Abstract
Background:
The zebrafish is a powerful neurobehavioral genetics tool with which complex human brain disorders including alcohol abuse and fetal alcohol spectrum disorders may be modeled and investigated. Zebrafish innately form social groups called shoals. Previously, it has been demonstrated that a single bath exposure (24 hours postfertilization) to low doses of alcohol (0, 0.25, 0.50, 0.75, and 1% vol/vol) for a short duration (2 hours) leads to impaired group forming, or shoaling, in adult zebrafish.
Methods:
In the current study, we immersed zebrafish eggs in a low concentration of alcohol (0.5% or 1% vol/vol) for 2 hours at 24 hours postfertilization and let the fish grow and reach adulthood. In addition to quantifying the behavioral response of the adult fish to an animated shoal, we also measured the amount of dopamine and its metabolite 3,4-dihydroxyphenylacetic acid from whole brain extracts of these fish using high-pressure liquid chromatograph.
Results:
Here we confirm that embryonic alcohol exposure makes adult zebrafish increase their distance from the shoal stimulus in a dose-dependent manner. We also show that the shoal stimulus increases the amount of dopamine and 3,4-dihydroxyphenylacetic acid in the brain of control zebrafish but not in fish previously exposed to alcohol during their embryonic development.
Conclusions:
We speculate that one of the mechanisms that may explain the embryonic alcohol-induced impaired shoaling response in zebrafish is dysfunction of reward mechanisms subserved by the dopaminergic system.
Keywords: embryonic alcohol exposure, fetal alcohol spectrum disorders, zebrafish, abnormal social behavior, dopamine, DOPAC
Introduction
Alcohol (ethyl alcohol, ethanol, or EtOH) consumption during pregnancy is known to have deleterious effects on the developing human fetus. The exposed children, depending on the severity of the symptoms, may be diagnosed as suffering from fetal alcohol syndrome (FAS), partial FAS, alcohol-related neurodevelopmental disorders (ARNDs), or alcohol-related birth defects, collectively known as fetal alcohol spectrum disorder (FASD) (Stratton et al., 1996). In the United States, FASD has a prevalence rate of 9 per 1000 live births (Sampson et al., 1997a). Heavy alcohol consumption during pregnancy can lead to FAS, a severe form of the disease characterized by facial abnormalities, growth retardation, and central nervous system impairments (Stratton et al., 1996). FAS children suffer from aberrations in executive function, learning and memory, language, visual-spatial ability, motor function, attention, activity levels, and overall intellectual performance (Mattson et al., 2011). Although FAS is the most severe outcome of prenatal alcohol exposure, it is not the most prevalent disorder within FASD (Sampson et al., 1997b). ARND represents two-thirds of FASD cases (May et al., 2009). Individuals with ARND may exhibit observable behavioral anomalies that are similar to those seen in individuals with FAS; however, they lack the physical abnormalities necessary for the FAS diagnosis (Stratton et al., 1996).
Animal models have been proposed to facilitate the understanding of the biological mechanisms of these disorders (Cole et al., 2012). For example, mice (Schneider et al., 2011a), rats (Slawecki et al., 2004), and nonhuman primates (Schneider et al., 2011b) have been successfully used to model FAS and/or ARND. The zebrafish is another animal model that has started to aid this research (Fernandes and Gerlai, 2009; Buske and Gerlai, 2011; Mahabir et al., 2013).
Zebrafish strike a balance between complexity and practicality. They are vertebrates that share a number of characteristics with mammals such as basic anatomical layout of their brain (Tropepe and Sive, 2003) and typical vertebrate neurotransmitter systems (Chatterjee and Gerlai, 2009). The nucleotide sequence of their genes has also been found to be highly similar to that of mammalian homologs (Howe et al., 2013). Zebrafish are small (3–4 centimeters long) and can be housed in groups, making their maintenance inexpensive. Furthermore, the zebrafish has developmental characteristics that are especially useful in the study of ARND (Bilotta et al., 2004). Zebrafish eggs are fertilized externally (Nüsslein-Volhard and Dahm, 2002), and fry develop outside of the mother and independent of any parental care (Engeszer et al., 2007). Therefore, complications associated with the intra-uterine environment or with maternal care that are characteristic of mammalian species can be avoided using zebrafish (Bilotta et al., 2004). Exposure of zebrafish embryos to the desired alcohol concentration may be achieved with high temporal precision, because the chorion of the zebrafish egg is partially permeable to alcohol (Loucks and Carvan, 2004). The alcohol exposure procedure is simple. It involves submerging the eggs in the desired concentration of alcohol for the desired length of time at the desired stage of development (Fernandes and Gerlai, 2009). Also, because zebrafish are prolific breeders (females can produce 200 eggs in a single spawning) and the eggs are small, a large number of eggs can be exposed to alcohol at precisely the same developmental stage and for the same duration, a procedure that is expected to reduce error variation among the exposed subjects (Fernandes et al., 2014). Last, a successful zebrafish ARND model has been published (Fernandes and Gerlai, 2009; Buske and Gerlai, 2011; Fernandes et al., 2014).
Patients suffering from ARND display a variety of behavioral abnormalities, many of which are associated with impaired social skills (Kodituwakku, 2007; Rasmussen et al., 2010). Zebrafish, innately form social groups called shoals (Pitcher, 1983), making them a potentially suitable model organism for the study of impaired social behavior. When zebrafish are presented with a shoal (computer-generated images of or live conspecifics), they approach the shoal stimulus and stay in close proximity to it (Qin et al., 2014). The decrease in the distance between the experimental subject and the shoal stimulus is thus a natural response that offers precise and easy quantification. Using a computer-animated shoal stimulus, Fernandes and Gerlai (2009) found a dose-dependent decrease in shoaling behavior in fish exposed to alcohol during embryonic development. Adult zebrafish treated with system water during their embryonic development approached and stayed close to the animated shoal, whereas fish that received alcohol for 2 hours at their 24th postfertilization hour stage of development lacked the response (highest alcohol dose) or showed a diminished reaction (lower alcohol doses). Employing the same dosing regime, another study confirmed the above findings but with a different experimental procedure (Buske and Gerlai, 2011). In this latter study, alcohol-exposed fish were found to be further away from their nearest neighbor in freely moving shoals compared with controls. We stress the fact that the latter 2 studies demonstrated the first time that bath immersion in even low doses of alcohol (0, 0.25, 0.50, 0.75, and 1.0% vol/vol) administered for only a short duration (2 hours) during embryonic development could have significant effects. Notably, the actual alcohol concentration reaching the embryo inside the egg was found to be one-twentieth to one-thirtieth of the external bath concentration, and these very low doses led to the significant behavioral abnormalities without physical malformations or growth retardation (Fernandes and Gerlai, 2009; Buske and Gerlai, 2011), findings that demonstrated the potential utility of zebrafish in modeling human ARND. The mechanisms of the observed behavioral changes, however, remained unknown.
To start unraveling these mechanisms, dopamine and its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), were analyzed for a number of reasons. Previously, prenatal alcohol exposure has been shown to impair the dopaminergic system in a variety of species (Schneider et al., 2011a). The sight of conspecific shoals has been shown to have rewarding properties in zebrafish (Al-Imari and Gerlai, 2008), and presentation of animated conspecific images (artificial shoals) has been found to increase the levels of dopamine and DOPAC in the brain of the stimulated zebrafish (Saif et al., 2013). Importantly, dopaminergic dysfunction induced by low to moderate embryonic alcohol exposure has now been shown in zebrafish too (Buske and Gerlai, 2011; Mahabir et al., 2013). Buske and Gerlai (2011) found decreased shoal cohesion as well as decreased dopamine and DOPAC levels in alcohol-exposed fish, providing the first piece of evidence suggesting that the embryonic alcohol-induced impairment of shoaling behavior correlates with abnormalities in the dopaminergic system in zebrafish. The limitation of this latter study, however, was that tissue (brain) samples required for high-precision liquid chromatography (HPLC) analysis of neurochemicals could not be correlated with the individual’s behavioral responses because the behavior of the fish were quantified in groups, that is, in freely moving live shoals. Furthermore, embryonic alcohol-induced abnormalities in shoaling-elicited dopaminergic responses could not be separated from potential baseline (prestimulation) dopaminergic impairment. Mahabir et al (2013) also demonstrated reduction of dopamine levels in response to embryonic alcohol exposure; however, this study did not include any behavioral measures.
The current study is aimed at addressing some of the above limitations. Here, a computer-animated shoal was used to elicit the behavioral response from a single subject at a time. The behavioral responses of the subject were recorded by a camera and analyzed using an automated tracking software application. Whole brain extracts were analyzed using HPLC to quantify dopamine and DOPAC levels from the same fish that were behaviorally tested. Importantly, the HPLC analysis was conducted for adult fish both before and after the shoal stimulus presentation. This allowed us to quantify the potential effects of embryonic alcohol exposure on the functioning of dopaminergic system at baseline (without shoaling stimulation) and when the neurotransmitter system is engaged (with stimulation). We confirm that presentation of the shoal stimulus increases dopamine and DOPAC levels in the brain of the test fish. Furthermore, and more importantly, we report no embryonic alcohol-induced impairment in dopamine and DOPAC levels before shoal stimulation (baseline), but find abolishment of the shoal stimulation induced increase of dopamine and DOPAC levels in the embryonic alcohol-treated fish.
Methods
Animals and Housing
Sexually mature adult zebrafish of the AB strain (ZFIN Center, Eugene, OR) were bred at the University of Toronto Mississauga, Vivarium (Mississauga, Ontario, Canada) to obtain fertilized eggs. AB is a frequently studied zebrafish strain (Lockwood et al., 2004). A total of 120 fertilized eggs were collected 2 hours postfertilization from the same parents and washed with system water as described (Fernandes and Gerlai, 2009).
An equal number of eggs was placed in containers with 100mL of solution of the corresponding alcohol concentration (0.00, 0.50, or 1.00% vol/vol) at the 24th hour postfertilization for 2 hours, a procedure and timing of alcohol exposure previously employed (Fernandes and Gerlai, 2009; Buske and Gerlai, 2011; Mahabir et al., 2013). The 24th hour postfertilization represents the end of the segmentation and the beginning of the pharyngula stage of zebrafish development, which corresponds approximately to the late first trimester or early second trimester of human fetal development (Kimmel et al., 1995; also see http://zfin.org/zf_info/zfbook/stages/ and http://www.ehd.org/virtual-human-embryo/). After the 2-hour–long alcohol exposure, eggs were washed with system water and maintained in 1.3-L tanks of a nursery rack (Aquaneering Inc., San Diego, CA). The developing fish were maintained as described elsewhere (Fernandes and Gerlai, 2009). Zebrafish were housed in the system rack in groups of 10 until they were at least 8 months old (fully developed sexually mature young adults, about 50% male and female). The sample sizes of treated fish were as follows: 0.00% EtOH control, n=29; 0.50% EtOH, n=30; and 1.00% EtOH, n=29 (these sample sizes are slightly lower than the number of eggs treated, because not all collected eggs were fertilized).
Behavioral Apparatus
The experimental set-up was a 37-l tank (50×25×30cm, length × width × height) with a flat LCD computer screen (17-inch Samsung SyncMaster 732N monitor) placed on the left and right sides of the tank. Each monitor was connected to a Dell Vostro 1000 laptop running a custom-made software application (Saverino and Gerlai, 2008) that allowed the presentation of animated fish images. The experimental tank was illuminated by a 15-W fluorescent light-tube from above. The back and bottom of the tank were coated with white corrugated plastic sheets to increase contrast and reduce glare for video-tracking. Two identical experimental set-ups were used in parallel. The behavior of experimental fish was recorded onto the hard drive of video cameras (one camera per test tank, JVC GZ -MG37u and GZ-MG50) placed in front of the test tank. This way, movements of the fish on the vertical plane perpendicular to the line of sight of the camera could be evaluated. The digital recordings were transferred to a desktop computer (Dell, Dimension 8400) and later replayed and analyzed using the Ethovision Color Pro Videotracking software (Version 8.5 XT, Noldus Info Tech, Wageningen, The Netherlands).
Behavioral Test Procedure
Behavioral testing was conducted as previously described (Fernandes and Gerlai, 2009). Briefly, to explore the potential effect of early developmental exposure to alcohol, we placed our adult experimental zebrafish in the test tank singly, and 30 seconds later we started a 23-minute–long recording session. During the recording session, the subject was first presented with a 10-minute no-stimulus interval (habituation period), followed by a 10-minute stimulus presentation interval (animated images of 5 zebrafish moving with a speed ranging between 1.5 and 4cm/second), after which a 3-minute no-stimulus interval commenced. The shoal stimulus was presented on only one side of the test tank for each experimental fish, but the side of presentation alternated randomly across experimental subjects. Subjects that were in the no-stimulus condition were placed in the test tank for 23 minutes and received blank stimulus screens.
Analysis of Neurochemicals Using HPLC
A subset of behaviorally tested fish (control, n=14; 0.50% EtOH, n=13, 1% EtOH, n=3) were promptly decapitated after the behavioral session and their brains were dissected on ice and sonicated. Notably, one-half of these fish in each alcohol treatment group received no shoal stimulus while the other one-half did. The details of sample preparation methods and the HPLC procedures have been published elsewhere (Chatterjee and Gerlai, 2009). Briefly, each sonicate represented a single zebrafish brain and was centrifuged. The supernatant was analyzed with HPLC using BAS 460 MICROBORE-HPLC with electrochemical detection (Bio-analytical Systems Inc., West Lafayette, IN) together with Uniget C-18 reverse phase microbore column as the stationary phase (BASi, cat no. 8949). Standard neurochemicals (Sigma) were used to quantify and identify the peaks on the chromatographs. Levels of dopamine and DOPAC were quantified. Results are expressed as nanogram of neurochemical per milligram of total brain protein.
Quantification of Behavior and Statistical Analysis
We quantified the swimming activity of the experimental fish (total distance moved) and the distance the fish maintained from the stimulus presentation computer screen. The former measure may reflect alcohol-induced activity changes, whereas the latter quantifies the strength of preference to stay in the proximity of conspecifics (group preference). We also quantified absolute angular velocity (the speed of turning irrespective of direction).
Data were analyzed using SPSS (version 14.1) for the PC. Repeated-measure variance analysis (ANOVA) was employed to investigate the effect of interval (23 one-minute intervals, the repeated measure factor), the effect of alcohol treatment (3 doses), and the interaction between these factors. Nonrepeated-measure ANOVA was also employed for measures derived from the interval data. Data from the mean absolute angular velocity were square root transformed to homogenize variances, and subsequently the results were analyzed using univariate ANOVA. A 3 (alcohol treatment)×2 (stimulus type: conspecific presentation or blank presentation) univariate ANOVA was used to examine dopamine and DOPAC levels. In case of significant effects, posthoc Tukey’s Honestly Significant Difference (HSD) multiple comparison test as well as paired t tests were conducted as appropriate.
Results
Statistical analysis of fish behavior demonstrated no significant sex differences [F(1, 62)=0.60, p=.44], sex × interval interaction [F(22, 1364)=0.97, P=.50], sex × alcohol treatment interaction [F(2, 62)=2.51, P=.089], or sex × interval × alcohol treatment interaction [F(44, 1364)=1.32, P=.08]. As a result, data are pooled for sex in subsequent analyses.
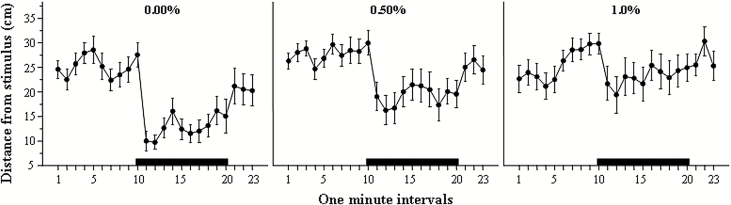
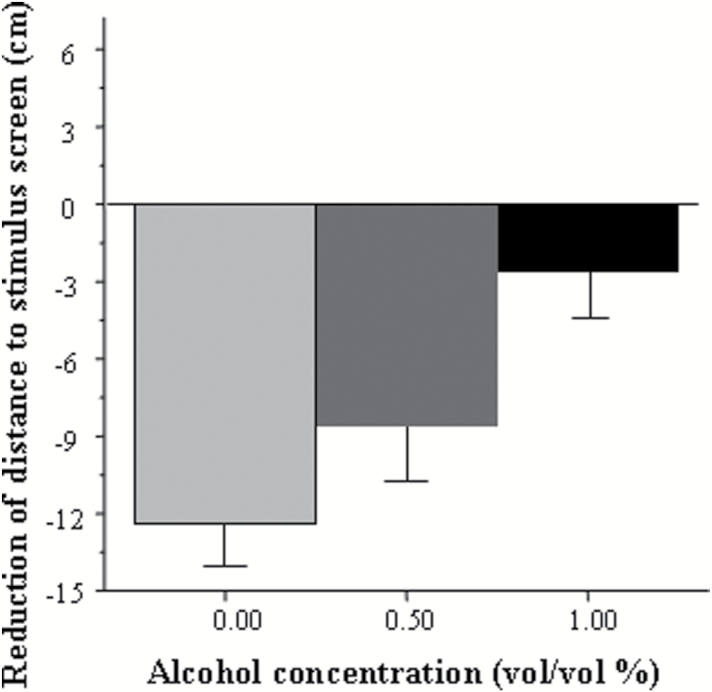
Figure 1 shows the distance experimental fish swam from the stimulus screen. When a single zebrafish was presented with the sight of conspecifics (shoal of live fish or an animated shoal; Qin et al., 2014), the subject approached the group and remained in proximity of the group (Saverino and Gerlai, 2008). The conspecifics-induced approach (shoaling) response is evident in Figure 1 and was particularly robust in fish that were not treated with alcohol (control). However, the response appears to be diminished in embryonic alcohol-treated fish in a dose-dependent manner, an observation supported by our statistical analyses. We found a significant main effect of interval [F(22, 1364)=9.09, P<.0001], main effect of alcohol treatment [F(2, 62)=4.33, P=.017], and also a significant interval × alcohol treatment interaction [F(44, 1364)=1.97, P<.0001], indicating that embryonic alcohol exposure affected the distance from the stimulus in an interval-dependent manner. To explore the significant interval × alcohol treatment interaction, we calculated the average distance from the stimulus screen for the entire 10-minute no-stimulus period (habituation period) and the entire 10-minute shoal presentation period (stimulus period) and subtracted the mean distance of the habituation period from the mean distance of the stimulus period (Figure 2), a measure we call stimulus-induced reduction of distance. This addressed the inappropriateness of posthoc multiple comparison tests such as Tukey’s HSD for the analysis of repeated-measure variables. We found the distance reduction to be the largest in control fish and smallest in the fish exposed to the highest concentration of alcohol during embryonic development (Figure 2). ANOVA revealed a significant effect of alcohol treatment [F(2, 67)=4.60, P=.010], and Tukey’s HSD posthoc test demonstrated that untreated (control) fish were significantly different (P=.007) from fish that were exposed to 1.0% ethanol. Next, we investigated whether the apparent reduction in the distance to the stimulus screen upon the conspecific presentation was statistically distinguishable from random chance, that is, from zero-centimeter reduction. One-sample t tests confirmed that all fish except those previously treated with 1.0% during development significantly reduced their distance from the stimulus (control t(21)=−7.24, P<.0001; 0.50% EtOH t(22)=−3.99, P=.0005; 1.0% EtOH t(22)=−0.89, P=.19).
Figure 1.
Average distance between the adult experimental zebrafish and the stimulus presentation computer screen plotted for 1-minute intervals of the 23-minute behavioral recording session. Mean ± SEM are shown. Sample sizes are given in the Methods section. The period of stimulus presentation is shown by the horizontal bar above the x-axis (no bar represents the period of no-stimulus presentation; black bar represents the period of presentation of animated images of 5 conspecifics). The alcohol concentration to which fish were exposed during development (one exposure for 2 hours at 24 hours postfertilization) is shown above the graphs. Note the robust reduction of the distance to the stimulus upon conspecific presentation in control fish and that this response diminishes as the alcohol dose employed during embryonic development increases.
Figure 2.
Reduction of distance between the adult experimental zebrafish and the stimulus screen in response to conspecific stimulus presentation. Mean ± SEM are shown. Sample sizes are given in the Methods section. Values represent the difference between the distances fish were from the stimulus presentation screen before and during conspecific stimulus presentation. Larger negative values mean larger reduction of distance, that is, stronger response to the conspecifics. The categories indicated for the x-axis represent the embryonic alcohol dose (vol/vol % bath concentration) administered at the 24th hour postfertilization for a 2-hour duration. Note the linear negative dose response relationship between the alcohol dose and the shoaling response.
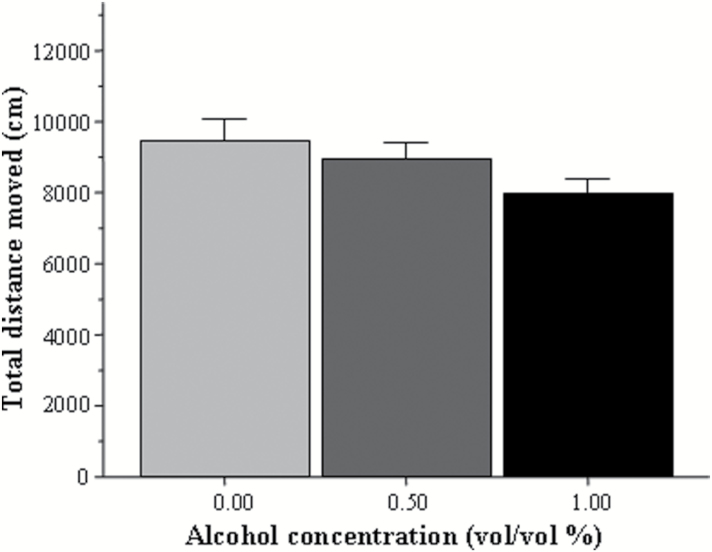
The above-described behavioral impairment may be the result of numerous performance deficits unrelated to shoaling tendency. One of these is motor function. To investigate this possibility, we compared the total distance moved by fish of each group during the entire 23-minute trial. Figure 3 demonstrates an apparent lack of difference in the activity of the fish between the alcohol-treated and control groups, an observation confirmed by ANOVA [F(2, 67)=1.88, P=.160].
Figure 3.
Total distance swam by the adult experimental zebrafish during the entire 23-minute recording session. Mean ± SEM are shown. Sample sizes are given in the Methods section. The concentration of alcohol treatment (one exposure for 2 hours, 24 hours postfertilization) is shown under the x-axis. Note that early developmental exposure to alcohol had no significant effect on general activity of the adult fish.
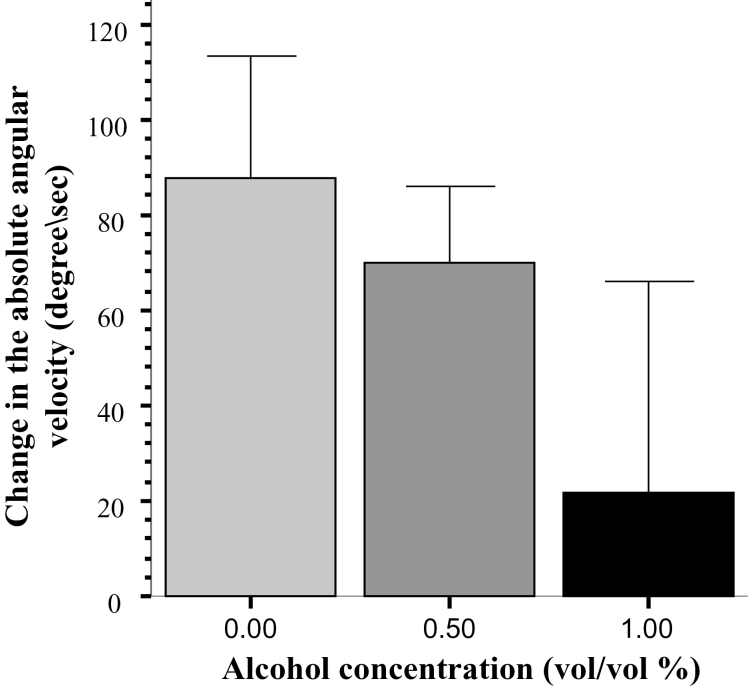
Another potential performance confound that may influence shoaling responses is perception. In the current study, the only modality that the test fish could use to detect the shoaling stimulus was vision. To investigate whether the animated images were perceived by the experimental fish, we examined the mean absolute angular velocity, a measure that quantifies the speed of turning (Ethovison Manual). Previously, we have found that presentation of moving images that do not resemble conspecifics increase angular velocity in zebrafish, a response we argued was unrelated to shoaling (Saif et al., 2013). Thus, quantification of image presentation-induced changes in angular velocity may allow us to dissociate behavioral responses unrelated to shoaling from shoaling. To calculate the change in the amount of turns, we first averaged the angular velocity values for the habituation period and also for the stimulus presentation period, and then we subtracted the former from the latter. A positive value thus represents a stimulus-induced increase in turning. Figure 4 shows the amount of increase in turning (angular velocity) in response to the shoal stimulus. ANOVA found no significant effect of alcohol treatment [F(2, 65) = 2.12, p = 0.13]. Next we examined whether the apparent increase in the angular velocity upon the conspecific presentation was significant, and compared the stimulus-induced change in angular velocity to “0” (no change). To homogenize variances, we performed a square root transformation before the statistical analysis. One-sample t-tests performed with the scale transformed data confirmed that fish from the control and 0.50% alcohol treated groups significantly increased their angular velocity in response to the stimulus (control t(21) = 3.321, p < 0.01; 0.50% EtOH t(22) = 4.110 p < 0.0001; 1.0% EtOH t(22) = -0.320, p > 0.75). The above results show that fish of the highest concentration group did not respond to the shoal images while the fish in the control and the lower concentration group did.
Figure 4.
Change of the absolute angular velocity upon the presentation of the animated shoal. Mean ± SEM are shown. Sample sizes are given in the Methods section. The concentration of alcohol treatment (one exposure for 2 hours, 24 hours postfertilization) is shown under the x-axis. Note that no significant differences were found among the treatment groups.
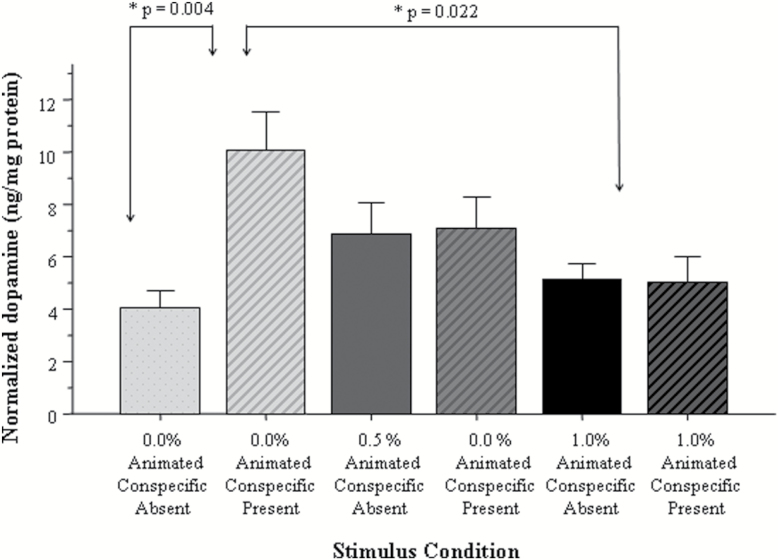
Next, we examined whether exposure to alcohol during development led to long-lasting changes in the dopaminergic system. We quantified the amount of dopamine and its metabolite DOPAC and normalized the measured neurochemical weights to the weight of the total brain protein across 6 conditions. Each of the 3 alcohol treatment groups (0, 0.50, and 1.0% EtOH vol/vol embryonic alcohol treatment) had 2 conditions: one in which the adult fish received presentation of the animated shoal (stimulated fish) and the other in which the shoal was absent (nonstimulated fish allowing for the quantification of baseline). Figure 5 shows the normalized dopamine levels across these 6 conditions. First, we conducted a univariate ANOVA examining the effect of alcohol treatment (0, 0.50, and 1.0% vol/vol) and stimulus (present vs absent) on dopamine. We found a significant main effect of stimulus [F(1, 34)=5.20, P=.029] and a nonsignificant main effect of alcohol treatment [F(2, 34)=2.09, P=.140]. The interaction between alcohol treatment × stimulus was also found to be significant [F(2, 34)=5.16, P=.011], suggesting that the effect of stimulus on dopamine levels was embryonic alcohol exposure dependent. To further explore this interaction, we conducted a Tukey’s HSD posthoc test and found that control fish (0% alcohol) responded with a significant (P=.004) increase of dopamine levels to the presentation of the shoal stimulus, but the embryonic alcohol-treated fish did not (the difference between stimulus absent and stimulus present conditions was not significant for these fish, P>.05). Last, Tukey’s HSD also detected no significant differences (P>.05) among any of the groups before stimulus presentation (baseline).
Figure 5.
The amount of dopamine normalized to total brain protein varies in a stimulus presentation and embryonic alcohol dose-dependent manner. Mean ± SEM are shown. Sample sizes are given in the Methods section. The group designations shown under the x-axis represent the bath concentration of alcohol employed at the 24th hour postfertilization for 2 hours. These designations also show whether the adult fish received the stimulus or not before tissue sampling. Note the shoal stimulus induced increase of dopamine in the adult control fish (no alcohol exposure) and the lack of such increase in the adult fish exposed to alcohol during their embryonic development.
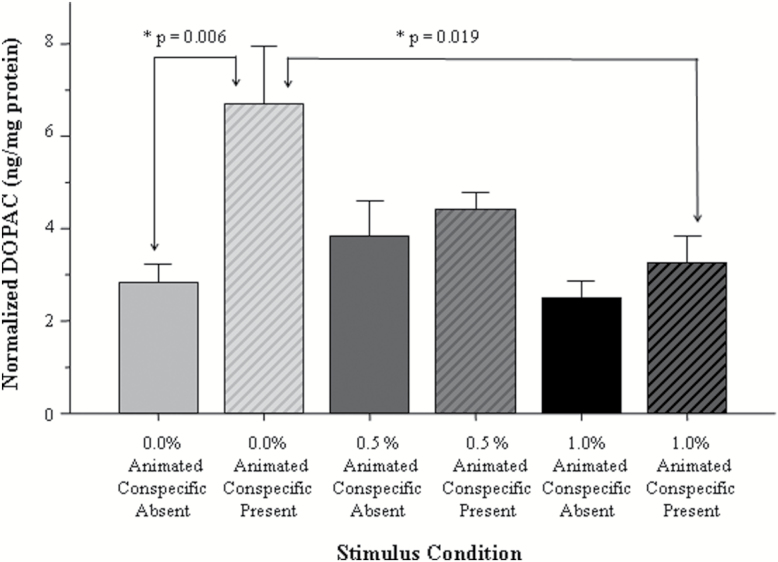
The results obtained for DOPAC mirrored those found for dopamine (Figure 6). ANOVA revealed a significant main effect of alcohol treatment [F(2, 34)=3.41, P=.045] and stimulus [F(1, 34)=8.45, P=.006] and also found the alcohol treatment × stimulus interaction to be significant [F(2, 34)=3.31, P=.049]. Tukey’s HSD revealed a significant (P=.006) stimulus-induced increase of DOPAC levels in control fish but found no such increase in any of the embryonic alcohol-treated fish (P>.05). Tukey’s HSD also found no differences (P>.05) among any of the groups that were not stimulated by the animated shoal (baseline).
Figure 6.
The amount of 3,4-dihydroxyphenylacetic acid (DOPAC) normalized to total brain protein varies in a stimulus presentation and embryonic alcohol dose-dependent manner. Mean ± SEM are shown. Sample sizes are given in the Methods section. The group designations shown under the x-axis represent the bath concentration of alcohol employed at the 24th hour postfertilization for 2 hours. These designations also show whether the adult fish received the stimulus before tissue sampling. Note the shoal stimulus-induced increase of dopamine in the adult control fish (no alcohol exposure) and the lack of such increase in the adult fish exposed to alcohol during their embryonic development.
Discussion
Prenatal alcohol exposure in the clinical population is known to cause a number of different deficits, including impairments in social behavior (Rasmussen et al., 2010). However, the mechanisms responsible for these aberrations remain unknown. Here, we employed zebrafish to begin the investigation of these mechanisms. We found zebrafish treated with alcohol during their embryonic development to exhibit a diminished shoaling response to animated images of conspecifics compared with unexposed control zebrafish. This result confirms the notion that even low doses of alcohol reaching the embryo once and for a short duration of time can have lasting deleterious consequences. Furthermore, our results show that the presentation of the conspecific images triggered an increase of dopamine and DOPAC levels in control fish, but it did not alter neurotransmitter levels in previously alcohol-treated zebrafish. Although only correlative, these results implicate the dopaminergic system in the embryonic alcohol exposure-induced impairment of shoaling response.
Zebrafish naturally swim in shoals in the wild (Spence et al., 2007) and also exhibit strong preference for conspecifics in the laboratory (Gerlai et al., 2000; Saverino and Gerlai, 2008; Fernandes and Gerlai, 2009; Saif et al., 2013; Qin et al., 2014). We utilized this innate behavior to quantify the effects of embryonic alcohol exposure on the functioning of the brain in the adult fish and found a significant and alcohol dose-dependent impairment in the reduction of distance to the images of conspecifics, that is, in the shoaling response. This result is in line with clinical data (Rasmussen et al., 2010; Kully-Martens et al., 2011) as well as with studies using animal models including zebrafish (Fernandes and Gerlai, 2009; Hamilton et al., 2010a 2010b; Buske and Gerlai, 2011; Schneider et al., 2011a; Parker et al., 2014a), which all found abnormal social behavior resulting from embryonic alcohol exposure. However, before concluding that the impaired response to the images of conspecifics we found in the alcohol-exposed fish was indeed due to their abnormal social behavior, we need to consider a number of alternative hypotheses.
Prenatal alcohol exposure has been found to affect motor activity (Schneider et al., 2011a). Therefore, one could argue that the impaired shoaling response we report here may be the result of motor dysfunction. However, our current results suggest this argument is not correct. We found no abnormalities in motor function of the alcohol-treated fish. For example, their general activity level (total distance swam) did not differ from control (Figure 3). Furthermore, the amount and speed of turning they performed was also statistically indistinguishable from control. Thus, we conclude that the impaired response to the images of conspecifics is not due to alterations in motor function.
Another possible reason for the impaired shoaling response may be abnormal vision. Matsui et al. (2006) and Arenzana et al. (2006) reported alcohol exposure impaired the visual system in zebrafish. However, these authors used higher alcohol concentrations than what we employed here, and they exposed the zebrafish embryos to alcohol for much longer periods of time than we did. Nevertheless, although we observed no aberrations in the gross structural features of the eyes of our exposed fish, exposure to even low concentrations of alcohol and for a short period of time may affect eye development and thus slightly impair vision. We argue, however, that visual impairment is unlikely to explain the abnormal shoaling response we report for 2 reasons. One, our current behavioral results show that the alcohol-exposed zebrafish do see. We base this conclusion on the turning frequency found in response to stimulus image presentation in the alcohol-treated fish, a response that was indistinguishable from that of control. Two, although this latter finding does not preclude the possibility of slight visual impairment, notably, Buske and Gerlai (2011) found impaired shoaling even in freely moving groups of zebrafish exposed to the same embryonic alcohol treatment employed here. In these shoals, modalities other than vision may also be used by shoal members. Notably, shoaling fish have been found to shoal normally even in the complete absence of visual cues (Timmermann et al., 2004), yet the embryonic alcohol-treated zebrafish exhibited impaired shoaling (Buske and Gerlai, 2011).
The questions of what central nervous system mechanisms embryonic alcohol exposure alters and whether these alterations may causally explain the observed behavioral changes reported here remain to be answered. Nevertheless, the literature and our current results implicate the dopaminergic system as one such mechanism. In the current study, we found the presentation of conspecific images induced increased levels of dopamine and DOPAC, but only in control fish. Al-Imari and Gerlai (2008) showed that zebrafish found the sight of conspecifics rewarding, andSaif and Gerlai (2013) found the appearance of an animated shoal increased dopamine and DOPAC in the brain of zebrafish. Scerbina et al. (2012) showed that dopamine D1-receptor antagonism disrupts shoaling. Buske and Gerlai (2011) and Mahabir et al. (2013) have found that embryonic alcohol exposure impaired the dopaminergic system, a result that is in line with what has been found in primates prenatally exposed to alcohol (Schneider et al., 2006). Last, Parker et al. (2014b), albeit using a different embryonic alcohol exposure regimen than we did, found alterations in expression of genes encoding, among other proteins, dopamine receptors. Given the aforementioned findings and the fact that dopamine is known to play an important role in reward (Kuhar et al., 1991), the correlation we found between abnormal shoaling responses and reduced dopamine and DOPAC levels is not surprising but pinpoints a possible mechanism for the action of alcohol exposure during embryogenesis. The observed lack of statistically appreciable differences in dopamine and DOPAC levels between control and alcohol-treated fish at baseline, that is, before the presentation of images of conspecifics, is also notable. In 2 previous studies (Buske and Gerlai, 2011; Mahabir et al., 2013), reduced dopamine and DOPAC levels were found in the fish that were exposed to alcohol during their embryonic development. In these studies, however, baseline responses could not be distinguished from stimulation-induced changes, as neurochemical levels were measured after the fish were exposed to either live conspecifics or animated images of conspecifics. In summary, we conclude that embryonic alcohol exposure impairs the dopaminergic response to social stimuli in zebrafish. Whether this impairment is specific to such stimuli, or more general, that is, it is associated with other rewards or reinforcers, is a question that will be investigated in the future.
The last comment we make concerns the methods we employed. One of the goals of establishing a zebrafish ARND model is to utilize this model for the identification of both the mechanisms and the potential biomarkers associated with the disease that would aid treatment and diagnosis. Establishment of the model is the first step in this research. Subsequent steps may include systematic mutation screens for the identification of genetic factors that may alter the sensitivity of the embryo to alcohol exposure or the outcome of this exposure at a later stage of development including adulthood. Similarly, large-scale drug screens may identify small molecules that modify the effect or the manifestation of embryonic alcohol exposure. Both for mutation and drug screening we expect the need of high throughput, because a potentially large number of fish will have to be tested to identify interesting mutants and/or drugs. Importantly, because the methods we employed include computerized stimulus delivery and also computerized behavioral response quantification, the experimenter does not need to be present during the behavioral session and thus the test paradigm is highly scaleable, that is, can be run in a massively parallel manner, which makes the employed behavioral method efficient. We argue, therefore, that our results and the presented methods together with the known translational relevance of the zebrafish will make this species a useful model for the analysis of ARND.
Ethical Statement
The research reported in this study was approved by the Local Animal Care Committee of the University of Toronto Mississauga and complies with the guidelines as set forth by the Canadian Council for Animal Care and is also in accordance with accepted guidelines such as Guiding Principles in the Care and Use of Animals (DHEW Publications, NIH, 80-23).
Statement of Interest
The authors claim no conflict of interest.
Acknowledgments
Supported by NIH/NIAAA (R01 AA14357-01A2) and NSERC (311637) grants to R.G.
References
- Al-Imari L, Gerlai R. (2008). Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio). Behav Brain Res 189:216–219. [DOI] [PubMed] [Google Scholar]
- Arenzana FJ, Carvan MJ, III, Aijón J, Sánchez-González R, Arévalo R, Porteros A. (2006). Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicol Teratol 28:342–348. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S. (2004). Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol 26:737–743. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. (2011). Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol Teratol 33:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. (2009). High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav Brain Res 200:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GJ, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay S. (2012). Effects of ethanol exposure on nervous system development in zebrafish. Int Rev Cell Mol Biol 299:255–315 [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Patterson LB, Rao AA, Parichy DM. (2007). Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. (2009). Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res 33:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Y, Tran S, Abraham E, Gerlai R. (2014). Embryonic alcohol exposure impairs associative learning performance in adult zebrafish. Behav Brain Res 265:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. (2000). Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Akers KG, Rice JP, Johnson TE, Candelaria-Cook FT, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. (2010a) Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res 207:290–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DA, Candelaria-Cook FT, Akers KG, Rice JP, Maes LI, Rosenberg M, Valenzuela CF, Savage DD. (2010b) Patterns of social-experience-related c-fos and Arc expression in the frontal cortices of rats exposed to saccharin or moderate levels of ethanol during prenatal brain development. Behav Brain Res 214:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. (1995). Stages of embryonic development of the zebrafish. Dev Dyn. 203:253–310. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. (2007). Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: a review. Neurosci Biobehav Rev 31:192–201. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. (1991). The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14:299–302. [DOI] [PubMed] [Google Scholar]
- Kully-Martens K, Denys K, Treit S, Tamana S, Rasmussen C. (2011). A review of social skills deficits in individuals with fetal alcohol spectrum disorders and prenatal alcohol exposure: profiles, mechanisms, and interventions. Alcohol Clin Exp Res 36:568–576. [DOI] [PubMed] [Google Scholar]
- Lockwood B, Bjerke S, Kobayashi K, Guo S. (2004). Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacol Biochem Behav 77:647–654. [DOI] [PubMed] [Google Scholar]
- Loucks E, Carvan MJ III. (2004). Strain-dependent effects of developmental ethanol exposure in zebrafish. Neurotoxicol Teratol 26:745–755. [DOI] [PubMed] [Google Scholar]
- Mahabir S, Chatterjee D, Gerlai R. (2013). Strain dependent neurochemical changes induced by embryonic alcohol exposure in zebrafish. Neurotoxicol Terato l:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI. (2006). Effects of ethanol on photoreceptors and visual function in developing zebrafish. Invest Ophthalmol Vis Sci 47:4589–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. (2011). Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. (2009). Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies Paley B, ed. Dev Disabil Res Revs 15:176–192. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Dahm R. (2002). Zebrafish. Oxford: Oxford University Press. [Google Scholar]
- Parker MO, Annan LV, Kanellopoulos AH, Brock AJ, Combe FJ, Baiamonte M, Teh MT, Brennan CH. (2014a) The utility of zebrafish to study the mechanisms by which ethanol affects social behavior and anxiety during early brain development. Prog Neuropsychopharmacol Biol Psychiatry. 55:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Evans AM-D, Brock AJ, Combe FJ, Teh M-T, Brennan CH. (2014b) Moderate alcohol exposure during early brain development increases stimulus-response habits in adulthood. Addict Biol. Advance online publication. Retrieved 23 Oct 2014. 10.1111/adb.12176. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ. (1983). Heuristic definitions of fish shoaling behaviour. Anim Behav 31:611–613. [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R. (2014). Induction of social behaviour in zebrafish: live versus computer-animated fish as stimuli. Zebrafish 11:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C, Becker M, McLennan J, Urichuk L, Andrew G. (2010). An evaluation of social skills in children with and without prenatal alcohol exposure. Child Care Health Dev 37:711–718. [DOI] [PubMed] [Google Scholar]
- Saif M, Chatterjee D, Buske C, Gerlai R. (2013). Sight of conspecific images induces changes in neurochemistry in zebrafish. Behav Brain Res 243:294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM. (1997a) Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology 56:317–326. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. (2008). The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res 191:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbina T, Chatterjee D, Gerlai R. (2012). Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids 43:2059–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barnhart TE, Larson JA, DeJesus OT, Mukherjee J, Nickles RJ, Converse AK, Roberts AD, Kraemer GW. (2006). Moderate-level prenatal alcohol exposure alters striatal dopamine system function in rhesus monkeys. Alcohol Clin Exp Res 29:1685–1697. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Adkins MM. (2011a) The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev 21:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Barr CS, Larson JA, Kraemer GW. (2011b) Moderate prenatal alcohol exposure and serotonin genotype interact to alter CNS serotonin function in rhesus monkey offspring. Alcohol Clin Exp Res 35:912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawecki CJ, Thomas JD, Riley EP, Ehlers CL. (2004). Neurophysiologic consequences of neonatal ethanol exposure in the rat. Alcohol 34:187–196. [DOI] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. (2007). The behaviour and ecology of the zebrafish, Danio rerio. Biological Reviews 83:13–34. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Howe CJ, Battaglia FC.Institute of Medicine (U.S.). Division of Biobehavioral Sciences and Mental Disorders. Committee to Study Fetal Alcohol Syndrome, National Institute on Alcohol Abuse and Alcoholism (U.S.) (1996) Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington DC: National Academy Press.
- Timmermann M, Schlupp I, Plath M. (2004). Shoaling behaviour in a surface-dwelling and a cave-dwelling population of a barb Garra barreimiae (Cyprinidae, Teleostei). Acta Ethologica 7:59–64. [Google Scholar]
- Tropepe V, Sive HL. (2003). Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav 2:268–281. [DOI] [PubMed] [Google Scholar]