with BD (16 females and 6 males, aged 22–43 years old) in a depressive episode who successfully completed the 6-week lithium trial.
Seventeen healthy volunteers (9 females and 8 males, aged 18–43 years old) free of any psychiatric disorder (lifetime DSM-IV criteria) and with no history of mental disorders among first-degree relatives were recruited through advertisements in the local community and constituted our control group.
The exclusion criteria common for both patients and controls were: (a) presence of neurological disorders or any medical disorder that could affect the central nervous system; (b) mental retardation; and (c) contraindications for MRI scanning.
The study was approved by local ethics committees, and all subjects provided informed written consent.
Clinical Assessment Scales
All clinical assessments were performed by experienced psychiatrists. Psychiatric diagnosis was assessed in both patients and healthy controls at baseline (week 0) using the Structured Clinical Interview for DSM-IV (First et al., 1995). Moreover, a general medical history, including information about medication use, was obtained directly from each participant.
At each follow-up visit (weeks 1, 2, 4, and 6), the severity of depressive and manic symptoms was assessed using, respectively, the HDRS and the Young Mania Rating Scale (Young et al., 1978). Clinical response was considered a decrease ≥50% in the HDRS, while remission was defined as a HDRS score ≤8. The global functioning (baseline) and improvement (endpoint) were also evaluated using the Clinical Global Improvement scale (Guy, 1976). Inter-rater reliability was over 0.9 for all of them.
Proton Magnetic Resonance Spectroscopy (1H-MRS)
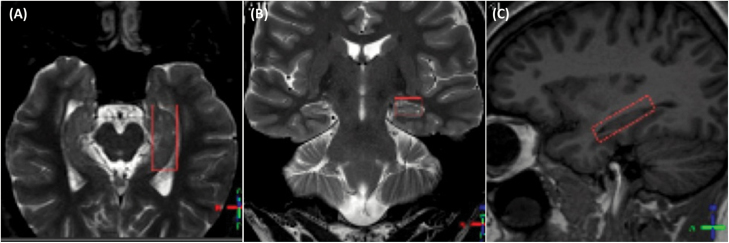
1H-MRS imaging sequences were performed using a 3.0T magnet (Intera Achieva, Philips) and an eight-channel head coil. Metabolite levels were obtained using the PRESS sequence (TE/TR = 35/1500ms) with 160 scan averages. A voxel of 10 x 15 x 40mm (6cm3 of volume) was positioned along the longest axis of the the left hippocampus, as shown in Figure 1.
Figure 1.
Axial (A), coronal (B), and sagittal (C) planes demonstrating the positioning of the VOI in the left hippocampus. Images are displayed in radiological convention (the left side of the brain corresponds to the right side of the figure).
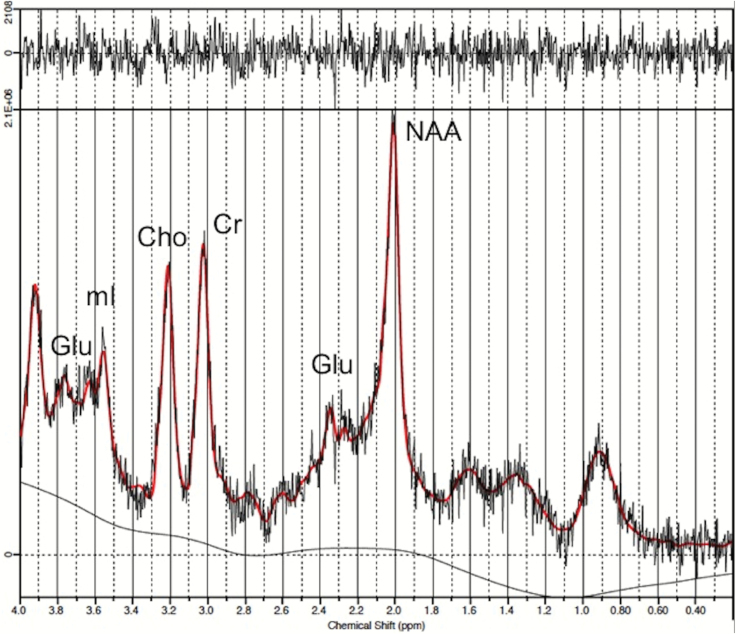
Spectra were quantified with LCModel software (Provencher, 1993), using internal water as a reference (metabolite levels with Cramer-Rao lower bounds >20% were excluded from the analysis). The concentrations of the following metabolites were obtained: Cr, Glu, mI, NAA, and Cho (Figure 2).
Figure 2.
Example of spectra showing the glutamate peak. Cho, choline; Cr, creatine; Glu, glutamate; mI, myo-inositol; NAA, n-acetyl-aspartate.
The metabolite concentrations were corrected by the water content of the voxel in order to avoid the influence of potential differences in the voxel composition between the groups under study (due to regional atrophy, for example). To investigate brain tissue composition of the voxel of interest, three-dimensional volumetric images were obtained using the 3D-T1-FFE technique (FA = 8°; TE/TR/TI = 3.2/7/900ms) with an isotropic voxel size of 1mm3. Briefly, the brain tissue was extracted using the brain extraction tool (BET) and, subsequently, segmentation into white matter (WM), gray matter (GM) and cerebrospinal fluid (CSF) was achieved using the automated brain segmentation tool FAST (Zhang et al., 2001); both BET and FAST are part of the FSL suite (http://www.fmrib.ox.ac.uk/fsl). Finally, the volume of interest (VOI) of the hippocampus was overlaid to the segmented image using a Python-based script developed in house. Metabolite concentrations were corrected, taking into account the different water content and relaxation properties in CSF, GM, and WM, as proposed by Gasparovic et al (2006). In brief, firstly the fraction of the observed water signal for CSF, GM, and WM was calculated according to the different water content in the tissues (97%, 78%, and 65% for CSF, GM, and WM, respectively). Subsequently, the observed signal was corrected for water T1 and T2 relaxation effects, taking into account the calculated water fractions for each tissue and the different T1 and T2 water relaxation times at 3T for CSF (3.0 s and 0.2 s), GM (1.47 s and 0.11 s), and WM (1.06 s and 0.074 s) values obtained from the literature (Posse et al., 2007). No correction for metabolite relaxation contribution was applied. Hence, the obtained metabolite values represent an approximation to the absolute concentration and are reported in institutional units (I.U.).
Data Analysis
The Kolmogorov-Smirnov test was used to verify the normality of the data within each study group. Between-group comparisons of continuous variables with a Gaussian distribution were performed with the student’s t-test, while the Mann-Whitney test was used for non-normal data. The chi-square test was used for the comparison of categorical variables. Changes in hippocampal metabolites and severity of clinical symptoms of BD patients over the 6 weeks of treatment with lithium were assessed with the paired-samples t-test (normal distribution) or with the non-parametric Wilcoxon Rank Test (non-normal distribution).
BD patients were also divided into two subgroups according to the blood lithium levels at endpoint (week 6): lower lithium levels were classified as 0.2–0.49 mmol/L and standard lithium levels as ≥0.5 mmol/L. The mixed-effects model was used to evaluate differences in the changes in hippocampal metabolite concentrations (target variable) over the follow-up between the following subgroups of BD subjects: type I versus type II; patients in remission versus those not in remission at endpoint; and lower versus standard lithium levels at endpoint.
The Spearman correlation test was employed in order to ascertain whether hippocampal metabolite levels were significantly correlated to clinical variables at baseline, and also to verify possible significant correlations between changes in hippocampal metabolite levels, plasma lithium levels, and depressive symptom scores over time. For correlation analyses, the changes in hippocampal metabolite levels and clinical measures over time were derived as follows: (week 6–week 0)/week 0.
Statistical significance was set at p ≤ 0.05. In order to control for family-wise type I statistical errors, the p-value of all analyses was adjusted using the sequential Bonferroni procedure proposed by Hochberg (1988).
Results
Demographic and Clinical Data
Demographic and clinical data are summarized in Table 1. No significant differences in age or gender distribution were observed between BD patients and healthy controls. Fifteen BD patients (78.9%) had never received any psychopharmacological treatment. Five BD subjects (26.3%) had a positive history of tobacco use (none of the healthy controls had). BD patients with (n = 5) and without (n = 14) a history of smoking showed similar concentrations of all hippocampal metabolites (Mann-Whitney test, all p ≥ 0.444; data not shown).
Table 1.
Demographic and Clinical Information of Patients with Bipolar Disorder (BD) and Healthy Controls (HC)
| BD (n = 19) | HC (n = 17) | Statistical tests | |
|---|---|---|---|
| Age (mean ± sd) | 28.7±5.4 | 27.4±6.5 | t = -0.61, df = 34, p = 0.547 |
| Gender (no. females;%) | 13 (68.4%) | 9 (52.9%) | χ2 = 0.90, df = 1, p = 0.342 |
| BD subtype (no. type II; %) | 14 (73.7%) | - | - |
| Duration of illness (months; mean ± sd) |
34.2±18.0 | - | - |
| Treatment-naïve (no. patients; %) |
15 (78.9%) | - | - |
| History of psychosis (no. patients; %) |
1 (5.3%) | - | - |
| Response rate at week 6a
(no. patients; %) |
17 (89.5%) | - | - |
| Remission rate at week 6b
(no. patients; %) |
13 (68.4%) | - | - |
| Plasma lithium levels at week 6 (mmol/L; mean ± sd) | 0.49±0.19 | - | - |
| Lower vs standard lithium levels
c
(no. patients Li 0.2–0.5; %) |
9 (47.4%) |
sd, standard deviation
aReduction ≥ 50% in Hamiton Depression Rating Scale (HDRS) scores relative to the baseline.
bHDRS score ≤ 8.
cLower levels = 0.2–0.49 mmol/L and standard levels ≥ 0.50 mmol/L.
At the endpoint, 17 subjects (89.5%) responded to the treatment and 13 patients (68.4%) achieved remission (Table 1).
Hippocampal Metabolites in Unmedicated Bipolar Disorder Versus Healthy Controls
Subjects with BD in a depressive episode and healthy controls showed similar concentrations of all hippocampal metabolites (Table 2). Importantly, no significant differences in the GM and WM fractions within the hippocampal VOI were observed between BD patients and healthy controls (Table 2).
Table 2.
Metabolite Levels and Voxel Composition in the Left Hippocampus of Patients With Bipolar Disorder (BD) and Healthy Controls (HC) at Baseline
| BD (n = 19) | HC (n = 17) | Statistical testsa | |
|---|---|---|---|
| Cr (mean I.U. ± sd) | 2.63±0.31 | 2.68±0.48 | t = -0.38, df = 34, p = 0.707 |
| Glu (mean I.U. ± sd) | 3.75±0.66 | 3.55±0.74 | t = -0.88, df = 34, p = 0.382 |
| mI (mean I.U. ± sd) | 2.29±0.36 | 2.25±0.45 | t = -0.28, df = 34, p = 0.782 |
| NAA (mean I.U. ± sd) | 3.03±0.31 | 3.03±0.37 | t = --0.06, df = 34, p = 0.951 |
| Cho (mean I.U. ± sd) | 0.85±0.10 | 0.85±0.18 | t = -0.04, df = 34, p = 0.967 |
| GM Fraction (mean ± sd) | 0.57±0.03 | 0.57±0.05 | t = -0.30, df = 34, p = 0.764 |
| WM Fraction (mean ± sd) | 0.35±0.05 | 0.33±0.05 | t = -1.32, df = 34, p = 0.193 |
Cho, choline; Cr, creatine; Glu, glutamate; GM, gray matter; I.U., institutional units; mI, myo-inositol; NAA, n-acetyl-aspartate; sd, standard deviation; WM, white matter.
aAs no significant result was observed, only unadjusted p-values are reported.
At baseline, no significant correlation was found between any of the hippocampal metabolites and duration of illness and HDRS, Young Mania Rating Scale, or Clinical Global Improvement scores in the BD group (all p ≥ 0.250, corrected for multiple comparisons; Supplementary Table S1). Also, the concentrations of hippocampal metabolites at baseline did not predict remission status at week 6 (all t ≤ 1.54, df = 17, all unadjusted p ≥ 0.141; data not shown).
Hippocampal Metabolites After Treatment with Lithium
In the whole group of BD patients, an increase in Cho levels was observed after 6 weeks of treatment with lithium, but the statistical significance of the change did not survive correction for multiple comparisons (Table 3). There was no significant change in the brain tissue composition within the VOI (i.e., GM and WM fractions) over the 6 weeks of follow-up (Table 3).
Table 3.
Metabolite Levels and Voxel Composition in the Left Hippocampus and Clinical Scales Before (Week 0) and after (Week 6) Lithium Treatment in Patients with Bipolar Disorder
| Week 0 | Week 6 | Statistical tests (paired-sample) | Adjusted-p a | |
|---|---|---|---|---|
| Cr (mean I.U. ± sd) | 2.63±0.31 | 2.74±0.32 | t = 1.60, df = 18, p = 0.126 | p = 0.378 |
| Glu (mean I.U. ± sd) | 3.75±0.66 | 3.61±0.57 | t = -0.72, df = 18, p = 0.477 | p = 0.477 |
| mI (mean I.U. ± sd) | 2.29±0.36 | 2.55±0.60 | t = 1.90, df = 18, p = 0.073 | p = 0.292 |
| NAA (mean I.U. ± sd) | 3.03±0.31 | 3.14±0.33 | t = 1.08, df = 18, p = 0.295 | p = 0.477 |
| Cho (mean I.U. ± sd) | 0.85±0.10 | 0.92±0.10 | t = 2.56, df = 18, p = 0.020 | p = 0.100 |
| GM Fraction (mean ± sd) | 0.57±0.03 | 0.57±0.04 | t = 0.08, df = 18, p = 0.935 | - |
| WM Fraction (mean ± sd) | 0.35±0.05 | 0.34±0.04 | t = -0.52, df = 18, p = 0.609 | - |
| HDRS scores (mean ± sd) | 22.42±2.98 | 6.42±3.96 | t = -15.74, df = 18, p < 0.001 | - |
| YMRS scores (mean ± sd) | 5.42±4.57 | 2.63±3.50 | Wilcoxon test, p= 0.002 | - |
| CGI scores (mean ± sd) | 4.00±0.33 | 1.95±0.78 | Wilcoxon test,p < 0.001 | - |
CGI, Clinical Global Improvement scale; Cho, choline; Cr, creatine; Glu, glutamate; GM, gray matter; HDRS, 21-item Hamilton Depression Rating Scale; I.U., institutional units; mI, myo-inositol; NAA, n-acetyl-aspartate; sd, standard deviation; WM, white matter; YMRS, Young Mania Rating Scale.
aCorrected for multiple comparisons using the sequential Bonferroni procedure proposed by Hochberg (1988).
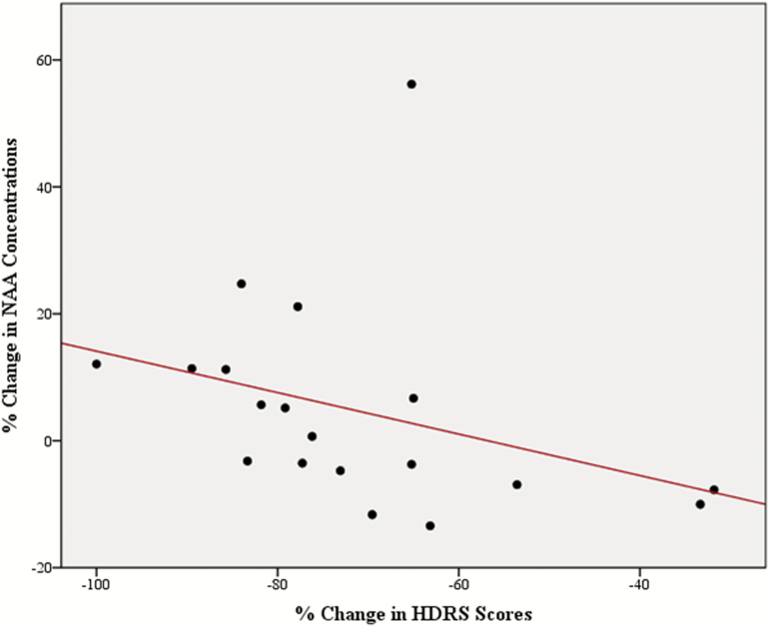
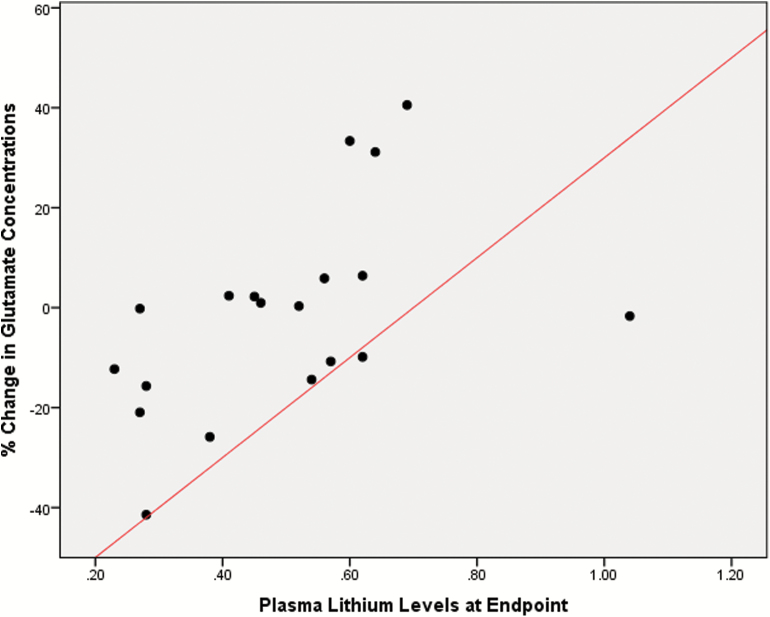
A negative correlation between reduction in HDRS scores and changes in NAA levels (Spearman rho = -0.651, 95% CI -0.89 to -0.21, p = 0.012, corrected for multiple comparisons; Figure 3); and a significant positive correlation between plasma lithium levels at endpoint and changes in Glu levels over time (Spearman rho = 0.606, 95% CI 0.2–0.84, p = 0.029, corrected for multiple comparisons) were found (Figure 4). The results of the other correlation analyses between the changes in hippocampal metabolites and clinical variables over time are detailed in Supplementary Table S2.
Figure 3.
Scatterplot showing an inverse correlation between reductions in the 21-item Hamilton Depression Rating Scale (HDRS) scores in patients with bipolar disorder (BD) and the rate of change in hippocampal N-acetyl aspartate (NAA) concentrations over the 6 weeks of follow-up.
Figure 4.
Scatterplot depicting a positive correlation between plasma levels of lithium at endpoint and the rate of change in glutamate (Glu) concentrations over the 6 weeks of follow-up in patients with bipolar disorder (BD).
In the mixed-effect model analyses, no significant interactions of remission status at endpoint and BD subtype with the change in the concentration of any of the hippocampal metabolites were observed (all p ≥ 0.159; Supplementary Tables S3 and S4). A significant association was found between plasma lithium levels at week 6 and the changes in Glu concentration over time (coefficient = 0.861; F = 6.46, df = 34, p = 0.016; Supplementary Table S5): levels of 0.2 to 0.49 mmol/L (n = 9) were associated with a decrease in glutamate concentrations, whereas the subgroup of BD subjects with standard lithium levels (≥0.50 mmol/L; n = 10) showed an overall increase in glutamate concentrations over time. BD patients with lower and standard lithium levels had similar age and gender distribution, duration of illness, and clinical scores both at baseline and endpoint, as well as response rates at week 6 (Supplementary Table S6).
No significant differences in the levels of hippocampal metabolites between BD patients in remission versus those not in remission were observed at endpoint (all t ≤ 1.48, df = 17, all unadjusted p ≥ 0.156; Supplementary Table S7).
Discussion
To the best of our knowledge, this is the first study to assess hippocampal metabolites in medication-free individuals with bipolar depression and also to evaluate lithium’s effects on hippocampal NAA, Cr, mI, Cho, and Glu concentrations using a longitudinal design.
The most novel finding observed in the present investigation was the bimodal effect of lithium plasma levels on hippocampal Glu concentrations, which was corroborated by the observation of a positive correlation between plasma lithium levels at endpoint and the change in Glu levels over time. Consistently with our report, the only study assessing Glu levels in the hippocampus of BD patients so far (Colla et al., 2009) evaluated 21 individuals on chronic lithium therapy with a mean plasma level of 0.7±0.03 mmol/L and found elevated Glu concentrations relative to healthy controls. Nevertheless, no work to date has investigated the effects of low-dose lithium in hippocampal metabolites and Glu levels. Different lines of evidence suggest that lithium modulates glutamatergic transmission, but the exact mechanism involved in this process is unknown (Machado-Vieira et al., 2012). Preclinical and clinical studies have shown that lithium modulates key mitochondrial proteins and neuronal factors involved in the regulation of cellular energy metabolism and neuroplasticity, including the enzyme glycogen synthase kinase 3 (GSK3; Quiroz et al., 2008; Machado-Vieira et al., 2009; Khairova et al., 2012; Soeiro-de-Souza et al., 2012). Both cellular energy production processes—through the coupling between glucose consumption and Glu-Gln cycling (Ende et al., 2000; Dager et al., 2008)—and GSK3 activity (Du et al., 2010) may influence glutamatergic transmission. While it has been hypothesized that depressive and manic episodes may be characterized by modulation of the Gln/Glu ratio in opposite directions, and also that BD would be associated with an overall increase in brain Glu or Glx levels (Yüksel and Öngür, 2010), the studies supporting this view have mostly enrolled patients under mood-stabilizer medication and/or with a chronic course (>10 years) of illness (Frye et al., 2007; Colla et al., 2009; Gigante et al., 2012). Moreover, as low-dose lithium has been shown to be as effective as standard-dose therapy for the treatment of bipolar depression, but associated with a lower incidence of side effects (Kleindienst et al., 2005; Machado-Vieira et al., 2009; Machado-Vieira, Zanetti, et al., 2014), the present finding raises the possibility that the use of lithium dosages with plasma levels higher than 0.50 mmol/L increases the risk of regional toxic effects in the hippocampus due to overexposure to high Glu levels (Machado-Vieira et al., 2012).
The present study showed similar levels of NAA, Cr, mI, Cho, and Glu in the left hippocampus of medication-free bipolar depression relative to healthy controls at baseline. Conflicting results in previous studies may be due to heterogeneous technical approaches for spectra quantification, poor study design, and enrollment of medicated patients at different phases of the illness (Capizzano et al., 2007; Kraguljac et al., 2012). Our results are in line with the largest 1H-MRS investigation conducted to date in BD (Gigante et al., 2014), which found no differences with regard to NAA, Cr, mI, Cho, and Glx levels in BD type I patients (medicated and at variable mood states on the day of 1H-MRS scanning) compared to healthy controls.
After 6 weeks of lithium monotherapy, no significant changes were observed in hippocampal metabolites. However, there was a significant inverse correlation between the change in NAA levels and the reduction in HDRS scores. The fact that we observed a significant inverse correlation between reductions in depressive symptoms and increases in hippocampal NAA levels over time but not a significant overall change in NAA concentrations before and after lithium monotherapy suggests that increases in NAA concentrations in the hippocampus of BD patients occur in proportion to the amelioration of depression symptoms rather than simply reflecting direct effects of lithium on this brain structure. As the hippocampus is known to be vulnerable to the effects of stress and excessive glucocorticoid release (Teicher et al., 2012), a plausible hypothesis would be that these increases in NAA levels might represent an improvement in local neuronal plasticity or neuron-glia homeostasis following the attenuation in the hypothalamic-pituitary-adrenal axis hyperactivity commonly observed in patients who are remitting from a depressive episode. Consistent with this idea, Colla et al. (2009) reported that remitted patients with BD type I exhibited a significant inverse relationship between diurnal saliva cortisol levels and hippocampal NAA concentrations.
We also observed that remission status at week 6 (i.e., achieving or not achieving remission) did not produce a significant influence on the levels of any of the hippocampal metabolites over time, and there were no significant differences between remitted and non-remitted BD patients at week 6. This might be seen as contradictory with the finding of inverse correlation found between the change in NAA levels and the change in HDRS scores discussed above. However, it is important to mention that, although not sufficient to yield remission criteria, a significant reduction in HDRS scores after 6 weeks of lithium treatment was also noticed in the non-remitting subgroup of BD patients (n = 6; data not shown). This, together with the relatively modest sample size of our study groups, might have increased the risk of type II statistical errors in the subgroup analyses on remission status and, thus, these findings should be considered preliminary.
The fact that we recruited a sample of medication-free BD patients with a relatively short duration of illness (mean of 36 months) and with no current drug abuse/dependence reduces the possibility that our results have been influenced by confounding factors such as psychotropic medication use, drug abuse, and illness course. Nevertheless, there are some methodological limitations that should be acknowledged, in particular the modest sample size of our study. Also, the 6-week period of follow-ups adopted in our study might be seen as relatively short to precisely define clinical response and/or remission in bipolar depression. Finally, we corrected the metabolite values presented here for different tissue composition and water relaxation properties, assuming water T1 and T2 values reported in the literature for CSF, GM, and WM. Ideally, T1 and T2 should be measured individually for each patient by acquiring spectra with different repetition and echo times in order to account for differences in relaxation properties between individuals or groups (i.e., patients versus controls). This process is extremely time consuming and was not feasible in our study. Nevertheless, previous relaxation studies showed that individual and regional differences are small, and the factor with the largest impact on the measured metabolite signal intensities using internal water as a reference is the difference of water content and relaxation in CSF compared to water in GM or WM (Wansapura et al., 1999). We corrected for this effect by taking into account the segmentation results in metabolite quantitation, as described above. For all these reasons, our results should be considered preliminary and further replication by studies recruiting larger samples is desirable.
In conclusion, the present study suggests that lithium has a bimodal action in hippocampal glutamate concentrations, depending on the plasma levels. This finding may help to explain the high frequency of adverse effects and drop-out rates observed in clinical trials targeting standard lithium levels and highlight the importance of differing lithium plasma levels in neurobiological studies of this agent.
Statement of Interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
This study was sponsored by the Sao Paulo Research Foundation (FAPESP, Brazil, 2009/14891–9, RM-V). The Laboratory of Neuroscience, LIM27, is also supported by the Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS). Dr Zanetti is funded by FAPESP, Brazil (no. 2013/03905-4). This is clinical trial number NCT01919892.
References
- Amsterdam JD, Shults J. (2008). Comparison of short-term venlafaxine versus lithium monotherapy for bipolar II major depressive episode: a randomized open-label study. J Clin Psychopharmacol 28:171–181. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. (2007). Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology 32:1888–1902. [DOI] [PubMed] [Google Scholar]
- Bustillo JR. (2013). Use of proton magnetic resonance spectroscopy in the treatment of psychiatric disorders: a critical update. Dialogues Clin Neurosci 15:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzano AA, Jorge RE, Acion LC, Robinson RG. (2007). In vivo proton magnetic resonance spectroscopy in patients with mood disorders: a technically oriented review. J Magn Reson Imaging 26:1378–1389. [DOI] [PubMed] [Google Scholar]
- Colla M, Schubert F, Bubner M, Heidenreich JO, Bajbouj M, Seifert F, Luborzewski A, Heuser I, Kronenberg G. (2009). Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol Psychiatry 14:696–704. [DOI] [PubMed] [Google Scholar]
- Dager SR, Corrigan NM, Richards TL, Posse S. (2008). Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Top Magn Reson Imaging 19:81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Wei Y, Liu L, Wang Y, Khairova R, Blumenthal R, Tragon T, Hunsberger JG, Machado-Vieira R, Drevets W, Wang YT, Manji HK. (2010). A kinesin signaling complex mediates the ability of GSK-3beta to affect mood-associated behaviors. Proc Natl Acad Sci USA 107:11573–11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. (2000). The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry 57:937–943. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (1995). Structured clinical interview for DSM-IV axis I disorders, patient edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatry Institute. [Google Scholar]
- Frye MA, Watzl J, Banakar S, O’Neill J, Mintz J, Davanzo P, Fischer J, Chirichigno JW, Ventura J, Elman S, Tsuang J, Walot I, Thomas MA. (2007). Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology 32:2490–2499. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, and Morrison LA. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55:1219–1226. [DOI] [PubMed] [Google Scholar]
- Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. (2012). Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord 14:478–487. [DOI] [PubMed] [Google Scholar]
- Gigante AD, Lafer B, Yatham LN. (2014). (1)H-MRS of hippocampus in patients after first manic episode. World J Biol Psychiatry 15:145–154. [DOI] [PubMed] [Google Scholar]
- Guy W. (1976). The clinical global impression scale. In: ECDEU assessment manual for psychopharmacology-revised, pp 218–222. Rockville, MD: US Dept. of Health, Education and Welfare, ADAMHA, MIMH Psychopharmacology Research Branch. [Google Scholar]
- Hamilton M. (1960). Rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y. (1988). A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–802. [Google Scholar]
- Howells FM, Ives-Deliperi VL, Horn NR, Stein DJ. (2013). Increased thalamic phospholipid concentration evident in bipolar I disorder. Prog Neuropsychopharmacol Biol Psychiatry 41:1–5. [DOI] [PubMed] [Google Scholar]
- Khairova R, Pawar R, Salvadore G, Juruena MF, de Sousa RT, Soeiro-de-Souza MG, Salvador M, Zarate CA, Gattaz WF, Machado-Vieira R. (2012). Effects of lithium on oxidative stress parameters in healthy subjects. Mol Med Rep 5:680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindienst N, Severus WE, Möller HJ, Greil W. (2005). Is polarity of recurrence related to serum lithium level in patients with bipolar disorder? Eur Arch Psychiatry Clin Neurosci 255:72–74. [DOI] [PubMed] [Google Scholar]
- Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. (2011). Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry 68:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Reid M, White D, Jones R, den Hollander J, Lowman D, Lahti AC. (2012). Neurometabolites in schizophrenia and bipolar disorder–a systematic review and meta-analysis. Psychiatry Res 203:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Manji HK, Zarate CA., Jr (2009). The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord 11(Suppl 2):92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Ibrahim L, Henter ID, Zarate CA., Jr (2012). Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav 100:678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Soeiro-De-Souza MG, Richards EM, Teixeira AL, Zarate CA., Jr (2014). Multiple levels of impaired neural plasticity and cellular resilience in bipolar disorder: developing treatments using an integrated translational approach. World J Biol Psychiatry 15:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Zanetti MV, de Sousa RT, Soeiro-de-Souza MG, Moreno RA, Busatto GF, Gattaz WF. (2014). Lithium efficacy in bipolar depression with flexible dosing: a six-week, open-label, proof-of-concept study. Exp Ther Med 8:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Kristiansen LV, Beneyto M, Scarr E, Dean B, Meador-Woodruff JH. (2007). Decreased NR1, NR2A, and SAP102 transcript expression in the hippocampus in bipolar disorder. Brain Res 1127:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 13:829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. (2014). A Critical Appraisal of Neuroimaging Studies of Bipolar Disorder: Toward a New Conceptualization of Underlying Neural Circuitry and a Road Map for Future Research. Am J Psych 171:829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry P-G, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, and Alger JR. (2007). Proton Echo-Planar Spectroscopic Imaging of J-Coupled Resonances in Human Brain at 3 and 4 Tesla. Magn Reson Med 58:236–244. [DOI] [PubMed] [Google Scholar]
- Provencher SW. (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30(6):672–679. [DOI] [PubMed] [Google Scholar]
- Quiroz JA, Gray NA, Kato T, Manji HK. (2008). Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology 33:2551–2565. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Twardowska K. (1999). The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J Psychiatr Res 33:363–370. [DOI] [PubMed] [Google Scholar]
- Senaratne R, Milne AM, MacQueen GM, Hall GB. (2009). Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: a proton magnetic resonance spectroscopy study. Psychiatry Res 172:205–209. [DOI] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Dias VV, Figueira ML, Forlenza OV, Gattaz WF, Zarate CA, Jr, Machado-Vieira R. (2012). Translating neurotrophic and cellular plasticity: from pathophysiology to improved therapeutics for bipolar disorder. Acta Psychiatr Scand 126:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suppes T, Marangell LB, Bernstein IH, Kelly DI, Fischer EG, Zboyan HA, Snow DE, Martinez M, Al Jurdi R, Shivakumar G, Sureddi S, Gonzalez R. (2008). A single blind comparison of lithium and lamotrigine for the treatment of bipolar II depression. J Affect Disord 111:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. (2012). Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci USA 109:E563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball Jr WS. (1999). NMR relaxation times in the human brain at 3.0 Tesla. J Magn Reson Imaging 9:531–538. [DOI] [PubMed] [Google Scholar]
- Watson S, Gallagher P, Ritchie JC, Ferrier IN, Young AH. (2004). Hypothalamic-pituitary-adrenal axis function in patients with bipolar disorder. Br J Psychiatry 184:496–502. [DOI] [PubMed] [Google Scholar]
- Young AH, McElroy SL, Bauer M, Philips N, Chang W, Olausson B, Paulsson B, Brecher M. (2010). A double‑blind, placebo‑controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I). J Clin Psychiatry 71:150–162. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. (1978). A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 133:429–435. [DOI] [PubMed] [Google Scholar]
- Yüksel C, Öngür D. (2010). Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry 68:785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag 20:45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.