Abstract
Background:
Accumulating evidence supports a role for the immune system in the pathogenesis of Parkinson’s disease. Importantly, recent preclinical studies are now suggesting a specific contribution of inflammation to the α-synuclein-induced pathology seen in this condition.
Methods:
We used flow cytometry and western blots to detect toll-like receptor 2 and 4 expression in blood and brain samples of Parkinson’s disease patients and mice overexpressing human α-synuclein. To further assess the effects of α-synuclein overexpression on the innate immune system, we performed a longitudinal study using Thy1.2-α-synuclein mice that expressed a bicistronic DNA construct (reporter genes luciferase and green fluorescent protein) under the transcriptional control of the murine toll-like receptor 2 promoter.
Results:
Here, we report increases in toll-like receptors 2 and 4 expression in circulating monocytes and of toll-like receptor 4 in B cells and in the caudate/putamen of Parkinson’s disease patients. Monthly bioluminescence imaging of Thy1.2-α-synuclein mice showed increasing toll-like receptor 2 expression from 10 months of age, although no change in toll-like receptor 2 and 4 expression was observed in the blood and brain of these mice at 12 months of age. Dexamethasone treatment starting at 5 months of age for 1 month significantly decreased the microglial response in the brain of these mice and promoted functional recovery as observed using a wheel-running activity test.
Conclusion:
Our results show that toll-like receptors 2 and 4 are modulated in the blood and brain of Parkinson’s disease patients and that overexpression of α-synuclein leads to a progressive microglial response, the inhibition of which has a beneficial impact on some motor phenotypes of an animal model of α-synucleinopathy.
Keywords: α-synuclein, microglia, dexamethasone, TLR2, TLR4, bioluminescence imaging
Introduction
In recent years, it has become clear that Parkinson’s disease (PD) pathophysiology involves aspects of the immune response as evidenced by changes in the blood and central nervous system. It has in fact long been recognized that PD brains are characterized by the presence of a microglial reaction (McGeer et al., 1988; McGeer and McGeer, 2004), which increases with disease duration (Croisier et al., 2005). Positron emission tomography studies have similarly reported increased microglial responses in early stages of the disease, correlating with severity of motor impairments (Ouchi et al., 2005). More targeted studies have revealed a possible role for toll-like receptors (TLRs) in the pathological processes underlying neurodegenerative disorders, including PD (Panaro et al., 2008; Ros-Bernal et al., 2011; Cote et al., 2011; Drouin-Ouellet et al., 2011; Drouin-Ouellet and Cicchetti, 2012; Kim et al., 2013) For example, α-synuclein (αSyn) can act as a damage-associated molecular patterns for TLR2 (Kim et al., 2013) while TLR4 activation promotes microglial αSyn clearance in α-synucleinopathies (Stefanova et al., 2011).
TLRs are pattern recognition receptors that not only play a key role in the innate immune response to micro-organisms, but also to tissue injury. Upon stimulation, TLRs trigger signalling pathways that result in the activation of NF-κB, p38 mitogen-activated protein kinase, and Jun-N-terminal kinase (JNK) (Dunne and O’Neill, 2003; West et al., 2006). These pathways in turn recruit adaptor proteins, the most common being myeloid differentiation primary response gene (88) (MyD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF).
In PD, there is a degeneration of the nigrostriatal dopaminergic pathway with formation of Lewy bodies, which together form the pathological hallmarks of the disease (Lang and Lozano, 1998). Lewy bodies are composed of protein aggregates, of which a main constituent is αSyn (Spillantini et al., 1997). The involvement of αSyn to PD pathogenesis is further supported by the fact that overexpression of this protein, as a consequence of either a duplication or triplication of the gene, leads to early-onset PD (Singleton et al., 2003; Chartier-Harlin et al., 2004). While this protein may directly drive pathology within the affected neurons, in vitro and in vivo observations also suggest that αSyn can directly activate microglia. In particular, aggregated forms of this protein secreted by neurons of the substantia nigra (SN) could do this, leading to the production of proinflammatory mediators and thereby exacerbating neuronal death (Zhang et al., 2005; Su et al., 2008; Theodore et al., 2008; Lee et al., 2010).
Although the contribution of TLRs to the pathology of αSyn is not clear, a few reports have revealed an upregulation of TLRs in animal models of PD and more specifically in models of α-synucleinopathies such as the (thy1)-[A30P]-αSYN, the (PLP)-αSYN, and Thy1.2-αSyn mice (Stefanova et al., 2007; Letiembre et al., 2009; Watson et al., 2012). More recently, it was shown that the depletion of the TLR2 gene in primary microglial cultures can prevent the induction of proinflammatory factors upon exposure to αSyn and that certain forms of αSyn oligomers could trigger microglial activation by acting as endogenous ligands of this receptor (Kim et al., 2013). This increased microglial proliferation and production of cytokines induced by the neuronal release of αSyn has further been demonstrated to be under the control of TLR2 (Kim et al., 2014). Additionally, TLR4 has been implicated in αSyn-induced inflammatory responses and is suggested to be a mediator of the microglial phagocytosis of αSyn in a model of multiple system atrophy (Stefanova et al., 2011). In fact, multiple system atrophy patients express elevated levels of TLR4 mRNA in the SN, striatum, cortex, and nucleus dentatus (Brudek et al., 2013).
A few studies have also shown an increased expression of TLRs in toxin-induced animal models of PD (Panaro et al., 2008; Ros-Bernal et al., 2011), while a single study has reported increased expression of TLR9 in the striatum of PD patients (Ros-Bernal et al., 2011), although the expression of other TLRs was not investigated. In previous work, we have explored the role of the MyD88-dependant pathways in the context of toxin-induced animal models of PD (Cote et al., 2011; Drouin-Ouellet et al., 2011; Drouin-Ouellet and Cicchetti, 2012). These studies revealed an important dichotomy for the role of MyD88 in dopaminergic cell death in the enteric vs central nervous system (Cote et al., 2011; Drouin-Ouellet et al., 2011). Building on our previous observations, we wanted to further understand the role of the immune system driven by the TLR2 and TLR4 pathways, and we have done this in PD patients and a transgenic αSyn mouse model that expressed the main pathological hallmarks of the disease, that is αSyn, but that also equated, given its subtle dopaminergic neuronal death, premanifest PD.
Methods
Human Study
Study Population
Local regional ethical approval was given for blood sampling (REC 03/303) from patients with PD and controls and was undertaken in accordance with the Declaration of Helsinki. Twelve patients with idiopathic PD were recruited from the PD research clinic at the John van Geest Centre for Brain Repair (Cambridge, UK), and age-matched healthy controls (n=9) were recruited at the Herchel Smith Building for Brain and Mind Sciences (Cambridge, UK) (Table 1). All individuals involved provided informed consent, and none of the subjects included in the study were on anti-inflammatory drug treatment.
Table 1.
Demographic of Individuals Providing Blood and Postmortem Brain Samples
| Blood samples | ||||||
|---|---|---|---|---|---|---|
| Age (y) | Gender | Age (y) | Gender | Disease duration (y) | ||
| Control cases | 74 | F | PD cases | 80 | M | >1 |
| 75 | F | 67 | M | 3 | ||
| 61 | F | 81 | M | 3 | ||
| 77 | F | 70 | M | >1 | ||
| 72 | F | 64 | M | >1 | ||
| 60 | F | 82 | F | 3.5 | ||
| 69 | F | 77 | M | 9 | ||
| 64 | F | 71 | M | 1 | ||
| 77 | M | 65 | M | 3 | ||
| 66 | F | 4 | ||||
| 75 | F | 4 | ||||
| 76 | F | 10 | ||||
| Mean±SD | 69.9±6.7 | 72.8±6.5 | ||||
| M:F ratio | 1:8 | 8:4 | ||||
| Postmortem samples | ||||||
| Age (y) | Gender | Age (y) | Gender | Disease duration (y) | ||
| Control cases | 88 | M | PD cases | 79 | M | 12 |
| 61 | F | 75 | M | NK | ||
| 61 | M | 75 | F | 10 | ||
| 75 | M | 80 | M | 19 | ||
| 61 | F | 78 | F | 19 | ||
| 67 | M | 82 | M | 11 | ||
| 75 | M | 8 | ||||
| Mean±SD | 68.8±10.9 | 77.4±2.8 | ||||
| M:F ratio | 4:2 | 5:2 | ||||
Abbreviations: F, female; M, male; NK, not known; PD, Parkinson’s disease; SD, standard deviation.
Isolation of PBMCs and Flow Cytometry
Peripheral blood mononuclear cells (PBMCs) were isolated from 12 mL of peripheral venous blood samples collected in K3EDTA tubes (BD vacutainer) by centrifugation using a Ficoll-Paque gradient and were frozen at −80°C. Prior to flow cytometry analyses, cells were thawed, and PBMCs subtypes were distinguished using the following antibodies: anti-CD45-Pacific orange (Invitrogen), anti-CD3-V450 (BD Biosciences), anti-CD4-PE-texas red (Invitrogen), anti-CD8-APC-H7 (BD Biosciences), anti-CD19-PE-Cy7 (BD Biosciences), anti-CD14-PerCP-Cy5 (BD Biosciences), anti-CD16-FITC (BD Biosciences), anti-TLR2-PE (eBiosciences), and anti-TLR4-APC (eBiosciences) (all made in mouse). Data acquisition was performed using a BD LSR II flow cytometer, and the results were analyzed with BD eDiva Software (version 6.1.2, BD Biosciences) and FCS Express 4 Flow Cytometry (De Novo Software).
Western Blots on Brain Samples
Human brain tissue was obtained from the Queens Square UK Brain Bank and used under local regional ethical approval (REC 01/177). Frozen samples of caudate/putamen from idiopathic PD patients (n=7) and age- and gender-matched healthy controls (n=6) were used. All patients had been diagnosed according to the diagnostic criteria of the UK PD Society Brain Bank (Hughes et al., 1992). An amount of 50 to 100mg of caudate/putamen per case was sequentially homogenized in Tris-buffered saline (TBS) and lysis buffer as previously described (Tremblay et al., 2007, 2011). Protein concentration was determined using a bicinchoninic acid assay (Pierce). Proteins (20 µg/sample) were subsequently heated at 95°C for 5 minutes in Laemmli’s loading buffer and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis on a 10% polyacrylamide gel before being transferred to a polyvinylidene fluoride membrane (Immobilon-P, Millipore Corporation). The targeted proteins were detected using the following primary antibodies: anti-TLR2 (Abcam), anti-TLR4 (Abcam), anti-MyD88 (Abcam), anti-TRIF (Abcam), anti-p65 (Santa-Cruz Biotechnology, Inc.) (all 1:1000 and all made in rabbit). Membranes were also probed for β-actin (1:10,000; Applied Biological Materials) as a control for protein load. This was followed by incubation with horseradish peroxidase-coupled secondary antibody (Jackson Immunoresearch) and chemiluminescence reagent (Luminata Western HRP Substrate, EMD Millipore). Chemiluminescence was measured using a KODAK Imaging Station 4000 MM Digital Imaging System (Molecular Imaging Software version 4.0.5f7; Carestream Healthand a myECL Imager (Thermo Scientific)). Immunoblot band intensity was quantified with ImageJ Analysis Software (National Institutes of Health, http://imagej.nih.gov/ij).
Mouse Study
Thy1.2-aSyn×TLR2-GFP-Fluc Mouse Model
All animal experiments were performed in accordance with the Canadian Guide for the Care and Use of Laboratory Animals, and the Institutional Animal Care Committee of Laval University approved all procedures. Animals were bred and housed in standard laboratory conditions in a controlled-temperature environment with a 12-hlight/dark cycle with free access to food and water. Adult male TLR2-green fluorescent protein (GFP)-firefly-luciferase (fluc) (that do not express the human αSyn transgene; hαS-/-) mice on a C57BL/6 background (i.e, backcrossed with C57BL/6 mice for at least 9 generations) were crossed with adult female Thy1.2-αSyn mice overexpressing human wild-type αSyn (line 61 developed previously by the laboratory of Dr. E. Masliah at UCSD; hαS+/-) kept on a fully backcrossed C57BL/6 background. Transgenic TLR2-GFP-fluc mice bear a bicistronic DNA construct (reporter genes fluc and GFP), which is under the transcriptional control of the murine TLR2 promoter and enables an in vivo read out of the microglial response (Lalancette-Hebert et al., 2009). Transgenic TLR2-GFP-fluc, Thy1.2-αSyn, and TLR2-GFP-fluc×Thy1.2-αSyn mice were identified by polymerase chain reaction detection of luciferase and human αSyn. Given that males and females did not show differences in any of the analyses performed, both were included in the study.
Bioluminescence Imaging
TLR2-GFP-fluc mice (hαS-/- and hαS-/+) were scanned every month from the age of 2 to 12 months. Images were collected using an IVIS 200 Imaging System (CaliperLS-Xenogen) as previously described (Lalancette-Hebert et al., 2009; Drouin-Ouellet et al., 2011).
Dexamethasone Administration
Both non-transgenic×TLR2-GFP-fluc and Thy1.2-αSyn×TLR2-GFP-fluc mice received a daily subcutaneous injection of dexamethasone (3mg/kg, Sigma-Aldrich) for 30 days starting at 5 months of age (n=7–8 animals/group). This age was chosen because by this time, Thy1.2-αSyn mice exhibit an αSyn-associated pathology as well as brain microglial activation (Chesselet et al., 2012; Watson et al., 2012). Control groups of each genotype were given vehicle (saline 0.9%) instead of dexamethasone.
Behavioral Tests
Behavioral tests were conducted in mice assigned to the dexamethasone protocol at the end of the treatment course. These behavioral measures were collected using the open-field and wheel activity systems.
Open field: Locomotor activity was evaluated using an open field system (San Diego Instruments) consisting of 10 Plexiglas chambers (40×40cm) during a 1-hour session. Horizontal voluntary fine and ambulatory movements, distance traveled, time spent in each delimited space, and the number of entries into each of these areas was detected using a photobeam activity system.
Wheel activity system: Spontaneous running activity was evaluated during the active cycle of the mouse using activity wheels, as previously described (Drouin-Ouellet et al., 2012).
Flow Cytometry Analyses
Blood was collected through the submandibular vein into heparin tubes. Twenty-five µL of total blood was mixed with rat anti-mouse CD16/CD32 (2.4G2) in 75 µL of phosphate buffered saline (PBS) and incubated on ice for 15 minutes to block Fc receptors. The blood was stained with the following antibodies for 30 minutes: rat anti-CD45-V500 (BD Biosciences), rat anti-CD3-APC (eBiosciences), rat anti-CD4-V450 (BD Biosciences), rat anti-CD8a-APC-Cy7 (BD Biosciences), mouse anti-CD19-PE (eBiosciences), rat anti-CD115-APC (eBiosciences), rat anti-CD11b-APC-Cy7 (BD Biosciences), rat anti-Ly-6c-V500 (BD Biosciences), rat anti-Ly-6G-V450 (BD Biosciences), mouse anti-TLR2-PE-Cy7 (eBiosciences), and mouse anti-TLR4-AF488 (Life Technologies). Red blood cells were then lysed with 1mL of BD Pharm Lyse lysing solution (BD Biosciences) for 15 minutes and washed with PBS. Data acquisition and analyses were performed as for the human study.
Brain Tissue Preparation
Animals were sacrificed under deep anesthesia with ketamine/xylazine and perfused using a transcardiac infusion of PBS 1X (BioShop) containing protease (Sigma-Aldrich) and phosphatase inhibitors (sodium pyrophosphate 1mM and sodium fluoride 50mM). Brains were collected and either post-fixed in a solution containing 4% paraformaldehyde (pH 7.4) in PBS for 48 hours and subsequently cryoprotected using a 20% sucrose solution or snap-frozen and then stored at −80°C. Post-fixed coronal brain sections of 25-µm thickness were cut using a freezing microtome (Leica Microsystems). Samples of the striatum were extracted for western blot analyses.
Western Blots
Mouse striatum samples were homogenized in 8 volumes of TBS and radioimmunoprecipitation assay buffer lysis buffer using the same method as described in the human study sections, and proteins were detected using the same antibodies.
Immunofluorescence
Fluorescent immunostaining was used to visualize human αSyn and microglia. For αSyn staining, free-floating sections were treated with citrate buffer, pH 6.0, at 85°C for 15 minutes and left to cool down at room temperature (RT) for an additional 15 minutes. For visualization of proteinase K-resistant aggregates, sections were incubated in proteinase K (10 µg/mL) for 8 minutes at RT following previously published protocols (Fernagut et al., 2007). Sections were blocked for 30 minutes. After overnight incubation at 4°C with either a human specific mouse anti-αSyn antibody [Syn211] (1:100; Abcam) or a rabbit anti-Iba1 (ionized calcium binding adaptor molecule 1; 1:1000; Wako Pure Chemicals Industries), sections were incubated for 2 hours at RT in a solution containing the secondary antibody donkey Alexa Fluor 488-conjugated anti-mouse (1:500; Invitrogen) or the donkey Alexa Fluor 546-conjugated anti-rabbit. Sections were subsequently placed in a solution containing 4′,6-diamidino-2-phenylindole (0.022%) for 7 minutes at RT, mounted on slides, and coverslipped. Photomicrographs were taken with the Simple PCI version 6.0 (Hamamatsu) software linked to a Nikon eclipse i90 microscope (Nikon Instruments).
Statistical Analysis
Statistical analysis was performed using PRISM 6 (Graphpad Software). All data are expressed as group means±SEM. The analyses were carried out using an unpaired Student’s t test and one- and two-way analysis of variance followed by the appropriate posthoc test (eg, Tukey). In case of unequal variance, an unpaired t test with Welch correction was done. Normality was evaluated using a d’Agostino-Pearson omnibus normality test and in case a Gaussian distribution could not be assumed, data were transformed or a nonparametric test was used. Statistical significance was determined at an α level of 0.05.
Results
TLR2 and TLR4 Expression in Peripheral Immune Blood Cells of PD Patients
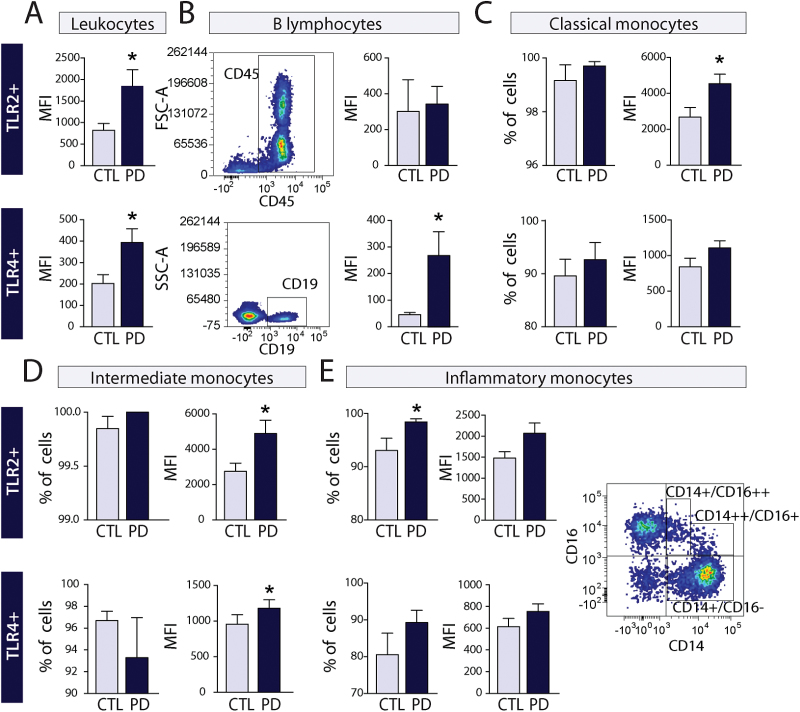
Flow cytometry analyses were performed on PBMCs isolated from PD patients with a disease duration of 10 years or less (Table 1). TLR2 mean fluorescence intensity (MFI; i.e., expression levels per cell) was increased in CD45+ leukocytes of PD patients compared to age-matched controls (P<.05) (Figure 1A). TLR2 MFI was similar to control samples in B lymphocytes (CD45+/CD19+) (Figure 1B), T helper lymphocytes (CD45+/CD3+/CD4+), and T cytotoxic lymphocytes (CD45+/CD3+/CD8+) (data not shown).
Figure 1.
Toll-like receptor (TLR)2 and TLR4 expression in peripheral blood cells of Parkinson’s disease (PD) patients. (A) Quantification of mean fluorescence intensity (MFI) of TLR2 and TLR4 shows an increase of CD45+ leukocytes in PD patients (n=12) vs controls (CTL) (n=9). (B-E) Subpopulation analysis reveals an increase in TLR4 MFI in CD45+/CD19+ B cells and of both receptors in CD14+ monocytes. Gating strategy is shown for each cell type analyzed. All data are expressed as group means±SEM. Statistical analyses were performed using an unpaired Student’s t test with a Welch correction in case of unequal variance. *P<.05.
TLR2 MFI was increased in the classical (CD45+/CD14++/CD16−; P<.05) and intermediate monocytes (CD45+/CD14++/CD16+; P<.05), but only a trend towards an increase was observed in inflammatory monocytes (CD45+/CD14+/CD16++; P=.087) (Figure 1C-E). In contrast, while the percentage of classical and intermediate monocytes was similar to controls, a significant increase in inflammatory monocytes expressing TLR2 (5.44%; P<.05) was found in PD blood samples (Figure 1E). Classification of blood monocytes follows a time course of activation (Ziegler-Heitbrock and Hofer, 2013) for which classical monocytes make up the vast majority of monocytes under physiological conditions, and which are associated with a resting state Intermediate monocytes exhibit a phenotype between classical and proinflammatory with increased antigen presenting activity (Grage-Griebenow et al., 1993) as well as higher proangiogenic capacity (Ziegler-Heitbrock et al., 1994). In contrast, functional studies have revealed that inflammatory monocytes produce higher levels of the inflammatory cytokines tumor necrosis factor-α (Belge et al., 2008) and interleukin (IL)-12 (Szaflarska et al., 2004).
As for TLR2, TLR4 MFI was increased in leukocytes (P<.05) (Figure 1A). TLR4 MFI assessment of populations of lymphocytes revealed an increase in B lymphocytes (P<.05; Figure 1B) but not in the populations of T lymphocytes assessed (T helper [CD45+/CD3+/CD4+] and T cytotoxic [CD45+/CD3+/CD8+]), which had similar MFI levels to control samples. Intermediate monocytes showed a significant increase (P<.05; Figure 1D), and a strong trend towards an increased TLR4 MFI was observed in classical monocytes (P<.057; Figure 1C). In contrast to TLR2, there was no difference between PD and controls in the number of inflammatory monocytes expressing TLR4 (Figure 1E). Changes observed in both TLR2 and TLR4 MFI in leukocytes of PD patients seem to be largely due to an increase in the expression of these receptors in monocytes.
TLR2 and TLR4 Expression in the Caudate/Putamen of PD Patients
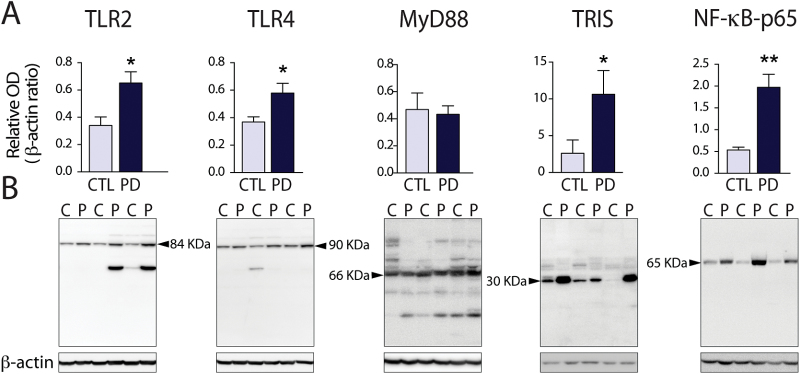
We next measured the expression of the proteins involved in TLR2- and TLR4-mediated signalling pathways in the striatum of PD patients and compared them to age- and gender-matched controls (Table 1). While both TLR2 and TLR4 protein expression were increased in PD patients (P<.05), the expression level of their common adaptor protein, MyD88, was unchanged (Figure 2A-B). TRIS- a 30-KDa splice variant of TRIF that is involved in TLR3- and TLR4-mediated signalling by interacting with TRIF to activate NF-κB, the interferon response element, and the IFNγ promoter (Han et al., 2010) was detected using an antibody against the N-terminal region of TRIF and showed a 4-fold increase in the caudate/putamen of PD patients (P<.05). Finally, NF-κBp65 (RelA), a subunit of the NF-κB transcription complex that is activated upon induction of TLR2 and TLR4, was also increased by 4-fold (P<.01) (Figure 2A). Taken together, these results reveal that TLR2 and TLR4, as well as some of their signalling-associated molecules, are increased in the caudate/putamen of PD patients.
Figure 2.
Toll-like receptor (TLR)2 and TLR4 expression in the brain of Parkinson’s disease (PD) patients. (A-B) Western blot quantification of TLR2, TLR4, and molecules involved in their downstream pathways (myeloid differentiation primary response gene (88) [MyD88], TIR-less splice variant of TIR-domain-containing adapter-inducing interferon-β [TRIS], nuclear factor κB [NF-κB]in the striatum of PD patients (n=7) compared to age- and gender-matched controls (CTL or C) (n=6). Data are expressed as a ratio over β-actin. All data are expressed as group means±SEM. Statistical analyses were performed using an unpaired Student’s t test with a Welch correction in case of unequal variance. *P<.05; **P<.01.
Generation of the Thy1.2-αSyn×TLR2-GFP-fluc Mouse Model
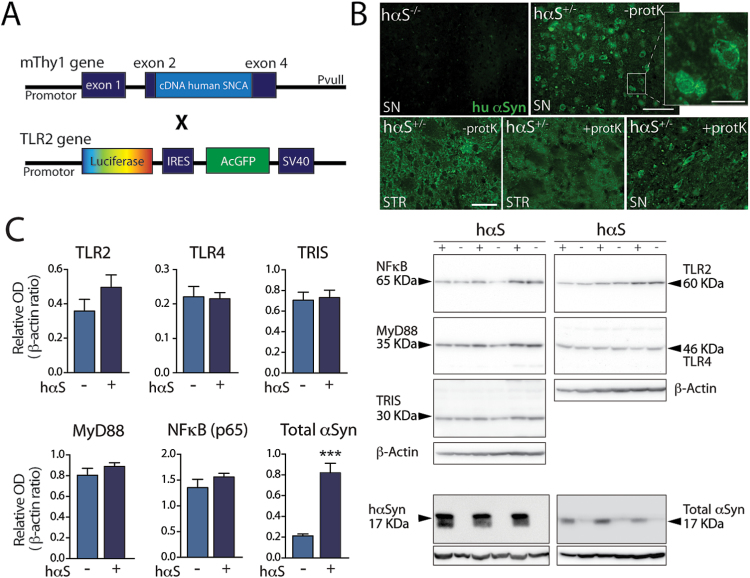
To better understand the relationship between TLR2 and TLR4 increased expression levels and the pathology created by an overexpression of αSyn, we crossed Thy1.2-αSyn with TLR2-GFP-fluc mice (Figure 3A). In accordance with the Thy1.2-αSyn mouse model (Chesselet et al., 2012), Thy1.2-αSyn×TLR2-GFP-fluc mice overexpressed hαSyn in several structures of the brain, including the SN and the striatum at 12 months of age. However, no overt gliosis could be detected at 6 months of age in either of these structures. In addition, proteinase K-resistant hαSyn aggregates were observable in these two structures at 12 months of age (Figure 3B).
Figure 3.
Toll-like receptor (TLR)2 and TLR4 expression in the brain of Thy1.2-α-synuclein (αSyn)× TLR2-GFP-fluc mice. Schematic diagram of the Thy1.2-αSyn (Rockenstein et al., 2002) and TLR2-GFP-fluc (Lalancette-Hebert et al., 2009) constructs that were used to generate the Thy1.2-αSyn×TLR2-GFP-fluc mice. (B) Immunofluorescent staining of human αSyn (hαSyn) showing none in a littermate nontransgenic for the hαSyn gene and strong expression in the transgenic mouse. Treatment with Proteinase K confirms the presence of αSyn in the substantia nigra of Thy1.2-αSyn×TLR2-GFP-fluc mice. (C) Western blot quantification of TLR2, TLR4, and molecules involved in their pathways (myeloid differentiation primary response gene (88) [MyD88], TIR-less splice variant of TIR-domain-containing adapter-inducing interferon-β [TRIS], Nuclear factor κB [NF-κB]-p65), as well as hαSyn and total αSyn, in the striatum of Thy1.2-αSyn×TLR2-GFP-fluc mice (n=9) compared to wild type×TLR2-GFP-fluc littermates (n=9) at 12 months of age. Western blot data are expressed as a ratio over β-actin. All data are expressed as group means±SEM. Statistical analyses were performed using an unpaired Student’s t test with a Welch correction in case of unequal variance. hαSyn-/-, TLR2-GFP-fluc mice; hαSyn-/-, Thy1.2-αSyn×TLR2-GFP-fluc mice; SNCA, synuclein, Alpha (Non A4 Component Of Amyloid Precursor); TRIS, TIR-less splice variant of TIR-domain-containing adapter-inducing interferon-β (TRIF). Scale bar=100 µm, inset=25 µm, lower row=50 µm.
TLR2 and TLR4 Expression in Peripheral Immune Blood Cells and the Striatum of Thy1.2-αSyn × TLR2-GFP-fluc Mice
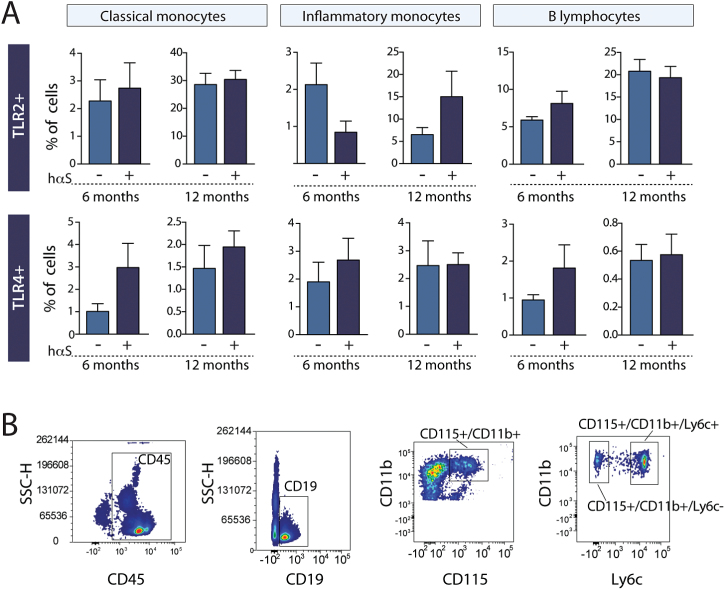
Following our observations of increased expression of TLR2 and TLR4 in the blood and caudate/putamen of PD patients, we set out to evaluate whether such changes were also present in our Thy1.2-αSyn×TLR2-GFP-fluc mouse model in late stage disease. In contrast to PD patients, we did not observe changes in TLR2, TLR4, MyD88, TRIS, and NF-κB p65 protein expression in the striatum of these mice despite a 3.9-fold overexpression of monomeric αSyn protein in that structure (Figure 3C). Similarly, no change in the percentage of TLR2 and TLR4 was detected in classical monocytes (CD115+/CD11b+/Ly6c−), inflammatory monocytes (CD115+/CD11b+/Ly6c+) or B lymphocytes (CD45+/CD19+) (Figure 4A-B).
Figure 4.
Toll-like receptor (TLR)2 and TLR4 expression in peripheral blood cells of Thy1.2-αSyn×TLR2-GFP-fluc mice. (A) Quantification of mean fluorescence intensity (MFI) of TLR2 and TLR4 shows no change in CD45+/CD19+ B cells or in CD115+/CD11b monocytes (n=8–9 mice per group). (B) Gating strategy is represented for each cell type assessed using flow cytometry. All data are expressed as group means ±SEM. Statistical analyses were performed using an unpaired Student’s t test with a Welch correction in case of unequal variance. hαS, human α-synuclein.
Progressive Microglial Activation in Thy1.2-αSyn×TLR2-GFP-fluc Mice
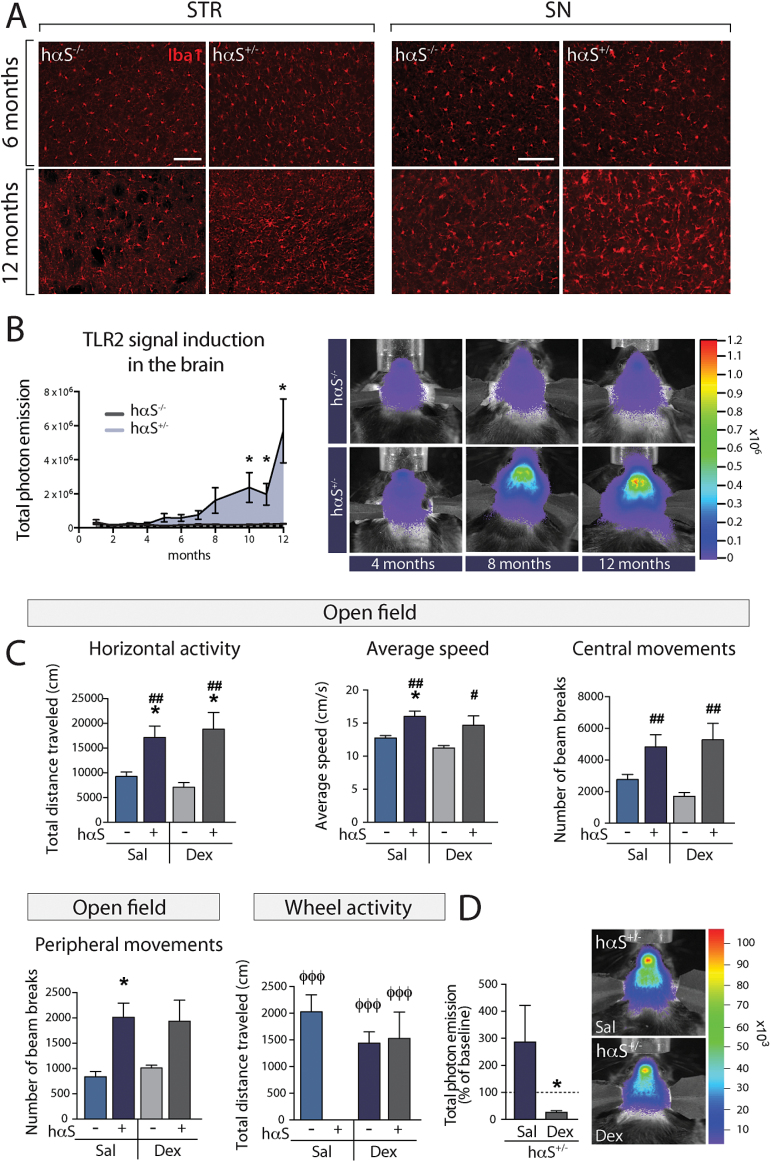
Similarly to previous reports that found a striatal microglial response in the Thy1.2-αSyn mice as early as 1 month of age and at 5 to 6 months of age in the SN (Watson et al., 2012), we observed microgliosis in the striatum of 12-month-old Thy1.2-αSyn×TLR2-GFP-fluc mice, as well as in the SN, although to a lesser extent (Figure 5A). We thus sought to follow the progression of the microglial response using in vivo bioluminescence detection of TLR2 mRNA signal, which has been shown to correlate with the microglial response (Lalancette-Hebert et al., 2009). Thy1.2-αSyn×TLR2-GFP-fluc mice were imaged every month from early adulthood (2 months) and although a strong TLR2 signal could be detected as early as 8 months, this increase became statistically significant from 10 months of age onwards (Figure 5B).
Figure 5.
Microglial response in the Thy1.2-αSyn×TLR2-GFP-fluc mouse model and its impact on motor deficits. (A) Immunofluorescent staining of Iba1 (microglia) showing a strong microglial response in the striatum and substantia nigra (SN) of the Thy1.2-αSyn transgenic mouse as compared to non-transgenic littermates at 12 months, but not at 6 months. (B) Longitudinal measurements of the brain microglial response using bioluminescent imaging displayed a significant increase in the toll-like receptor (TLR)2 signal at 10 months onwards (n=3–21 per group). (C) The decrease in total distance traveled in a 12-hour nocturnal session of wheel activity was reversed with dexamethasone treatment (n=7–8 per group). Locomotor activity assessed in a 1-hour session using the open field test confirms hyperactivity in Thy1.2-αSyn × TLR2-GFP-fluc mice, as evidenced by an increase in total distance traveled, average speed, as well as central and peripheral movements. However, these motor features were not reversed by dexamethasone. All data are expressed as group means ± SEM. Statistical analyses were performed using a 2-way ANOVA. P<.05 compared with saline-treated TLR2-GFP-fluc mice; #P<.05 and ##P<.01 compared with dexamethasone-treated TLR2-GFP-fluc mice; φφφ = p < .001 compared with dexamethasone-treated Thy1.2-αSyn × TLR2-GFP-fluc mice. (D) Bioluminescent imaging corroborates a decrease in TLR2 signal in Thy1.2-αSyn×TLR2-GFP-fluc mice that received dexamethasone treatment (n=6 per group). Statistical analyses were performed using Mann-Whitney U test. *P<.05. hαS-/-, TLR2-GFP-fluc mice; hαS-/+, Thy1.2-αSyn x TLR2-GFP-fluc mice; Dex, dexamethasone; Sal, saline. Scale bar (A) = 100 µm.
Effect of an Anti-inflammatory Drug Treatment on Motor Impairments
To evaluate the contribution of the observed inflammatory response in this mouse model, we administered dexamethasone, which has long been used clinically for the treatment of neuroinflammation (Anderson and Cranford, 1979; Norris and Hachinski, 1986). Thy1.2-αSyn×TLR2-GFP-fluc mice traveled very little distance as measured in the wheel activity test as compared to wild-type littermates (15.37±11.76 cm vs 2502± 409.2 cm). Although daily dexamethasone treatment starting at 5 months of age did not yield any changes in the expression of TLR2, TLR4, and NF-κB (data not shown), the treatment prevented deficits in nocturnal wheel activity but not in hyperactivity in the open field (Figure 5C). The inhibition of the microglial response was confirmed in vivo using bioluminescence imaging following the last injection of dexamethasone, revealing a decrease of TLR2 signal in Thy1.2-αSyn× TLR2-GFP-fluc mice that received dexamethasone (Figure 5D). Taken together, these results suggest that the microglial response could impact some aspects of the motor phenotype induced by the neuronal overexpression of hαSyn.
Discussion
Very few studies have examined the role of innate immunity in PD. Previously, we have demonstrated that the MyD88-dependent pathway significantly contributes to MPTP-induced toxicity in the enteric nervous system (Cote et al., 2011) but not in the brain (Drouin-Ouellet et al., 2011), uncovering a different role of MyD88 in the MPTP-mediated neuronal loss in the enteric vs central nervous system. Building on these observations, we sought to investigate the role of specific TLRs (2 and 4) in PD using blood samples of early-stage patients and their respective controls as well as postmortem brain tissue samples. Having established the various expression patterns for these receptors in both blood and brain, we crossed mice overexpressing the hαSyn protein with a TLR2-GFP-fluc mouse, allowing us to follow the inflammatory response with the development of an αSyn pathology by live bioluminescence imaging. Despite the fact that we do not have evidence for a direct interaction between αSyn and TLRs, this in vivo study enabled us to follow the progression of TLR2 expression in vivo and target the inflammatory response using the anti-inflammatory drug dexamethasone, which restored some of the behavioral deficits characterizing this mouse model of α-synucleinopathy.
An important new finding reported here is the expression patterns of TLR2 and 4 on blood cells. The pattern of TLR expression that we report in PD blood samples is at 2 distinct levels: 1) the changes in expression were assessed as the total percentage of cells expressing a specific receptor as well as 2) the levels of TLR expression within a single cell. Although the current literature presents inconsistent results regarding changes in circulating blood cells, some populations (including CD4+, CD8+ T cells, and B cells) (Bas et al., 2001; Baba et al., 2005; Niwa et al., 2012; Stevens et al., 2012) are characterized by changes in their total number in sporadic forms of PD. Very few studies have assessed changes in the number of monocytes, but one has described an increase, whereas the other has reported no change (Stevens et al., 2012; Funk et al., 2013).
A recent study has further highlighted the importance of monocytes in PD by reporting a monocyte-specific expression quantitative trait locus among PD variants (Raj et al., 2014). In our study, we found increased TLR4 expression on intermediate monocytes in PD patients, although the significance of this to disease pathogenesis is unclear. Of interest, IL-1 has been shown to activate monocytes via TLR4 (Ward et al., 2009), and elevated IL-1 levels have been reported in the cerebrospinal fluid of PD patients (Blum-Degen et al., 1995). We also observed that TLR2 expression is increased on monocytes of PD patients, and previously others have shown that monocytes collected from sporadic PD cases express higher levels of αSyn, which correlate with defective phagocytosis (Gardai et al., 2013). While αSyn has been demonstrated to activate microglia via TLR2, it is not known whether the same phenomenon occurs in the blood, but this could certainly contribute to the inflammatory response observed in PD patients.
Some studies have also reported a reduction in B lymphocytes in the blood of PD patients (Bas et al., 2001; Niwa et al., 2012; Stevens et al., 2012). The most important function of TLR4 in B lymphocytes in inflammatory disease is its ability to decrease the production of the anti-inflammatory cytokine IL-10 (Jagannathan et al., 2009), which is elevated in PD (Rentzos et al., 2009). It has been reported that initial Levodopa treatment impacts the total number of CD19+ B cells, although the cumulative effect of treatment was not significant (Bas et al., 2001; Stevens et al., 2012). While we cannot exclude the possibility that dopaminergic therapies affected TLR4 expression in B cells in our study, this is unlikely given that 7/12 patients were not receiving therapy and we did not observe differences in TLR4 expression between treated vs untreated patients.
Our cohort is also composed of more females in the control group than in the PD group. To our knowledge, differences in B lymphocyte count between males and females has never been reported, but gender has been seen to affect the number of monocytes (Stevens et al., 2012), although published results are, again, inconsistent. We thus focused our analysis on the percentage of cells expressing TLRs to avoid gender bias related to cell number. For example, testosterone decreases the expression of TLR4 in monocytes (Frisancho-Kiss et al., 2007). Given that there were more males in our PD group, it is, however, unlikely that the increased TLR4 MFI observed is due to gender differences.
We also observed elevated levels of TLR2 and TLR4 as well as TRIS and NF-κBp65 in the striatum of PD patients. This likely reflects an activation of these pathways in the disease, although we could not directly assess this using postmortem samples. Activation of NF-κB, which is believed to occur in PD, leads to the production of a range of inflammatory molecules, including reactive oxygen species, TNFα, and IL-1β. Furthermore, activation of TLR4 may play a major role in facilitating the immediate microglial innate immune and oxidative stress responses upon αSyn, as is the case with amyloid β in AD (Reed-Geaghan et al., 2009).
Based on our results in PD patient samples and on the immune-related effects of neuronal release of αSyn on microglia, specifically via TLR2 and TLR4, we sought to investigate the effects of hαSyn overexpression in mice. We qualitatively observed an increase in microglial density and morphological changes associated with a microglial reaction in the striatum of Thy1.2-αSyn×TLR2-GFP-fluc (and as reported in the Thy1.2-αSyn (Watson et al., 2012)). These mice express small aggregates that are resistant to proteinase K in the striatum. It has recently been reported that oligomers of αSyn, but not aggregates, can act as TLR2 ligands (Kim et al., 2013). It is therefore conceivable that the strong microglial response observed in the striatum is due to the increased presence of small oligomers in this structure, although this remains speculative. While bioluminescent TLR2 signal was measured throughout the entire brain and was not restricted to the striatum, the greatest photon emission of TLR2 mRNA signal was observed at the antero-posterior level of this region. However, bioluminescence imaging does not allow us to pinpoint specific regions of the brain. Unlike in human PD cases, we did not find any changes in TLR2 and TLR4 expression in circulating leukocytes of Thy1.2-αSyn×TLR2-GFP-fluc mice, nor in the striatum, although this may not be surprising. While hαSyn overexpression in mice models mimics a number of features of PD, it does not recapitulate the disease and at best, offers a window into premanifest stages of the disease.
The Thy1.2-αSyn mice exhibit a significant increase in striatal activated microglial cells as early as one month of age, a response which is maintained up to 14 months. In contrast, the microglial response in the SN appears at 5 to 6 months and is not sustained at later stages (Watson et al., 2012). This same group has reported an increase in TLR2 mRNA SN levels at 14 months of age, but not at 1 or 5 to 6 months, which is in line with our observation using in vivo bioluminescence imaging. However, it is currently not known to what extent this creates an inflammatory response that contributes to the pathophenotypes observed in the animals. To tackle this issue, we next sought to investigate the impact of inhibiting the microglial response during specific times of both microglial activation and behavioral impairments, but at which the pathology is still moderate (Chesselet et al., 2012). Administration of dexamethasone during a 1-month period at 6 months of age restored some functions as assessed using the wheel activity test. Dexamethasone blocks microglial activation by inhibiting major histocompatibility complex class II expression through downregulating cyclooxygenase 2 and inducible nitric oxide synthase production (Minghetti et al., 1999). This glucocorticoid has also been shown to prevent neuronal death triggered by the injection of the TLR4 agonist lipopolysaccharide (Castano et al., 2002).
The αSyn-associated pathology in the Thy1.2-αSyn mouse model at 6 months of age affects the striatal dopamine release, leading to high levels of extracellular dopamine and translating into changes in motor activity (Chesselet and Richter, 2011). The Thy1.2-αSyn mice thus display a striking reduction in activity over a 24-hour period in free-running wheels with lower night-time activity as early as 3-4 months of age, which seems to relate to selective deficits in the expression of circadian rhythms of locomotor activity (Kudo et al., 2011). The impairments in diurnal open-field and nocturnal wheel-running activity seem to recruit different mechanisms, which could explain the discrepancy in the effects of dexamethasone observed on these 2 behaviors.
Here we show that (1) there are increased levels of TLR2 and TLR4 in the blood and brain of PD patients, (2) neuronal overexpression of hαSyn triggers a microglial response that can be detected in the entire mouse brain at 10 months of age, and (3) this microglial response is likely to contribute to some motor deficits, although it does not seemingly involve TLR2 and TLR4 pathways. Further studies are warranted to investigate the temporal expression of these receptors in leukocytes and the contribution that this has to PD pathology, which in turn could lead to the discovery of biomarkers as well as the development of anti-inflammatory therapeutic strategies specific for these receptors. A better understanding of the events leading up to the activation of this microglial response and how it can influence the pathology generated by αSyn will be particularly important in the future treatment of PD.
Statement of Interest
None.
Acknowledgments
This work was supported by a grant from the Parkinson Society Canada to F.C. who is also recipient of a National Researcher career award from the Fonds de recherche du Québec - santé (FRQS) providing salary support and operating funds. J.D.O. is supported by a FRQS postdoctoral fellowship. I.S.A. is supported by a Canadian Institutes of Health Research – Huntington Society of Canada postdoctoral fellowship. R.A.B. is supported by a National Institute for Health Research award of a Biomedical Research Centre to the University of Cambridge and Addenbrooke’s Hospital. The authors thank the Parkinson’s UK Tissue Bank at Imperial College and the UK Multiple Sclerosis tissue Bank for providing the brain tissue.
References
- Anderson DC, Cranford RE. (1979). Corticosteroids in ischemic stroke. Stroke 10:68–71. [DOI] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. (2005). Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord 11:493–498. [DOI] [PubMed] [Google Scholar]
- Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, Buendia E. (2001). Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol 113:146–152. [DOI] [PubMed] [Google Scholar]
- Belge G, Meyer A, Klemke M, Burchardt K, Stern C, Wosniok W, Loeschke S, Bullerdiek J. (2008). Upregulation of HMGA2 in thyroid carcinomas: a novel molecular marker to distinguish between benign and malignant follicular neoplasias. Genes Chromosomes Cancer 47:56–63. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. (1995). Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20. [DOI] [PubMed] [Google Scholar]
- Brudek T, Winge K, Agander TK, Pakkenberg B. (2013). Screening of Toll-like receptors expression in multiple system atrophy brains. Neurochem Res 38:1252–1259. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. (2002). The degenerative effect of a single intranigral injection of LPS on the dopaminergic system is prevented by dexamethasone, and not mimicked by rh-TNF-alpha, IL-1beta and IFN-gamma. J Neurochem 81:150–157. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. (2004). Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364:1167–1169. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. (2011). Modelling of Parkinson’s disease in mice. Lancet Neurol 10:1108–1118. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. (2012). A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote M, Drouin-Ouellet J, Cicchetti F, Soulet D. (2011). The critical role of the MyD88-dependent pathway in non-CNS MPTP-mediated toxicity. Brain Behav Immun 25:1143–1152. [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. (2005). Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Gibrat C, Bousquet M, Calon F, Kriz J, Cicchetti F. (2011). The role of the MYD88-dependent pathway in MPTP-induced brain dopaminergic degeneration. J Neuroinflammation 8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Cicchetti F. (2012). Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol Sci 33:542–551. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, LeBel M, Filali M, Cicchetti F. (2012). MyD88 deficiency results in both cognitive and motor impairments in mice. Brain Behav Immun 26:880–885. [DOI] [PubMed] [Google Scholar]
- Dunne A, O’Neill LA. (2003). The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE 2003:re3. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. (2007). Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse 61:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. (2007). Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol 178:6710–6714. [DOI] [PubMed] [Google Scholar]
- Funk N, Wieghofer P, Grimm S, Schaefer R, Buhring HJ, Gasser T, Biskup S. (2013). Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov Disord 28:392–395. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Mao W, Schule B, Babcock M, Schoebel S, Lorenzana C, Alexander J, Kim S, Glick H, Hilton K, Fitzgerald JK, Buttini M, Chiou SS, McConlogue L, Anderson JP, Schenk DB, Bard F, Langston JW, Yednock T, Johnston JA. (2013). Elevated alpha-synuclein impairs innate immune cell function and provides a potential peripheral biomarker for Parkinson’s disease. PLoS One 8:e71634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grage-Griebenow E, Lorenzen D, Fetting R, Flad HD, Ernst M. (1993). Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol 23:3126–3135. [DOI] [PubMed] [Google Scholar]
- Han KJ, Yang Y, Xu LG, Shu HB. (2010). Analysis of a TIR-less splice variant of TRIF reveals an unexpected mechanism of TLR3-mediated signaling. J Biol Chem 285:12543–12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. (2009). TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J Immunol 183:7461–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Ho DH, Suk JE, You S, Michael S, Kang J, Joong Lee S, Masliah E, Hwang D, Lee HJ, Lee SJ. (2013). Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat Commun 4:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Cho ED, Kim HK, You S, Lee HJ, Hwang D, Lee SJ. (2014). beta1-integrin-dependent migration of microglia in response to neuron-released alpha-synuclein. Exp Mol Med 46:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. (2011). Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol 232:66–75. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Phaneuf D, Soucy G, Weng YC, Kriz J. (2009). Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain 132:940–954. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. (1998). Parkinson’s disease. First of two parts. N Engl J Med 339:1044–1053. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Woo MS, Moon PG, Baek MC, Choi IY, Kim WK, Junn E, Kim HS. (2010). Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol 185:615–623. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. (2009). Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging 30:759–768. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38:1285–1291. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. (2004). Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord 10 Suppl 1:S3–S7. [DOI] [PubMed] [Google Scholar]
- Minghetti L, Nicolini A, Polazzi E, Greco A, Perretti M, Parente L, Levi G. (1999). Down-regulation of microglial cyclo-oxygenase-2 and inducible nitric oxide synthase expression by lipocortin 1. Br J Pharmacol 126:1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa F, Kuriyama N, Nakagawa M, Imanishi J. (2012). Effects of peripheral lymphocyte subpopulations and the clinical correlation with Parkinson’s disease. Geriatr Gerontol Int 12:102–107. [DOI] [PubMed] [Google Scholar]
- Norris JW, Hachinski VC. (1986). High dose steroid treatment in cerebral infarction. Br Med J (Clin Res Ed) 292:21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. (2005). Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol 57:168–175. [DOI] [PubMed] [Google Scholar]
- Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, Nicolardi G. (2008). Expression of TLR4 and CD14 in the central nervous system (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol Immunotoxicol 30:729–740. [DOI] [PubMed] [Google Scholar]
- Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, Feng T, Lee M, Asinovski N, Frohlich I, Imboywa S, Von Korff A, Okada Y, Patsopoulos NA, Davis S, McCabe C, Paik HI, Srivastava GP, Raychaudhuri S, Hafler DA, Koller D, Regev A, Hacohen N, Mathis D, Benoist C, Stranger BE, De Jager PL. (2014). Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science 344:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. (2009). CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci 29:11982–11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D. (2009). Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol Scand 119:332–337. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. (2002). Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res 68:568–578. [DOI] [PubMed] [Google Scholar]
- Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol JC, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Hoglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. (2011). Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Natl Acad Sci U S A 108:6632–6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. (2003). alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. (1997). Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Reindl M, Neumann M, Kahle PJ, Poewe W, Wenning GK. (2007). Microglial activation mediates neurodegeneration related to oligodendroglial alpha-synucleinopathy: implications for multiple system atrophy. Mov Disord 22:2196–2203. [DOI] [PubMed] [Google Scholar]
- Stefanova N, Fellner L, Reindl M, Masliah E, Poewe W, Wenning GK. (2011). Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol 179:954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CH, Rowe D, Morel-Kopp MC, Orr C, Russell T, Ranola M, Ward C, Halliday GM. (2012). Reduced T helper and B lymphocytes in Parkinson’s disease. J Neuroimmunol 252:95–99. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. (2008). Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging 29:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarska A, Baj-Krzyworzeka M, Siedlar M, Weglarczyk K, Ruggiero I, Hajto B, Zembala M. (2004). Antitumor response of CD14+/CD16+ monocyte subpopulation. Exp Hematol 32:748–755. [DOI] [PubMed] [Google Scholar]
- Theodore S, Cao S, McLean PJ, Standaert DG. (2008). Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol, 67:1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Pilote M, Phivilay A, Emond V, Bennett DA, Calon F. (2007). Biochemical characterization of Abeta and tau pathologies in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 12:377–390. [DOI] [PubMed] [Google Scholar]
- Tremblay C, St-Amour I, Schneider J, Bennett DA, Calon F. (2011). Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 70:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JR, Francis SE, Marsden L, Suddason T, Lord GM, Dower SK, Crossman DC, Sabroe I. (2009). A central role for monocytes in Toll-like receptor-mediated activation of the vasculature. Immunology 128:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. (2012). Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol 237:318–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Koblansky AA, Ghosh S. (2006). Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol 22:409–437. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. (2005). Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19:533–542. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Hofer TP. (2013). Toward a refined definition of monocyte subsets. Front Immunol 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Appl B, Kafferlein E, Loffler T, Jahn-Henninger H, Gutensohn W, Nores JR, McCullough K, Passlick B, Labeta MO, et al. (1994). The antibody MY4 recognizes CD14 on porcine monocytes and macrophages. Scand J Immunol 40:509–514. [DOI] [PubMed] [Google Scholar]