Abstract
Background:
Evidence suggests that mammalian target of rapamycin activation mediates ketamine’s rapid but transient antidepressant effects and that glycogen synthase kinase-3β inhibits this pathway. However, ketamine has associated psychotomimetic effects and a high risk of abuse. The mood stabilizer lithium is a glycogen synthase kinase-3 inhibitor with strong antisuicidal properties. Here, we used a mouse stress model to investigate whether adjunct lithium treatment would potentiate ketamine’s antidepressant-like effects.
Methods:
Mice received chronic restraint stress and long-term pre- or postketamine lithium treatment in drinking water. The effects of lithium on ketamine-induced antidepressant-like effects, activation of the mammalian target of rapamycin/brain-derived neurotrophic factor signaling pathways, oxidative stress, and dendritic spine density in the brain of mice were investigated.
Results:
Subtherapeutic (600mg/L) lithium-pretreated mice exhibited an antidepressant-like response to an ineffective ketamine (2.5mg/kg, intraperitoneally) challenge in the forced swim test. Both the antidepressant-like effects and restoration of dendritic spine density in the medial prefrontal cortex of stressed mice induced by a single ketamine (50mg/kg) injection were sustained by postketamine treatment with 1200mg/L of lithium for at least 2 weeks. These benefits of lithium treatments were associated with activation of the mammalian target of rapamycin/brain-derived neurotrophic factor signaling pathways in the prefrontal cortex. Acute ketamine (50mg/kg) injection also significantly increased lipid peroxidation, catalase activity, and oxidized glutathione levels in stressed mice. Notably, these oxidative stress markers were completely abolished by pretreatment with 1200mg/L of lithium.
Conclusions:
Our results suggest a novel therapeutic strategy and justify the use of lithium in patients who benefit from ketamine.
Keywords: ketamine, lithium, mTOR, GSK-3, BDNF
Introduction
Depression in individuals with either major depressive disorder (MDD) or bipolar disorder (BD) is one of the leading causes of the global disease burden. Almost all current antidepressant drugs in clinical use require weeks to months to take full effect (Adell et al., 2005), and a significant proportion of patients do not respond to available agents (Insel and Wang, 2009). Ketamine, a noncompetitive N-methyl-D-aspartate receptor antagonist, has been safely used as an anesthetic and analgesic agent for many years. Recent clinical and preclinical research indicates that, in treatment-resistant MDD and BD subjects, a single subanesthetic dose of ketamine not only produces a rapid antidepressant effect within hours of administration, but also improves suicidal ideation (Berman et al., 2000; Zarate et al., 2006; DiazGranados et al., 2010).
Activation of mammalian target of rapamycin (mTOR) and subsequent synaptogenesis in the prefrontal cortex (PFC) have been suggested to mediate ketamine’s rapid antidepressant effects (Li et al., 2010; Dwyer and Duman, 2013). The loss of synaptic function in the PFC, as well as the downregulation of synaptic proteins such as the postsynaptic density protein 95 (PSD95), were associated with depressive-like behaviors in a rodent model of chronic stress (Li et al., 2011). Through activation of the mTOR signaling pathway, acute injection of ketamine rapidly increased levels of these synaptic proteins and dendritic spine density; in contrast, inhibition of mTOR signaling prevented these synaptic actions and the antidepressant-like effects of ketamine in experimental animals (Li et al., 2010).
In clinical populations, repeated ketamine treatment is usually necessary to avoid subsequent relapse (Zarate et al., 2006; Ibrahim et al., 2012). However, ketamine has been used as a recreational drug and has a high risk of abuse. Repeated administration of ketamine can cause a variety of side effects, including hallucinations and cognitive impairments, and psychotomimetic symptoms (Krystal et al., 2005). In fact, ketamine was found to induce schizophrenia-like behaviors in humans (Krystal et al., 2003), and treating animals with subanesthetic doses of ketamine is a pharmacological model of schizophrenia (Gunduz-Bruce, 2009). In addition, administration of subanesthetic doses of ketamine increases oxidative stress in the rodent brain (Zuo et al., 2007; de Oliveira et al., 2009; da Silva et al., 2010). These adverse effects limit ketamine’s potential for widespread clinical use.
Accumulating evidence indicates that the mood stabilizer lithium has strong antisuicidal properties (Cipriani et al., 2005) and holds promise for treating other neurological and neurodegenerative diseases via its diverse mechanisms of action (Chiu and Chuang, 2010; Chiu et al., 2013). Among them, lithium’s ability to inhibit glycogen synthase kinase-3 (GSK-3), a ubiquitous serine-threonine kinase, has been considered critical to mediating its numerous mood-stabilizing and neuroprotective effects. Ketamine’s rapid antidepressant effects require GSK-3 inhibition (Beurel et al., 2011). In addition, GSK-3 negatively regulated mTOR in mouse brain (Sarkar et al., 2008). These findings indicate that lithium and ketamine may have a signaling convergence on the mTOR pathway and a possible mechanistic synergy on their antidepressant-like effects. The present study was undertaken to investigate whether combining ketamine with the mood stabilizer lithium could benefit ketamine’s antidepressant effects in mice as well as protect against the oxidative stress associated with ketamine use.
Methods
Animals and Chronic Restraint Stress
Male CD-1 mice were purchased from Charles River Laboratory (Wilmington, MA), and chronic restraint stress was performed as previously described (Omata et al., 2011). Briefly, mice were placed into a Plexiglas tube (2.5cm in diameter) individually for 2 hours once a day for 2 weeks (supplementary Figure S1a). All procedures for animal experiments were approved by the Animal Care and Use Committee of the National Institutes of Health (NIH).
Drug Treatment and Measurement of Serum Lithium Concentration
Ketamine hydrochloride (10mg/mL; Vedco, St. Joseph, MO) was freshly diluted in saline (0.9%) before use and injected intraperitoneally at a volume of 10mL/kg of body weight. To ensure a steady-state serum concentration, mice were pretreated with lithium chloride through drinking water (600 or 1200mg/L) for 3 weeks prior to acute saline or ketamine challenge (supplementary Figure S1b). For postketamine lithium treatment, another cohort of stressed mice received lithium chloride treatment (1200mg/L) in drinking water following a single ketamine challenge (supplementary Figure S1c). Serum lithium concentrations of mice were measured by MEDTOX Laboratories (Saint Paul, MN; test code 60063).
Behavioral Tests
Mice underwent the open-field test (OFT) 60 minutes after acute ketamine injection, followed by the forced swim test (FST) 30 minutes later. To investigate the behavioral effects of postketamine lithium treatment, both tests were repeated in the same order after 1 and 2 weeks of lithium treatment using the same group of mice (supplementary Figure S1).
OFT
Under bright illumination from regular ceiling lights, mice were placed individually in the center of a clear open-field chamber (27.9 cm×27.9 cm×20cm), and their horizontal locomotor activities were measured by using an automated photo-beam open-field system (Med-Associates, St. Albans, VT) for 30 minutes.
FST
Briefly, each mouse was placed for 6 minutes in a 25-cm–high, 16-cm–diameter transparent cylindrical tank filled with room temperature water (~22°C). Immobility was scored during the last 4 minutes of the session, and a decrease in swimming time was considered a measure of depressive-like behaviors. All test sessions were analyzed off-line by ANY-maze video-tracking system (Stoelting, Wood Dale, IL).
Tail-Suspension Test
Briefly, mice were suspended individually by the tail using nonirritating adhesive tape placed at about one-half the total tail length to a hook connected to a horizontal bar. Each test session lasted for 6 minutes and total immobility time was analyzed off-line by ANY-maze system (Stoelting).
Brain Sample Preparation and Western-Blot Analysis
Brain tissues were homogenized in 20mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid containing 0.32M sucrose and centrifuged at 700 g for 10 minutes at 4°C. The supernatants were centrifuged again at 14,000 g for 10 minutes at 4°C, and the pellets were resuspended in T-PER reagent (Thermo Scientific, Rockford, IL). Proteins were separated and transferred onto a nitrocellulose membrane. Blots were immunostained overnight at 4°C with primary antibody against total GSK-3β (BD, Franklin Lakes, NJ), phospho-GSK-3β at Ser9, total Akt (the serine/threonine kinase, also known as protein kinase B or PKB), phospho-Akt at Ser473, total extracellular signal-regulated kinases (ERKs), phospho-ERK at Thr202/Tyr204, total mTOR, phospho-mTOR at Ser2448, total P70S6 kinase (P70S6K), phospho-P70S6K at Thr389, total eukaryotic elongation factor-2 (eEF2), phospho-eEF2 at Thr56, PSD95 (all from Cell Signaling, Beverly, MA), total tropomyosin-related kinase B (TrkB; Millipore, Billerica, MA), phospho-TrkB at Tyr817, or the house-keeping gene β-actin (Abcam, Cambridge, MA). Membranes were then incubated with secondary antibodies (LI-COR, Lincoln, NE) for 1 hour at room temperature. Finally, blotted proteins were detected and quantified using the Odyssey infrared imaging system (LI-COR).
Analysis of Oxidative Stress
Mice were sacrificed by decapitation 20 minutes after acute ketamine challenge, and the brains were dissected and homogenized according to the buffer requirements of each assay.
Thriobarbituric Acid Reactive Substances Assay
Assay of thriobarbituric acid reactive substances, byproducts of lipid peroxidation, was performed according to the manufacturer’s instructions (Cayman Chemical, Ann Arbor, MI). The production of malondialdehyde was normalized by protein concentration.
Catalase Activity Assay
This assay was performed according to the manufacturer’s instructions (Cayman Chemical). The production rate of formaldehyde was normalized by protein concentration.
Glutathione Assay
Analyses of reduced and oxidized glutathione levels were conducted per the manufacturer’s instructions (Cayman Chemical). The oxidized glutathione content was expressed as the ratio to total (reduced and oxidized) glutathione.
Analysis of Dendritic Spine Density
Mice were sacrificed and brains were subjected to Golgi-staining (FD NeuroTechnologies, Columbia, MD) at the time indicated. Briefly, coronal sections of 100 µm in thickness were prepared, and both basal and apical dendrites (~50 and ~100 µm from soma, respectively) of pyramidal neurons in layer V of medial PFC (anterior cingulate and prelimbic) were chosen for quantitative analysis. Images were captured by an Olympus BX61 microscope, and the length of dendritic segments was determined by using ImageJ software from NIH. Spine numbers in ~30-µm segments were measured manually by investigators blind to the experimental conditions. Two segments from each neuron were analyzed, and the results were expressed as number of spines per µm.
Statistical Analyses
All statistical analyses were performed using GraphPad Prism (GraphPad, San Diego, CA). Data are expressed as mean±SEM and analyzed using t test or 1-way analysis of variance. When necessary, multiple comparisons between groups were assessed with posthoc Student–Newman–Keuls multiple comparison test. Statistical significance was considered at P<.05.
Results
Antidepressant-Like Effects of Ketamine in CD-1 Mouse Model of Stress
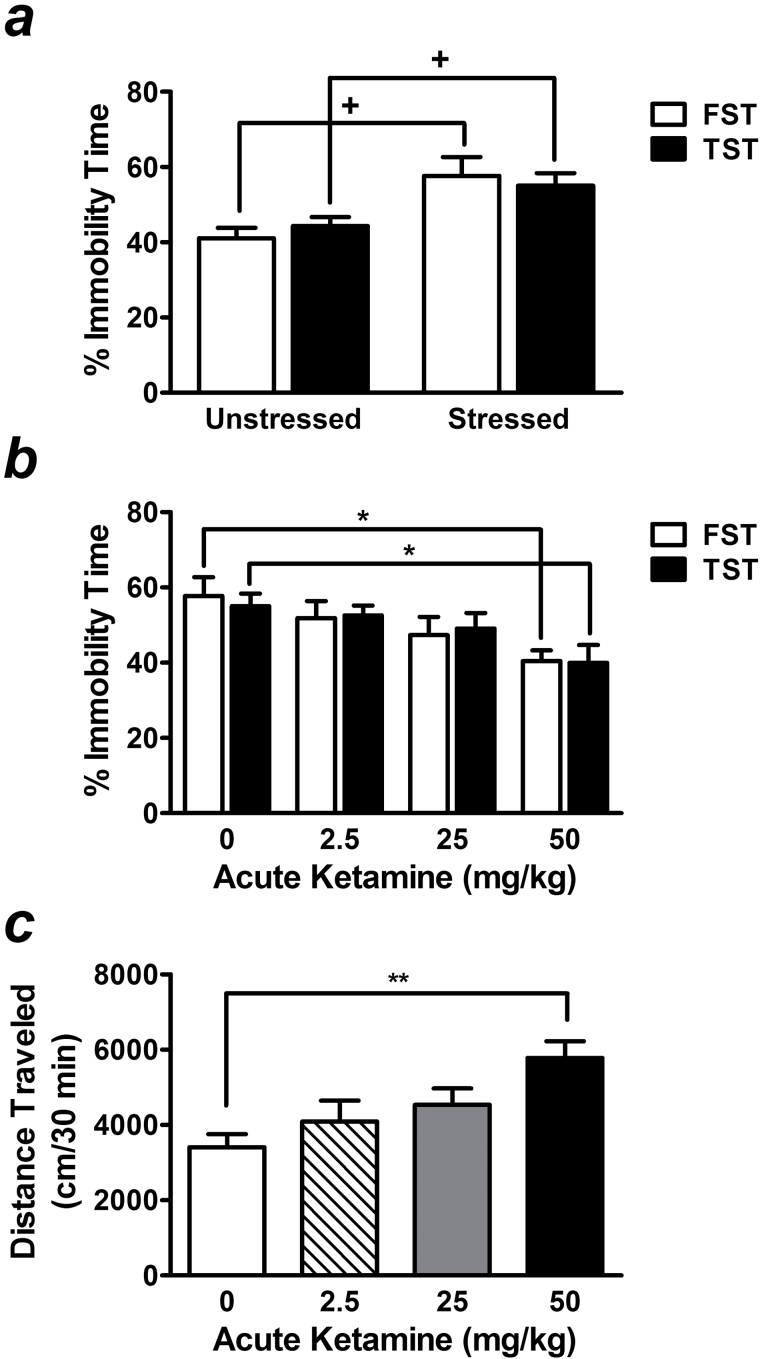
To mimic a clinical situation, the chronic restraint stress paradigm was used for the detection of antidepressant-like effects of ketamine and lithium. The depressive-like behaviors of this stress model can be suppressed by 20mg/kg of the antidepressant desipramine (Omata et al., 2011). In the present study, chronic restraint paradigm markedly increased immobility time in the FST and tail suspension test (TST) by 140.8± 12.1% (t(14)=2.93, P=.011) and 124.2±7.6% (t(14)=2.59, P=.021), respectively, compared with unstressed mice (Figure 1a). A wide range of ketamine doses (1–100mg/kg) has been reported to effectively produce antidepressant-like effects in various mouse strains (Mantovani et al., 2003; Hayase et al., 2006; Kos et al., 2006; Popik et al., 2008; Browne and Lucki, 2013). We thus performed a dose response test in our CD-1 mouse model of stress and found that the minimum dose for ketamine to suppress immobility time in the FST was 50mg/kg (72.2±6.5% of control, F[3, 26] =3.02, P<.05) (Figure 1b). This effective dose of ketamine was confirmed by a decreased immobility time in the TST performed 24 hours after FST (72.6±8.7% of control, F[3, 26] =0.046, P<.05) (Figure 1b). However, mice that received this dose of ketamine also exhibited hyperlocomotion as measured by the OFT (169.8±13.2% of control, F[3, 26] =5.03, P <.01) (Figure 1c).
Figure 1.
Dose response of acute ketamine challenge in stressed mice. Chronic restraint stress produced depressive-like behaviors in CD-1 mice, as assessed by increased immobility time in the forced swim test (FST) and tail-suspension test (TST) (a). In stressed mice, acute injection of ketamine at a dose of 50mg/kg not only significantly suppressed immobility in both tests (b), but also increased locomotor activity measured by the open-field test (OFT) (c). Data are mean±SEM (n =6–8). +P<.05, t test; *P<.05, **P<.01, compared with control groups, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA).
Preketamine Treatment with a Subtherapeutic Dose of Lithium Potentiates the Antidepressant-Like Effects Induced by a Low Dose of Ketamine
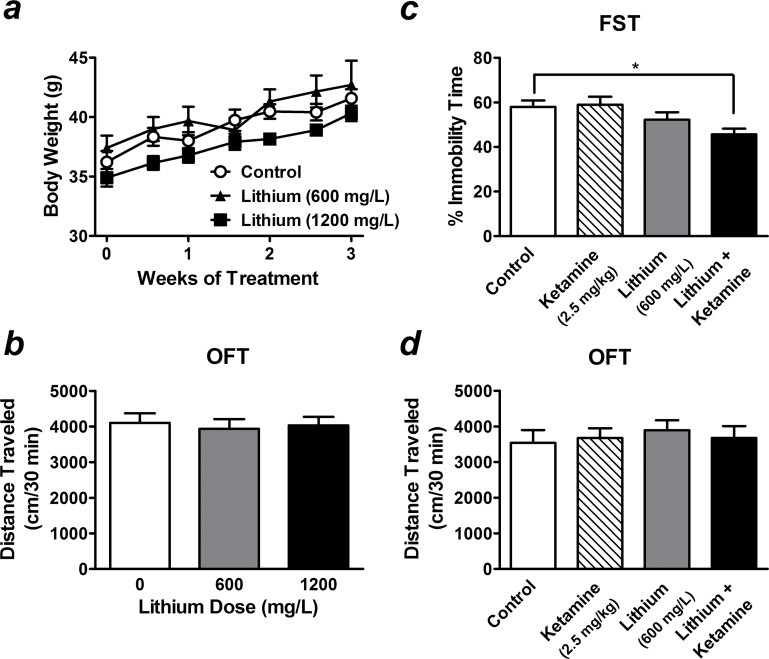
Long-term treatment is usually necessary for lithium to reach a steady-state serum level and exert its effects. Our pilot study indicated that the serum concentration of lithium in CD-1 mice after 3 weeks of treatment with 600mg/L of the drug in drinking water (0.202±0.029 mEq/L; n=8) was below the therapeutic levels for human patients with BD (~0.5–1.2 mEq/L) (American Psychiatric Association, 2002). No differences were observed for measures of body weight (Figure 2a) or locomotor activity (Figure 2b) between controls and mice that received this dose of lithium for 3 weeks.
Figure 2.
Preketamine treatment with a subtherapeutic dose of lithium potentiates a low dose of ketamine-induced antidepressant-like effects. In CD-1 mice, long-term treatment with a sub- (600mg/L) or low therapeutic dose (1200mg/L) of lithium chloride in drinking water for 3 weeks did not affect body weight (a) or locomotor activity (b). Pretreatment with 600mg/L of lithium alone had no effect on immobility time in saline-challenged stressed mice (lithium alone group), but significantly potentiated the response induced by a very low dose of ketamine (2.5mg/kg) in the forced swim test (FST) (lithium + ketamine group) (c). The locomotor activity of stressed mice measured by the open-field test (OFT) was not affected by any given treatment (d). Data are mean±SEM (n =6–12). *P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA).
In stressed mice, pretreatment with 600mg/L of lithium alone had no significant effects on immobility time in the FST (Figure 2c). Interestingly, stressed mice that had been pretreated with this subtherapeutic dose of lithium had a robust antidepressant-like response to acute challenge with a very low dose (2.5mg/kg) of ketamine (78.76±4.38% of control, F[5, 52] =4.34, P<.05) (Figure 2c) that, administered alone, had no significant antidepressant effect (Figure 1b). Treatment with this dose of lithium alone or together with a 2.5-mg/kg ketamine challenge did not affect locomotor activity of stressed mice (Figure 2d). These data show that the combination of “ineffective” doses of lithium and ketamine can produce synergy on antidepressant-like effects and suggest a signaling convergence between these two drugs.
Preketamine Treatment with a Subtherapeutic Dose of Lithium Potentiates the Activation of mTOR/BDNF-TrkB Signaling Pathways Induced by a Low Dose of Ketamine
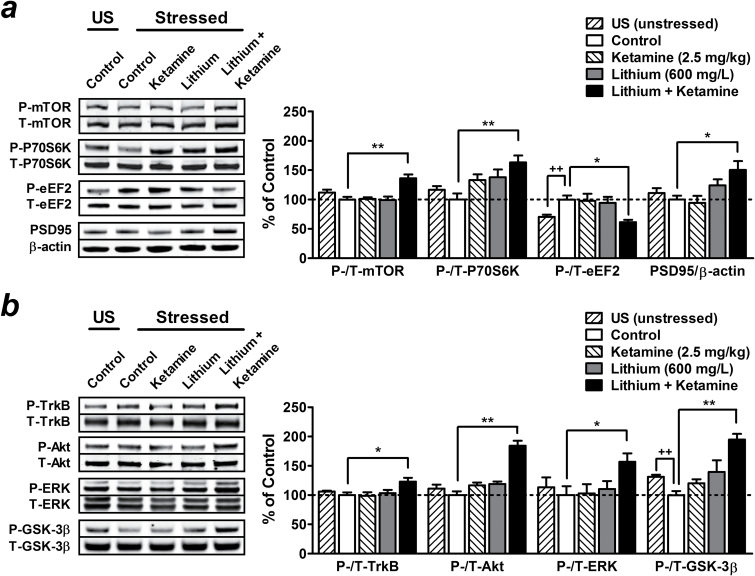
As mentioned above, activation of the mTOR pathway in the PFC is critical to ketamine’s rapid antidepressant-like effect. In the present study, mice that had undergone chronic restraint stress showed a trend towards decreased phosphorylation of mTOR and its downstream effector P70S6K in the PFC, though the effect was not statistically significant compared with unstressed mice (Figure 3a). In stressed mice, acute injection with a behaviorally ineffective dose (2.5mg/kg) of ketamine or pretreatment with 600mg/L of lithium alone had no significant effects, while a combination with these two treatments strongly enhanced the phosphorylation of mTOR (136.30±6.47%, P<.01) and P70S6K (163.25±11.58%, P<.01) in the PFC (Figure 3a ).
Figure 3.
Preketamine treatment with a subtherapeutic dose of lithium potentiates the activation of mammalian target of rapamycin (mTOR)/brain-derived neurotrophic factor (BDNF)-tropomyosin-related kinase B (TrkB) signaling pathways induced by acute challenge with a low dose of ketamine. Mice were pretreated with 600mg/L of lithium for 3 weeks, and brain tissues were collected 60 minutes after a single injection of saline (lithium alone group) or a very low dose (2.5mg/kg) of ketamine (lithium + ketamine group). Typical Western blots and quantified results are shown. The phosphorylation levels of mTOR, P70S6K, eukaryotic elongation factor-2 (eEF2), and the expression of postsynaptic density protein 95 (PSD95) (a), as well as the phosphorylation levels of TrkB, Akt, extracellular signal-regulated kinase (ERK), and glycogen synthase kinase-3β (GSK-3β) (b) in the prefrontal cortex (PFC) were normalized to the levels of total protein or β-actin and expressed as percentage of control group. Data are mean±SEM (n =4–8). ++P<.01, t test; *P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA). P, phosphorylated protein; T, total protein; US, unstressed.
We also found that stressed mice showed increased phosphorylation of eEF2 (145.37±9.96% of unstressed mice, t(12) =4.47, P<.01) (Figure 3a ), an essential component required for polypeptide elongation in protein synthesis (Wang et al., 2001). The activity of eEF2 is negatively regulated by its phosphorylation at threonine 56 induced by eEF2 kinase, a downstream effector of the mTOR pathway that is inhibited by P70S6K (Wang et al., 2001). Again, pretreatment with 600mg/L of lithium together with acute injection of 2.5mg/kg of ketamine robustly decreased eEF2 phosphorylation (60.25±4.46%, P<.05) and upregulated the expression of synaptic protein PSD95 (150.44±14.91%, P<.05) in the PFC compared with control stressed mice. Neither drug alone had significant effects (Figure 3a ).
In addition to the mTOR pathway, GSK-3β inhibition (Beurel et al., 2011) and acute increases in brain-derived neurotrophic factor (BDNF) protein levels (Garcia et al., 2008; Autry et al., 2011), including activation of the downstream effectors Akt and ERK (Li et al., 2010), are also necessary for ketamine’s rapid antidepressant actions. Similar to the observations described above, we found that only pretreatment with 600mg/L of lithium plus subsequent challenge with 2.5mg/kg of ketamine significantly increased phosphorylation levels of TrkB (a BDNF receptor; 123.02±6.49%, P<.05), Akt (184.18±8.57%, P<.01), ERK (156.59±14.37%, P<.05), and GSK-3β (206.21±10.40%, P<.01) (Figure 3b ) in the PFC compared with control stressed mice. Treatment with either drug alone had no significant effects. It is interesting to note that chronic restraint stress alone markedly decreased GSK-3β phosphorylation in this brain region (76.10±5.23%, t(12) =3.69, P<.01) compared with unstressed mice (Figure 3b ).
Preketamine Treatment with a Low Therapeutic Dose of Lithium Suppresses Acute Ketamine-induced Oxidative Stress
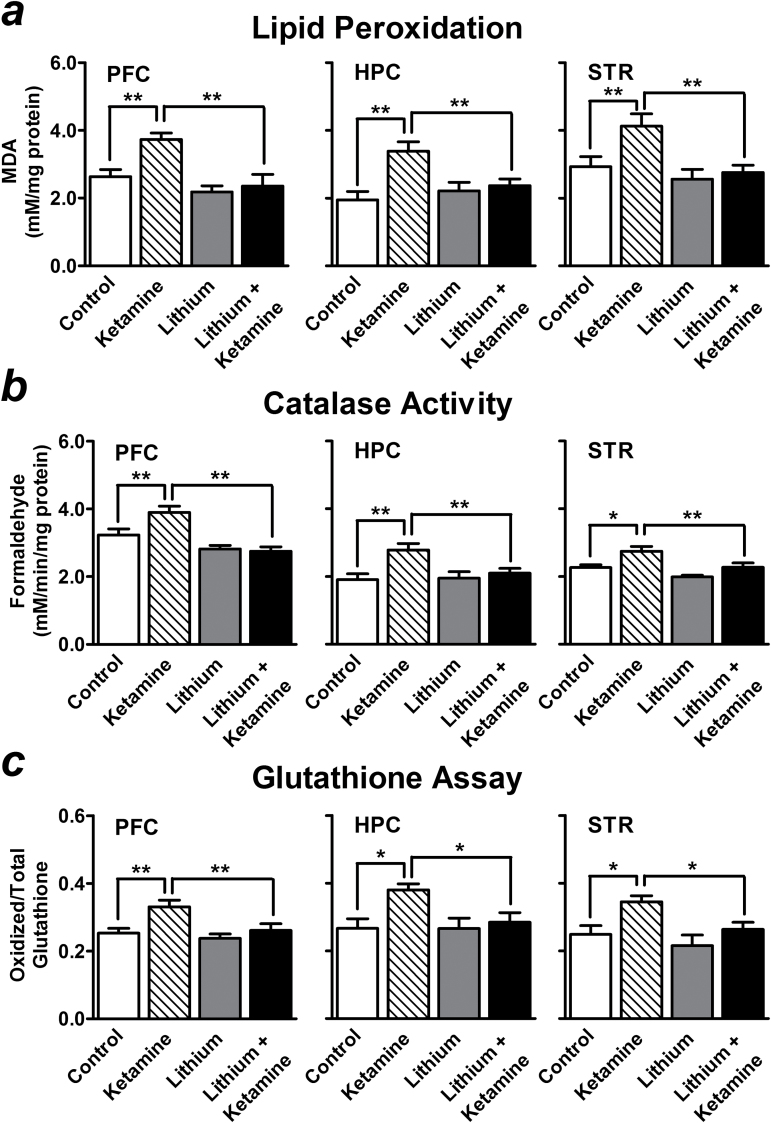
In our mouse model of stress, we found that acute injection with ketamine at a dose that produces an antidepressant-like effect (50mg/kg) markedly increased lipid peroxidation (141.49±7.29% of control, P<.01) (Figure 4a ), catalase activity (120.64±5.79% of control, P<.01) (Figure 4b ), and levels of oxidized glutathione (130.90±7.75% of control, P<.01) (Figure 4c ) in the PFC 20 minutes after injection. Similar results were also observed in the hippocampus (lipid peroxidation: 173.40±14.26%, P<.01; catalase activity: 145.69±9.92%, P<.01; oxidized glutathione: 142.60±6.62%, P<.05) and striatum (lipid peroxidation: 140.86±12.37%, P<.01; catalase activity: 121.37±6.14%, P<.05; oxidized glutathione: 138.70±7.27%, P<.05; compared with control stressed mice) (Figure 4).
Figure 4.
Preketamine treatment with a low therapeutic dose of lithium suppresses acute ketamine-induced oxidative stress. Acute ketamine injection at a dose that produces antidepressant-like effect (50mg/kg) markedly increased lipid peroxidation (a), catalase activity (b), and the levels of oxidized glutathione (c) in the prefrontal cortex (PFC), hippocampus (HPC), and striatum (STR) of stressed mice 20 minutes after injection. Compared with control stressed mice, preketamine treatment with 1200mg/L of lithium for 3 weeks robustly suppressed the oxidative metabolism markers induced by acute ketamine (lithium + ketamine group) in these brain regions. Data are mean±SEM (n =6–10). *P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a one-way analysis of variance (ANOVA).
To investigate whether lithium can protect against the oxidative stress induced by ketamine, mice were pretreated with a higher dose (1200mg/L) of lithium in drinking water for 3 weeks before ketamine challenge. The serum concentration of lithium after 3 weeks of treatment with this dose was at the lower end (0.483±0.052 mEq/L; n=8) of the therapeutic spectrum for human patients with BD. Long-term treatment with this dose of lithium did not affect either body weight (Figure 2a ) or locomotor activity of mice (Figure 2). Compared with control stressed mice, lithium pretreatment robustly suppressed ketamine-induced lipid peroxidation (89.35±13.09%, P<.01) (Figure 4a ), catalase activity (85.16±3.40%, P<.01) (Figure 4b ), and levels of oxidized glutathione (103.49±7.73%, P<.01) (Figure 4c ) in the PFC. These ketamine-induced oxidative metabolism markers in the hippocampus (lipid peroxidation: 121.23±10.34%, P<.01; catalase activity: 109.80±7.42%, P<.01; oxidized glutathione: 106.85±10.40%, P<.05) and striatum (lipid peroxidation: 94.04±7.48%, P<.01; catalase activity: 100.37±5.64%, P<.01; oxidized glutathione: 106.12±8.19%, P<.05, compared with control stressed mice) were also reduced by lithium pretreatment (Figure 4). Although stress is an important contributor to intracellular reactive oxygen species (ROS) generation, we did not observe a significant increase in ROS formation induced by chronic restraint stress (data not shown).
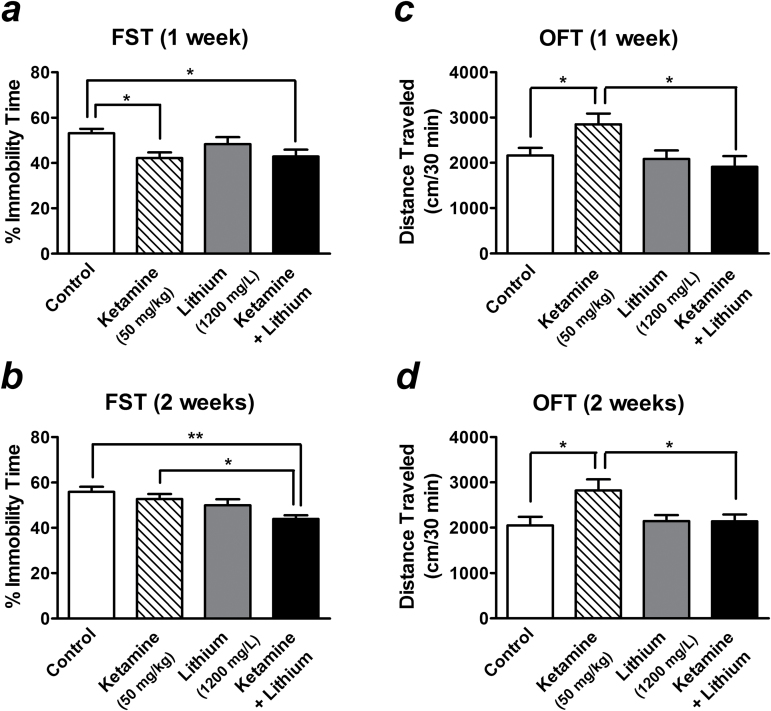
Postketamine Treatment with a Low Therapeutic Dose of Lithium Maintains the Antidepressant-Like Effect and Prevents the Hyperlocomotion Induced by a Single Injection of Ketamine
Although a single infusion of ketamine exerts rapid antidepressant effects, these typically last for only 1 week in human patients and experimental animals (Berman et al., 2000; Zarate et al., 2006; Autry et al., 2011; Ibrahim et al., 2012). We thus sought to investigate whether lithium treatment after ketamine injection might prolong its antidepressant-like effect in our mouse stress model, thereby assessing its potential as a relapse prevention therapeutic strategy.
To ensure the potential effects of lithium, the higher dose (1200mg/L) was preferred in this part of tests. Treatment with this dose of lithium that produced a low therapeutic serum concentration for 1 (Figure 5a ) or 2 weeks (Figure 5b ) produced no antidepressant-like effect in the FST in saline-challenged stressed mice. In contrast, the stressed mice that had received a single injection with 50mg/kg of ketamine still showed decreased immobility time in the FST after 1 week compared with control (F[3, 28] =3.69, P =0.024). Postketamine treatment with 1200mg/L of lithium for 1 week did not further suppress this effect (Figure 5a ). Consistent with findings in the literature, the ketamine-induced antidepressant-like effects were not sustained for 2 weeks (Figure 5b ). However, the immobility time of mice that received both ketamine injection and lithium treatment remained decreased after 2 weeks compared with control (F[3, 28] =5.18, P =.005) (Figure 5b ). On the other hand, the hyperlocomotion associated with ketamine injection lasted for at least 2 weeks (Figure 5c -d). Interestingly, this ketamine-induced long-lasting hyperlocomotion was completely prevented by postketamine lithium treatment measured at either 1 (F[3, 28] =3.88, P =.019) (Figure 5c ) or 2 weeks (F[3, 28] =3.73, P =.023) (Figure 5d ) following ketamine injection, while the same lithium treatment did not affect the OFT in saline-challenged mice.
Figure 5.
Postketamine treatment with a low therapeutic dose of lithium prolongs the antidepressant-like effects and prevents the hyperlocomotion induced by a single injection of ketamine. Stressed mice received long-term treatment with 1200mg/L of lithium in drinking water immediately after a single injection of saline (lithium alone group) or 50mg/kg of ketamine (ketamine + lithium group). The effects of lithium on ketamine-induced antidepressant-like effects (assessed by the forced swim test [FST]) (a-b) and locomotor activity (assessed by the open-field test [OFT]) (c-d) were measured after 1 (a, c) and 2 weeks (b, d) of lithium treatment. Data are mean±SEM (n =8).*P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA).
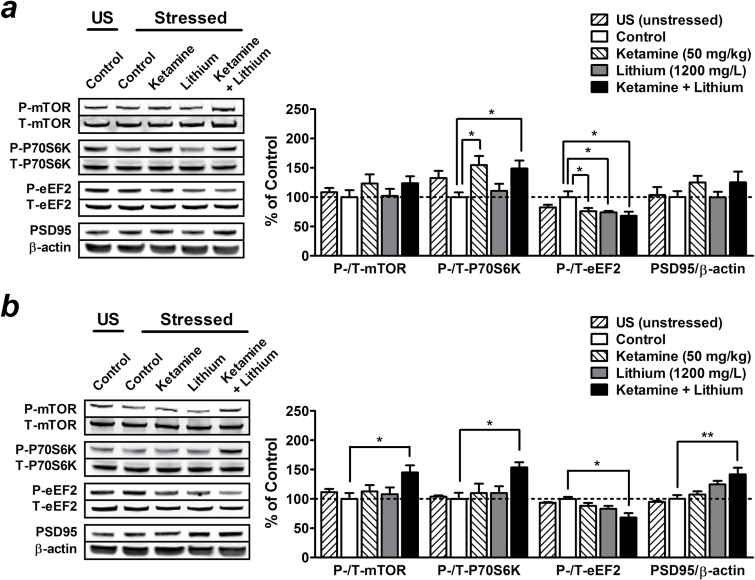
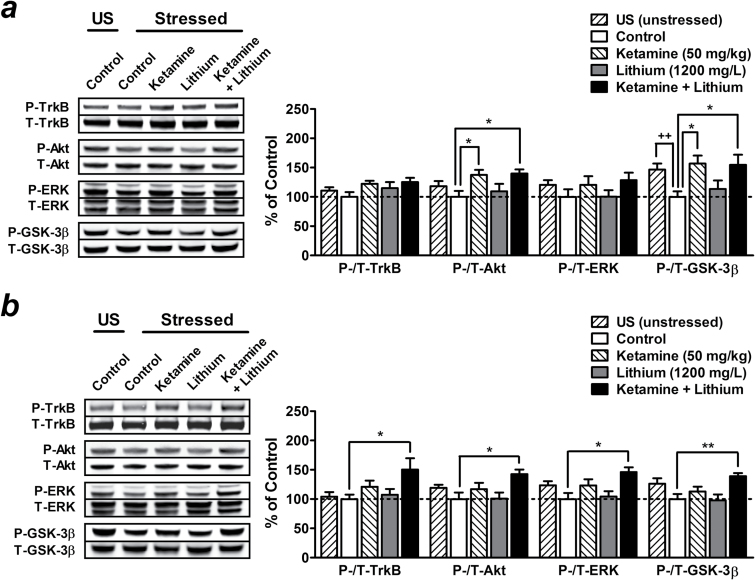
Postketamine Treatment with a Low Therapeutic Dose of Lithium Prolongs the Activation of mTOR/BDNF-TrkB Signaling Pathways Induced by a Single Injection of Ketamine
One week after a single injection of 50mg/kg ketamine, we observed a trend towards elevated phosphorylation of mTOR (123.17±15.73%, not significant), P70S6K (154.76±15.36%, P<.05) (Figure 6a ), TrkB (122.19±5.18%, not significant), Akt (137.52±8.42%, P<.05), ERK (120.39±14.93%, not significant), and GSK-3β (156.77±13.79%, P<.05) (Figure 7a ), while eEF2 phosphorylation was decreased (76.56±4.47%, P<.05) (Figure 6a ). Although not significant, PSD95 expression also appeared to be upregulated in the PFC (124.79±11.27%) compared with control stressed mice (Figure 6a ). Treatment with 1200mg/L of lithium for 1 week had no significant effects in saline-challenged stressed mice and did not affect the regulatory effects of ketamine described above (Figures 6a and 7a ).
Figure 6.
Postketamine treatment with a low therapeutic dose of lithium maintains the activation of the mammalian target of rapamycin (mTOR) signaling pathway induced by a single injection of ketamine. Stressed mice received long-term treatment with 1200mg/L of lithium in drinking water immediately after a single injection of saline (lithium alone group) or 50mg/kg of ketamine (ketamine + lithium group), and brain tissues were collected after 1 (a) or 2 weeks (b) of lithium treatment. Typical Western blots and quantified results are shown. Phosphorylation levels of mTOR, P70S6K, eukaryotic elongation factor-2 (eEF2), and the expression of postsynaptic density protein 95 (PSD95) in the prefrontal cortex (PFC) were normalized to the levels of total protein or β-actin and expressed as percentage of control group. Data are mean±SEM (n =6–8). *P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA). P, phosphorylated protein; T, total protein; US, unstressed.
Figure 7.
Postketamine treatment with a low therapeutic dose of lithium maintains the inhibition of glycogen synthase kinase-3β (GSK-3β) and activation of brain-derived neurotrophic factor (BDNF)-tropomyosin-related kinase B (TrkB) signaling pathway induced by a single injection of ketamine. Stressed mice received long-term treatment with 1200mg/L of lithium in drinking water immediately after a single injection of saline (lithium alone group) or 50mg/kg of ketamine (ketamine + lithium group), and brain tissues were collected after 1 (a) or 2 weeks (b) of lithium treatment. Typical Western blots and quantified results are shown. Phosphorylation levels of TrkB, Akt, extracellular signal-regulated kinase (ERK), and GSK-3β in the prefrontal cortex (PFC) were normalized to the levels of total protein and expressed as percentage of control group. Data are mean±SEM (n =6–8). ++P<.01, t test; *P<.05, **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA). P, phosphorylated protein; T, total protein; US, unstressed.
Compared with unstressed mice, GSK-3β phosphorylation remained significantly decreased in the PFC of stressed mice 1 week (68.23±6.46%, t(10) =3.33, P<.01) (Figure 7a ), but not 2 weeks, after chronic restraint stress (Figure 7b ). Similar to the observation in the behavioral study, the cellular changes induced by ketamine alone lasted for only approximately 1 week. No significant effects were obtained 2 weeks after a single injection with 50mg/kg of ketamine (Figures 6 and 7). However, postketamine treatment with lithium for 2 weeks prolonged and potentiated the regulatory effects of ketamine on the expression of PSD95 (141.91±11.25%, P<.01) as well as phosphorylation of mTOR (145.15±12.02%, P<.05), P70S6K (153.70±8.97%, P<.05), eEF2 (71.30±5.25%, P<.05) (Figure 6b), TrkB (150.38±19.44%, P<.05), Akt (142.66±7.60%, P<.05), ERK (146.24±7.86%, P<.05), and GSK-3β (139.08±5.20%, P<.05) (Figure 7b ) compared with control stressed mice. These results suggest that the behavioral effects of lithium treatment on ketamine’s antidepressant-like effects appear to be closely associated with GSK-3β inhibition and stimulation of the mTOR/BDNF-TrkB signaling pathways.
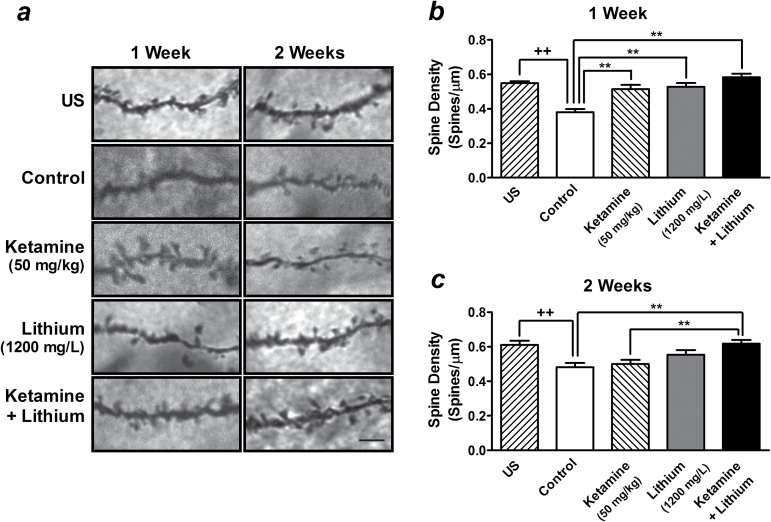
Postketamine Treatment with a Low Therapeutic Dose of Lithium Maintains the Restoration of Dendritic Spine Density Induced by a Single Injection of Ketamine
An increase in the density of dendritic spines in the medial PFC has been reported to be one of the mTOR-dependent mechanisms underlying ketamine’s antidepressant-like effects (Li et al., 2010; Liu et al., 2013). We thus analyzed the effects of postketamine lithium treatment on alterations in spine density induced by a single injection of ketamine. Compared with unstressed mice, the spine densities were found decreased in the medial PFC at 1 (69.55±3.20%, t(70) =7.78, P<.01) and 2 weeks (78.85±3.96%, t(70) =3.81, P<.01) after chronic restraint stress (Figure 8). Compared with control stressed mice, the spine densities in this brain region still remained increased 1 week after a single injection with 50mg/kg of ketamine (134.79±6.62% of control, F[3, 140] =15.80, P <.01), while this effect was not further potentiated by 1-week postketamine treatment with 1200mg/L of lithium (153.07±5.50% of control) (Figure 8a - b ). Ketamine-induced normalization in spine density was not sustained for 2 weeks (103.71±5.01% of control, not significant). However, the density of spines remained elevated in the medial PFC of mice that received both ketamine injection and lithium treatment for 2 weeks compared with control stressed mice (128.31±4.36%, F[3, 140] =6.26, P <.01) (Figure 8a , c ).
Figure 8.
Postketamine treatment with a low therapeutic dose of lithium maintains the dendritic spine density restored by a single injection of ketamine. Representative Golgi-stained sections of spines on dendrites of pyramidal neurons in layer V of medial prefrontal cortex (PFC) are shown (scale bar indicates 5 µm) (a). Stressed mice received long-term treatment with 1200mg/L of lithium in drinking water immediately after a single injection of saline (lithium alone group) or 50mg/kg of ketamine (ketamine + lithium group). Quantified data were obtained from brain samples of unstressed (US) and stressed mice after 1 (b) or 2 weeks (c) of lithium treatment. Data are mean±SEM (n =36). ++P<.01, t test; **P<.01, according to Student–Newman–Keuls multiple comparison test after a 1-way analysis of variance (ANOVA).
Discussion
The present study found that pretreatment of stressed mice with 600mg/L of lithium markedly potentiated the antidepressant-like effect induced by 2.5mg/kg of ketamine, which by itself was ineffective. This is particularly important, because this low-dose combination might avoid the possible adverse effects associated with individual drugs. Indeed, we observed an elevated level of oxidative stress in the mouse brain in conjunction with ketamine’s rapid antidepressant-like action, but this side effect was mitigated by pretreatment with 1200mg/L of lithium. Also notable was that the antidepressant-like effect induced by a single injection with 50mg/kg of ketamine was prolonged by postketamine treatment with 1200mg/L of lithium for at least 2 weeks. We demonstrated that the behavioral benefits of lithium treatment on ketamine’s antidepressant effects were associated with GSK-3β inhibition, stimulation of the mTOR/BDNF-TrkB signaling pathways, and restoration in dendritic spine density in the medial PFC. These data support a recent rat study showing that GSK-3 inhibition potentiates the antidepressant-like effects of subthreshold doses of ketamine (Liu et al., 2013), and are consistent with a clinical observation that lithium-treated BD patients expressed greater antianhedonic responses to ketamine (Lally et al., 2014).
Presumably due to significant strain differences in sensitivity to ketamine, the effective dose (50mg/kg) for ketamine to produce antidepressant-like effects in our mouse model of stress was high and caused hyperlocomotion. However, this dose was the result of our dose-response experiment and is also comparable with other studies. For example, acute ketamine was reported to induce antidepressant-like effects at doses of 30 to 100mg/kg in male ICR mice using FST (Hayase et al., 2006), 50 and 66mg/kg in male C57BL/6J/Han mice using TST (Kos et al., 2006), and 50mg/kg in male Swiss mice using FST (Popik et al., 2008). Mice that received 50mg/kg of ketamine challenge indeed appeared slightly impaired during the first 30 minutes after injection; however, no obvious lingering sedative or anesthetic conditions were observed while performing the OFT and FST. In addition, since hyperlocomotion is known to affect immobility in the FST, we performed TST as an additional indicator in the ketamine dose-response study. Although also dependent on a motor readout, the TST avoids the need of swimming and has been suggested to be more relevant for the study of animals with compromised motor coordination (Cryan et al., 2005). Most importantly, stressed mice that received both ketamine and lithium treatment showed decreased immobility time in the FST but did not exhibit hyperlocomotion (Figures 2 and 5).
Compared with other published reports (Dehpour et al., 1995, 2002; Ghasemi et al., 2009), the serum lithium concentrations of stressed mice in our study were relatively low. We speculated that this discrepancy might be caused by restraint stress, which may reduce water consumption, or because of the difference in the experimental conditions (eg, sucrose in water) or animals. As expected, long-term treatment with either 600 or 1200mg/L of lithium alone produced no effects on behavioral tests and most of the biochemical measurements (including GSK-3β phosphorylation). This may explain why the potentiation effects of postketamine lithium were obscure when ketamine’s effects were still evident (1 week) but became obvious after ketamine’s effect subsided (2 weeks). In fact, our preliminary data showed that treatment with 2400mg/L of lithium not only produced a therapeutic serum concentration (0.897±0.250 mEq/L, n =8) in stressed mice but also decreased immobility time in the FST (data not shown).
Ketamine-induced increase in dendritic spine density in the medial PFC was identified dependent on activation of mTOR pathway (Li et al., 2010) and can be mimicked by GSK-3 inhibition (Liu et al., 2013). Studies have noted that the expression of both mTOR and P70S6K is decreased in the postmortem brain of depressed subjects (Jernigan et al., 2011), suggesting a compromised mTOR pathway. We observed a trend towards decreased phosphorylation of these 2 proteins (Figure 3a ) and a sustained reduction in dendritic spine density in the PFC of stressed mice (Figure 8). In addition, rats that suffered from chronic mild stress showed increased GSK-3β expression in the hippocampus (Silva et al., 2008), and elevated GSK-3 activity was found in postmortem samples from individuals with MDD (Karege et al., 2007). Consistent with previous hippocampal data (Omata et al., 2011), chronic restraint stress also significantly decreased GSK-3β phosphorylation in the PFC (Figure 3b ), and this effect lasted for at least 1 week after chronic restraint stress ended (Figure 7). These findings provide additional justification for the use of this restraint paradigm in the present study. It is interesting to note that the increased GSK-3β phosphorylation in rat PFC observed during ketamine’s rapid antidepressant actions was not affected by mTOR or P70S6K antagonists (Zhou et al., 2014), further supporting the notion that GSK-3β is an upstream regulator of mTOR.
BDNF plays a central role in synaptic plasticity and mediates the clinical efficacy of antidepressants and anxiolytic drugs. Ketamine-induced antidepressant-like effects were absent in BDNF knockout mice (Autry et al., 2011) and abolished by inhibitors of Akt or ERK; both are upstream regulators of mTOR (Li et al., 2010). We found that lithium treatment also potentiated and prolonged acute ketamine’s effect on phosphorylation of TrkB, Akt, and ERK in stressed mice, suggesting the activation of this pathway. By inhibiting GSK-3β, lithium was demonstrated to activate BDNF promoter IV in primary cortical neurons (Yasuda et al., 2009) and upregulate BDNF expression in the rat brain (Fukumoto et al., 2001). Presumably through BDNF-TrkB receptor signaling, lithium treatment may subsequently enhance activation of its downstream effectors Akt and ERK. Moreover, Akt mediates the phosphorylation of GSK-3β while ketamine exerts its rapid antidepressant effects (Zhou et al., 2014). By disrupting the formation of β-arrestin 2/protein phosphatase 2A (PP2A)/Akt complex that inactivates Akt, lithium was reported to activate Akt and in turn inhibit GSK-3 (Beaulieu et al., 2008). These findings suggest a positive feedback loop and indicate that mTOR may act as a node receiving multiple upstream regulatory effects induced by lithium.
Under nutrient-deprived stress conditions, activated eEF2 kinase was found to block protein translation by phosphorylating eEF2 (Carlberg et al., 1990). We similarly observed an increased eEF2 phosphorylation in stressed mice (Figure 3a ). Echoing this finding, other studies reported reductions in eEF2 phosphorylation in the PFC (Carrier and Kabbaj, 2013) and hippocampus (Autry et al., 2011) of rodents following ketamine administration. Moreover, ketamine-induced augmentation of BDNF synthesis was found to be eEF2 dependent (Monteggia et al., 2013), and inhibitors of eEF2 kinase produced a fast-acting antidepressant-like effect in mice (Autry et al., 2011). These results suggest that eEF2 is sensitive to stress and involved in mediating ketamine’s antidepressant-like effects. In contrast to eEF2 kinase, eEF2 can be positively regulated by dephosphorylation with PP2A (Nairn and Palfrey, 1987). Lithium was found to upregulate PP2A activity in the rat brain (Tsuji et al., 2003). Therefore, lithium may have multiple regulatory effects on eEF2 dephosphorylation in addition to activation of the mTOR/P70S6K signaling pathway that inhibits eEF2 kinase.
Oxidative stress plays a significant role in the pathogenesis of many neurological and psychiatric diseases such as schizophrenia and BD (Kuloglu et al., 2002). Elevated ROS production was observed from 10 minutes in mouse brains (da Silva et al., 2010) to 30 minutes in rat brains (de Oliveira et al., 2009) after acute injection of subanesthetic doses of ketamine. Consistently, we found that ROS production was significantly elevated 20 minutes after ketamine injection at a dose that produces antidepressant-like effects (Figure 4). Many studies have shown that restraint stress increases ROS formation (Fontella et al., 2005). Perhaps due to the variations in severity of the restraint stress or the oxidative metabolism markers measured, we did find a trend towards increased ROS production in the brain of stressed mice, but the effect was not statistically significant.
Lithium has been reported to ameliorate ROS levels in an animal model of mania (Frey et al., 2006) and suppress elevated oxidative metabolism markers, including thriobarbituric acid reactive substances and catalase in unmedicated manic patients (Machado-Vieira et al., 2007). The present study is the first to demonstrate that the oxidative stress induced by ketamine can be completely blocked by pretreatment with a low therapeutic dose of lithium (Figure 4), underscoring lithium’s neuroprotective aspects. Similar to our observations, ketamine was found to produce hyperlocomotion in rodents at doses related to its antidepressant effects (da Silva et al., 2010). Interestingly, antioxidant treatment inhibited ketamine-induced hyperlocomotion in mice (de Araujo et al., 2011). In our study, treatment with a low therapeutic dose of lithium prevented ketamine-induced hyperlocomotor activity (Figure 5c - d ). Although further experiments are needed, these data suggest that lithium’s antioxidant effects may be part of its underlying mechanisms. Taken together, the antioxidant effects of lithium provide an additional benefit and justification for its adjunctive use with ketamine in the treatment of depression.
In conclusion, the present findings highlight the ability of lithium—when given in conjunction with ketamine—to augment and prolong both clinical efficacy and remission in the treatment of depression. Numerous clinical and preclinical studies have underscored ketamine’s remarkable antidepressant effects. Nevertheless, given the inevitable relapse of depressive symptoms and the potential for abuse, ketamine is not a viable long-term clinical option. Thus, in providing a novel therapeutic strategy to solve this important clinical issue, our results could be of significant clinical relevance.
Supplementary Material
For supplementary material accompanying this paper, visit http://www.ijnp.oxfordjournals.org/
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services (IRP-NIMH-NIH-DHHS). The authors thank Ioline Henter of the NIMH, NIH, for critical review and editorial assistance with this manuscript.
References
- Adell A, Castro E, Celada P, Bortolozzi A, Pazos A, Artigas F. (2005). Strategies for producing faster acting antidepressants. Drug Discov Today 10:578–585. [DOI] [PubMed] [Google Scholar]
- American Psychiatric A. (2002). Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 159:1–50. [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. (2008). A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132:125–136. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. (2011). Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 16:1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Lucki I. (2013). Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg U, Nilsson A, Nygard O. (1990). Functional properties of phosphorylated elongation factor 2. Eur J Biochem 191:639–645. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. (2013). Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70:27–34. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Chuang DM. (2010). Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther 128:281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Wang Z, Hunsberger JG, Chuang DM. (2013). Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Pretty H, Hawton K, Geddes JR. (2005). Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry 162:1805–1819. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. (2005). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625. [DOI] [PubMed] [Google Scholar]
- da Silva FCC, do Carmo de Oliveira Cito M, da Silva MIG, Moura BA, de Aquino Neto MR, Feitosa ML, de Castro Chaves R, Macedo DS, de Vasconcelos SMM, de França Fonteles MM, de Sousa FCF. (2010). Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15. [DOI] [PubMed] [Google Scholar]
- de Araujo FY, de Oliveira GV, Gomes PX, Soares MA, Silva MI, Carvalho AF, de Moraes MO, de Moraes ME, Vasconcelos SM, Viana GS, de Sousa FC, Macedo DS. (2011). Inhibition of ketamine-induced hyperlocomotion in mice by the essential oil of Alpinia zerumbet: possible involvement of an antioxidant effect. J Pharm Pharmacol 63:1103–1110. [DOI] [PubMed] [Google Scholar]
- de Oliveira L, Spiazzi CM, Bortolin T, Canever L, Petronilho F, Mina FG, Dal-Pizzol F, Quevedo J, Zugno AI. (2009). Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog Neuropsychopharmacol Biol Psychiatry 33:1003–1008. [DOI] [PubMed] [Google Scholar]
- Dehpour AR, Farsam H, Azizabadi-Farahani M. (1995). Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology 34:115–121. [DOI] [PubMed] [Google Scholar]
- Dehpour AR, Sadr SS, Azizi MR, Namiranian K, Farahani M, Javidan AN. (2002). Lithium inhibits the development of physical dependence to clonidine in mice. Pharmacol Toxicol 90:89–93. [DOI] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA., Jr (2010). Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 71:1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Duman RS. (2013). Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 73:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontella FU, Siqueira IR, Vasconcellos AP, Tabajara AS, Netto CA, Dalmaz C. (2005). Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res 30:105–111. [DOI] [PubMed] [Google Scholar]
- Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J. (2006). Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31:326–332. [PMC free article] [PubMed] [Google Scholar]
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. (2001). Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 158:100–106. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. (2008). Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32:140–144. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Sadeghipour H, Poorheidari G, Dehpour AR. (2009). A role for nitrergic system in the antidepressant-like effects of chronic lithium treatment in the mouse forced swimming test. Behav Brain Res 200:76–82. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H. (2009). The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev 60:279–286. [DOI] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. (2006). Behavioral effects of ketamine and toxic interactions with psychostimulants. BMC Neurosci 7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr (2012). Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Wang PS. (2009). The STAR*D trial: revealing the need for better treatments. Psychiatr Serv 60:1466–1467. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. (2011). The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35:1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. (2007). Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry 61:240–245. [DOI] [PubMed] [Google Scholar]
- Kos T, Popik P, Pietraszek M, Schafer D, Danysz W, Dravolina O, Blokhina E, Galankin T, Bespalov AY. (2006). Effect of 5-HT3 receptor antagonist MDL 72222 on behaviors induced by ketamine in rats and mice. Eur Neuropsychopharmacol 16:297–310. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. (2003). NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl) 169:215–233. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D’Souza DC. (2005). Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry 62:985–994. [DOI] [PubMed] [Google Scholar]
- Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. (2002). Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct 20:171–175. [DOI] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. (2014) Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Translational psychiatry 4:e469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. (2013). GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 38:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, Jr., da Silva Vargas R, Kapczinski F, Portela LV, Souza DO, Salvador M, Gentil V. (2007). Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neurosci Lett 421:33–36. [DOI] [PubMed] [Google Scholar]
- Mantovani M, Pértile R, Calixto JB, Santos ARS, Rodrigues ALS. (2003). Melatonin exerts an antidepressant-like effect in the tail suspension test in mice: evidence for involvement of N-methyl-d-aspartate receptors and the l-arginine-nitric oxide pathway. Neurosci Lett 343:1–4. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Gideons E, Kavalali ET. (2013). The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn AC, Palfrey HC. (1987). Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem 262:17299–17303. [PubMed] [Google Scholar]
- Omata N, Chiu CT, Moya PR, Leng Y, Wang Z, Hunsberger JG, Leeds P, Chuang DM. (2011). Lentivirally mediated GSK-3beta silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice. Int J Neuropsychopharmacol 14:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Kos T, Sowa-Kucma M, Nowak G. (2008). Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl) 198:421–430. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Krishna G, Imarisio S, Saiki S, O’Kane CJ, Rubinsztein DC. (2008). A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet 17:170–178. [DOI] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OF, Sousa N. (2008). Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience 152:656–669. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Morinobu S, Tanaka K, Kawano K, Yamawaki S. (2003). Lithium, but not valproate, induces the serine/threonine phosphatase activity of protein phosphatase 2A in the rat brain, without affecting its expression. J Neural Transm 110:413–425. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. (2001). Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J 20:4370–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang D-M. (2009). The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 14:51–59. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864. [DOI] [PubMed] [Google Scholar]
- Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, Zhou ZQ. (2014). Akt Mediates GSK-3beta phosphorylation in the rat prefrontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation 21:183–188. [DOI] [PubMed] [Google Scholar]
- Zuo DY, Wu YL, Yao WX, Cao Y, Wu CF, Tanaka M. (2007). Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol Biochem Behav 86:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.