Abstract
Background:
The acylethanolamides oleoylethanolamide and palmitoylethanolamide are endogenous lipid mediators with proposed neuroprotectant properties in central nervous system (CNS) pathologies. The precise mechanisms remain partly unknown, but growing evidence suggests an antiinflammatory/antioxidant profile.
Methods:
We tested whether oleoylethanolamide/palmitoylethanolamide (10mg/kg, i.p.) attenuate neuroinflammation and acute phase responses (hypothalamus-pituitary-adrenal (HPA) stress axis stress axis activation, thermoregulation, and anhedonia) induced by lipopolysaccharide (0.5mg/kg, i.p.) in rats.
Results:
Lipopolysaccharide increased mRNA levels of the proinflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6, nuclear transcription factor-κB activity, and the expression of its inhibitory protein IκBα in cytoplasm, the inducible isoforms of nitric oxide synthase and cyclooxygenase-2, microsomal prostaglandin E2 synthase mRNA, and proinflammatory prostaglandin E2 content in frontal cortex 150 minutes after administration. As a result, the markers of nitrosative/oxidative stress nitrites (NO2 -) and malondialdehyde were increased. Pretreatment with oleoylethanolamide/ palmitoylethanolamide reduced plasma tumor necrosis factor-α levels after lipopolysaccharide, but only oleoylethanolamide significantly reduced brain tumor necrosis factor-α mRNA. Oleoylethanolamide and palmitoylethanolamide prevented lipopolysaccharide-induced nuclear transcription factor-κB (NF-κB)/IκBα upregulation in nuclear and cytosolic extracts, respectively, the expression of inducible isoforms of nitric oxide synthase, cyclooxygenase-2, and microsomal prostaglandin E2 synthase and the levels of prostaglandin E2. Additionally, both acylethanolamides reduced lipopolysaccharide-induced oxidative/nitrosative stress. Neither oleoylethanolamide nor palmitoylethanolamide modified plasma corticosterone levels after lipopolysaccharide, but both acylethanolamides reduced the expression of hypothalamic markers of thermoregulation interleukin-1β, cyclooxygenase-2, and prostaglandin E2, and potentiated the hypothermic response after lipopolysaccharide. Interestingly, only oleoylethanolamide disrupted lipopolysaccharide-induced anhedonia in a saccharine preference test.
Conclusions:
Results indicate that oleoylethanolamide and palmitoylethanolamide have antiinflammatory/neuroprotective properties and suggest a role for these acylethanolamides as modulators of CNS pathologies with a neuroinflammatory component.
Keywords: OEA, PEA, lipopolysaccharide, neuroinflammation, anhedonia
Introduction
Endogenous lipid transmitters derived from membrane precursors are a current focus of investigation due to the wide range of biological functions in which they participate, including modulation of neurotransmitter release, neuroplasticity, synaptogenesis, neurogenesis, brain information processing, and cellular energetic systems (Orio et al., 2013). Fatty acid acylethanolamides are endogenous lipid mediators with multiple physiological functions that include the endocannabinoid anandamide (arachidonoylethanolamide [AEA]) and the noncannabimimmetic compounds N-oleoylethanolamide (OEA) and N-palmitoylethanolamide (PEA). Though involved in different functions, the acylethanolamides share biosynthetic and degradative mechanisms. They are synthesized on demand through a phospholipase D enzyme acting on a membrane phospholipid precursor, which is synthesized by a cAMP and Ca2+-dependent N-acyltransferase (Piomelli, 2003). Upon its release, they experience reuptake by a catalytically silent fatty acid amide hydrolase (FAAH)-1 variant (Fu et al., 2011) and are degraded through enzymatic hydrolysis by a specific FAAH (Schmid et al., 1985; Cravatt et al., 1996).
OEA and PEA are structurally related compounds that act mainly thought the nuclear peroxisome proliferator-activated receptor-alpha (PPAR-α) (Rodriguez de Fonseca et al., 2001; Fu et al., 2003; Lo Verme et al., 2005; Di Cesare Mannelli et al., 2013), although they might bind the transient receptor potential vanilloid type-1 (Overton et al., 2006; Almasi et al., 2008; Godlewski et al., 2009), the G protein-coupled receptors GPR55 and GPR119 (Overton et al., 2006; Godlewski et al., 2009), or other PPAR isoforms (Paternity et al., 2013; but see Fu et al. 2003; LoVerme et al., 2006). OEA is known as a satiety factor (Rodriguez de Fonseca et al., 2001; Fu et al., 2003), and both PEA and OEA act as analgesics in inflammatory and neuropathic pain (Lo Verme et al., 2005; Suardiaz et al., 2007; Di Cesare Mannelli et al., 2013).
Growing evidence indicates that OEA and PEA may have neuroprotective properties in neurological disorders such as stroke (Sun et al., 2007; Zhou et al., 2012; Ahmad et al., 2012a), traumatic brain injury (Ahmad et al., 2012b), Parkinson′s disease (Gonzalez-Aparicio et al., 2013; Gonzalez-Aparicio and Moratalla, 2013), or addiction (Melis et al., 2008; Plaza-Zabala et al., 2010; Bilbao et al., 2013; Coppola and Mondola, 2013). Some of the mechanisms implicated are the modulation of antioxidant responses, neuroinflammation, glial cell proliferation/differentiation, neurogenesis, and neurotransmission.
Given the significance and complexity of neuroinflammation in the physiopathology of central nervous system (CNS) diseases, we studied the role of OEA and PEA as modulators of the inflammatory/immune response after a lipopolysaccharide (LPS) challenge. LPS is a component of the outer membrane on gram-negative bacteria that is extensively used for neuroinflammation modeling. Systemic LPS injection to experimental animals elicits a multisystemic response that includes immune, endocrine, metabolic, and behavioral components known as the acute-phase response and sickness behavior (Hart, 1988; Konsman et al., 2002; Kushner and Rzewnicki, 1997).
We tested the efficacy of OEA and PEA to modulate the canonical proinflammatory pathway triggered by the activation of the nuclear factor-κB (NF-κB) (Madrigal et al., 2001) after LPS and evaluated the acute-phase responses described as activation of hypothalamo-pituitary axis (HPA) (increases in plasma corticosterone), changes in hypothalamic markers of thermoregulation (interleukin [IL]-1β, cyclooxygenase [COX]-2, and prostaglandin [PG]E2), and behavioral malaise (by checking motivational behavior).
Methods
Animals
Ninety-four male outbred Wistar Hannover rats (HsdRccHan:Wist, from Harlan, Spain), weighing 350 to 400g, were housed in groups (n=5–6) and maintained at a constant temperature of 24±2°C at a relative humidity of 70±5% in a 12-hour light–dark cycle (lights on at 8:00 am). Animals were fed a standard pellet chow (A04 SAFE, Scientific Animal Food and Engineering, Augy, France) with free access to fresh tap water and were maintained under constant conditions for 10 days prior to experiments.
All experimental protocols were approved and followed the guidelines of the Animal Welfare Committee of the Universidad Complutense of Madrid according to European legislation (2010/63/UE).
Drug Administration
LPS (serotype O111:B4, ref. L2630 Sigma, Spain) was dissolved in saline and injected i.p. at 0.5mg/kg. THe dose was chosen according to previous reports to induce neuroinflammation (MacDowell et al., 2013). OEA (10mg/kg, i.p.; synthesized in our laboratory; Giuffrida et al., 2000) and PEA (10mg/kg, i.p.; Tocris, Spain) were dissolved in vehicle (5% Tween 80 in saline) and injected 10 minutes before LPS administration. The doses were chosen according to previous studies in rodents reporting antiinflammatory/neuroprotective effects (Plaza-Zabala et al., 2010; Ahmad et al., 2012a, 2012b; Zhou et al., 2012).
Tissue Samples and Plasma Collection
Brain tissue samples were taken 150 minutes after LPS injection using a lethal dose of sodium pentobarbital (300mg/kg, i.p., Dolethal, Spain). The timing of sacrifice after LPS was chosen on the basis of previous studies showing an NF-κB-dependent proinflammatory response in the frontal cortex of Wistar rats at this time point (Perez-Nievas et al., 2010; MacDowell et al., 2013). Brains were isolated from the skull, and meninges and blood vessels were carefully discarded. The frontal cortex and hypothalamus were excised and frozen at -80°C until assayed. Blood was collected by cardiac puncture using trisodium citrate (3.15% wt/vol) as anticoagulant. Plasma was obtained by blood centrifugation (2000 g) 15 minutes at 4°C and stored at -20°C until determinations.
Rat brain frontal cortex was chosen because of its high levels of proinflammatory/antiinflammatory mediators and its susceptibility to the neuroinflammatory process elicited by LPS (Garcia-Bueno et al., 2008) and because this brain area is an important neural substrate for the regulation of the HPA axis response to an immune/inflammatory challenge (Radley et al., 2006). Hypothalamus is the main brain area involved in thermoregulation and fever (Saper, 1998).
Preparation of Nuclear and Cytosolic Extracts
A modified procedure based on the method of Schreiber and colleagues (Schreiber et al., 1989) was used. Briefly, brain frontal cortex and hypothalamus samples were homogenized in 300 μL buffer (10 mmol/L N-2-hydroxyethyl piperazine-N-2-ethanesulfonic acid (pH 7.9); 1 mmol/L ethylenediamine tetraacetic acid (EDTA), 1 mmol/L ethylene glycol tetraacetic acid (EGTA), 10 mmol/L KCl, 1 mmol/L dithiothreitol, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.1mg/mL aprotinin, 1mg/mL leupeptin, 1mg/mL Nap-tosyll-lysine-chloromethyl ketone, 5 mmol/L NaF, 1 mmol/L NaVO4, 0.5mol/L sucrose, and 10 mmol/L Na2MoO4). After 15 minutes, 0.5 % Nonidet P-40 (Roche, Mannheim, Germany) was added.
The tubes were vortexed and nuclei were collected by centrifugation at 8000 g for 5 minutes. Supernatants were considered as the cytosolic fraction. The pellets were resuspended in 100 μL buffer supplemented with 20% glycerol and 0.4mol/L KCl and shaken for 30 minutes at 4°C. Nuclear protein extracts were obtained by centrifugation at 13000 g for 5 minutes, and aliquots of the supernatant were stored at -80°C. All steps of the fractionation were carried out at 4°C.
Western-Blot Analyses
To determine the expression levels of the enzymes inducible nitric oxide synthase (iNOS) and COX-2, brain frontal cortices and hypothalamus were homogenized by sonication in 400 μL of phosphate-buffered saline (pH=7) mixed with a protease inhibitor cocktail (Complete, Roche, Madrid, Spain) followed by centrifugation at 12000 g for 10 minutes at 4°C. After adjusting protein levels in the supernatants, homogenates were mixed with Laemmli sample buffer (Bio Rad, CA) and 10 μL (1mg/mL) was loaded into an electrophoresis gel.
Membranes were blocked in 10mM Tris-buffered saline containing 0.1% Tween-20 and 5% skimmed milk/bovine serum albumin (BSA) and incubated with specific primary antibodies:IκBα (rabbit polyclonal antibody against an epitope mapping at the C-terminus of IκBα of human origin; dilution 1:1000 in 5% skimmed milk in BSA, Santa Cruz Biotechnology, CA); iNOS (rabbit polyclonal antibody against a peptide mapping at the amino terminus of iNOS of human origin; dilution 1:1000 in TBS-Tween, Santa Cruz Biotechnology, CA); COX-2 (goat polyclonal antibody against a peptide mapping at the C-terminus of COX-2 of human origin; dilution 1:750 in 5% BSA in TBS-Tween, Santa Cruz Biotechnology, CA). After washing with Tween 20, the membranes were incubated with the respective horseradish peroxidase-conjugated secondary antibodies for 90 minutes at room temperature. Blots were imaged using an Odyssey Fc System (Li-COR Biosciences), quantified by densitometry (NIH ImageJ software), and expressed in arbitrary units of optical density. The housekeeping gene β-actin was used as loading control.
Real Time-Polymerase Chain Reaction Analysis
Total cytoplasmic RNA was prepared from samples of frontal cortex or hypothalamus using TRIZOL reagent (Invitrogen, Grand Island, NY); aliquots were converted to complementary DNA using random hexamer primers. Quantitative changes in mRNA levels were estimated by real time-polymerase chain reaction (RT-PCR) using the following cycling conditions: 35 cycles of denaturation at 95°C for 10 seconds, annealing at 58–61°C for 15 seconds depending on the specific set of primers, and extension at 72°C for 20 seconds. Reactions were carried out in the presence of SYBR green (1:10000 dilution, Molecular Probes, Eugene, OR) in a 20-L reaction in a Rotor-Gene (Corbett Research, Mortlake, Australia). The primers used were to detect IL-1β, IL-6, TNF-α, NF-κB p65 subunit, IκBα, iNOS, COX-2, and m-PGES-1 (sequence details in Table 1). Relative mRNA concentrations were obtained by comparing the take-off point of the different samples using the software provided in the unit. It establishes an inverse correlation between the number of cycles before take-off and the concentration of mRNA, while assigning arbitrary units to the results. Tubulin and GADPH primer levels were used to normalize data (results are shown using tubulin normalization).
Table 1.
RT-PCR Primer Sequence Details
| Forward Primers (3’-5’) | Reverse Primers (5’-3’) | |
|---|---|---|
| IL-1β | ACCTGCTAGTGTGTGATGTTCCCA | AGGTGGAGAGCTTTCAGCTCACAT |
| IL-6 | AAGCTGAGCGACGAGTACAAGA | GTCAGCTCCAGCACCTTGTG |
| TNF-α | CTGGCCAATGGCATGGATCTCAAA | ATGAAATGGCAAATCGGCTGACGG |
| NFκB p65 | CATGCGTTTCCGTTACAAGTGCGA | TGGGTGCGTCTTAGTGGTATCTGT |
| IκBα | TGGCCTTCCTCAACTTCCAGAACA | TCAGGATCACAGCCAGCTTTCAGA |
| iNOS | GGACCACCTCTATCAGGAA | CCTCATGATAACGTTTCTGGC |
| COX-2 | CTTCGGGAGCACAACAGAG | GCGGATGCCAGTGATAGAG |
| m-PGES-1 | GGTGAAGCAAATGTTCCCAGCTCA | TTTAGCGGTTGGTCAAAGCCCATC |
| Tubulin | CCCTCGCCATGGTAAATACAT | ACTGGATGGTACGCTTGGTCT |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
Abbreviations: COX, cyclooxygenase; IL, interleukin; iNOS, inducible nitric oxide synthase; m-PGES-1, microsomal prostaglandin E2 synthase; NF, nuclear factor; RT-PCR, real time-polymerase chain reaction; TNF, tumor necrosis factor.
Plasma Cytokine Determination
IL- 1β and TNF-α plasma levels were determined using commercially available enzyme-linked immunosorbent assays (RayBiotech). Plasma samples were 1:2 diluted and assayed following the manufacturer′s guidelines. Quantification was performed using a standard curve of increasing cytokines′concentrations. The optical density was measured using a microplate reader (Synergy 2; BioTek Instruments) set to 450nm. The sensitivities of the assays were <80 pg/mL for IL-1β and <25 pg/mL for TNF-α. Intra-assay and inter-assay coefficients of variation were <10% and 12%, respectively, for both kits.
Nitrites (NO2 -) Levels
As the stable metabolites of the free radical nitric oxide (NO•), NO2 - were measured by using the Griess method (Green et al., 1982). In an acidic solution with 1% sulphanilamide and 0.1% N-(1-Naphthyl)ethylenediamine (NEDA), nitrites convert into a pink compound that is photometrically calculated at 540nm in a microplate reader (Synergy 2; BioTek).
Lipid Peroxidation
Lipid peroxidation was measured by a modification of the method of Das and Ratty (1987), whereby the thiobarbituric acid reacting substances, predominantly malondialdehyde (MDA), produced as a secondary product were quantified by use of the 2-thiobarbituric acid (TBA) color reaction. Brain tissue was homogenized in 10 volumes (wt/vol) of sodium phosphate buffer (pH 7.4). Assays contained tissue homogenate, trichloroacetic acid (40% wt/vol), HCl (5M), and TBA (2% wt/vol). Samples were heated for 15 minutes at 90°C and centrifuged at 12000 g for 10 minutes. The MDA-TBA adduct (pink chromogen) of the supernatant was measured spectrophotometrically (532nm) and the MDA concentration calculated by use of a standard curve prepared with MDA tetra-butylammonium salt. The results were expressed as nmol/mg protein.
Plasma Corticosterone
Corticosterone was measured in plasma by using a commercially available kit by RIA Coat-a-Count (Siemens, Los Angeles, CA). A gamma counter (Wallac Wizard 1470, Perkin Elmer, Waltham, MA) was used to measure radioactivity of the samples. The time of blood extraction and plasma collection oscillated between 1:00 pm and 3:00 pm.
NF-κB Transcription Factor Assay
NF-κB transcription factor activity was determined in nuclear extracts by using an enzyme-linked immunosorbent assay-based kit (Cayman Chemicals, Tallin, Estonia). Nuclear extracts were incubated with specific NF-κB p65 subunit response element probes, and p65 bound to its response element probe was detected using a specific antibody against this subunit. Horseradish peroxidase-labeled secondary antibody was added and the binding was detected by spectrophotometry. Measurement was performed according to the manufacturer’s instructions. This assay is specific for p65 activation, and it does not cross-react with other NF-κB subunits, such as p50.
PGE2 Determination
PGE2 levels were measured by commercially available enzyme immunoassay (PGE2 EIA Kit-Monoclonal; Cayman Chemical, Tallin, Estonia). Samples were sonicated in 400mL homogenization buffer (0.1M phosphate buffer, pH=7.4, 1mM EDTA, and 10mM indomethacin), purified in 4 volumes ethanol for 5 minutes at 4°C, centrifuged at 3000 g for 10 minutes, and acidified with glacial extracted using SPE (C-18) acetic acid (pH=3.5). PGE2 was extracted using SPE (C-18) columns (Waters, MA) rinsed with methanol and water. After sample′s application, columns were washed with water and hexane and PGE2 was eluted with ethyl acetate. Samples were evaporated to dryness under nitrogen and resuspended in enzyme immunoassay buffer. PGE2 levels were measured in a 96-well plate and read at 405nm following the manufacturer’s instructions (Synergy 2; BioTek Instruments). The sensitivity of the assay for PGE2 was 15 pg/mL; intra- and interassay coefficients of variation were 6.6% and 15.5%, respectively.
Protein Assay
Protein levels were measured using the Bradford method (Bradford, 1976).
Measurement of Rectal Temperature
Rectal temperature was measured by the use of a digital readout thermocouple (BAT12 thermometer, Physitemp) with a resolution of 0.1°C accuracy of ±0.1°C attached to a RET-2 Rodent Sensor, which was inserted 2.5cm into the rectum of the rat, the animal being lightly restrained by holding it in the hand of a trained individual to avoid stress-confounding factors. A steady readout was obtained within 10 seconds of probe insertion.
Saccharine Preference Test
Rats fed ad libitum were housed individually and were offered a free choice between 2 bottles located in the cages in a random manner, one with a 0.1% saccharin solution and another with tap water, during the time of the experiment (30 hours). Separated groups of animals were used to test the thermic response and the preference for saccharine. The consumption of water and saccharin solution was recorded at specific time intervals after pharmacological treatments. The preference for saccharin was calculated as consumed saccharin solution/total fluid intake. No previous food or water deprivation was applied before the test.
Statistical Analyses
Data in text and figures are expressed as mean ± SEM. Data were analyzed by 2-way ANOVA comparing 2 factors: inflammation (vehicle or LPS) and pretreatment (vehicle, OEA, PEA), followed by Bonferroni posthoc test when appropriate. Data on saccharine preference test, total fluid intake, and rectal temperatures were analyzed by 2-way repeated-measures ANOVA using treatment as a between-subjects factor and time as a repeated measure, followed by Bonferroni posthoc test. The behavioral experiments (rectal temperatures and saccharine preference test) and Western blots were performed independently for OEA and PEA, so the 2-way ANOVA were run accordingly (reported in results in this order: OEA and PEA). Additionally, in the behavioral experiments, we ran a 2-way ANOVA comparing the 2 factors (inflammation and pretreatment) at specific time points of the temporal curves: we chose 1 hour and 3 hours after LPS in the temperature curves, since the hypothalamic markers were studied at a time in between (2.5 hours); in the saccharine preference test, we chose the last time point of the test (30 hours), since it represents an accumulated measure over time. A P value ≤ .05 was considered statistically significant. Data were analyzed using GraphPad Prism version 5.04 (GraphPad Software Inc., San Diego, CA).
Results
Effect of OEA and PEA on Proinflammatory Cytokines in Frontal Cortex
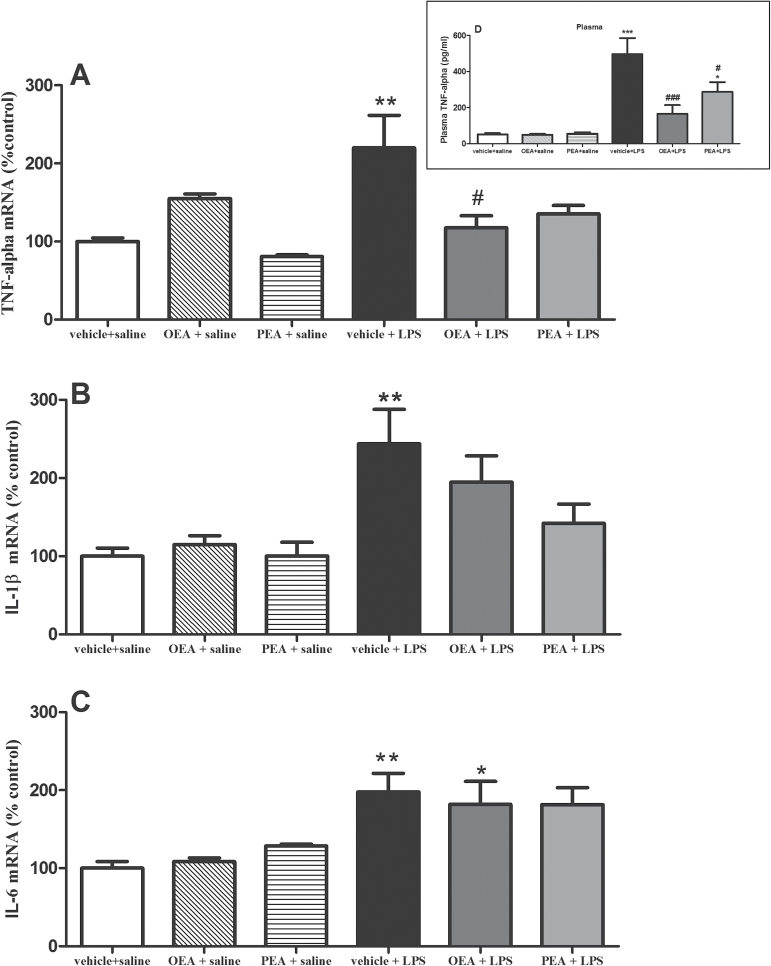
LPS administration increased mRNA expression of the proinflammatory cytokines TNF-α (Figure 1a; F(1,19) P=5.97, P=.0245), IL-1β (Figure 1B; F(1,24) P=10.34, P=.0037), and IL-6 (Figure 1C; F(1,19) P=22.93, P=.0001) in frontal cortex 150 minutes after administration. Pretreatment with OEA significantly reduced the increase in TNF-α mRNA levels induced by LPS (interaction effect: F(2,19)P=6.177) and had no significant effect on IL-1β and IL-6 in the presence or absence of LPS. Although PEA reduces LPS-induced increase in TNF-α and IL-1β (but not IL-6) mRNA, posthoc test revealed that these effects failed to reach statistical significance.
Figure 1.
Proinflammatory cytokines in frontal cortex (and plasma). Real time-polymerase chain reaction (RT-PCR) analysis of tumor necrosis factor (TNF)-α (A), interleukin (IL-)1β (B), and IL-6 (C) mRNAs in frontal cortex 150 minutes after lipopolysaccharide (LPS) administration. Data (n =4–7 per group) are normalized by tubulin and are presented as means ± SEM. Data in D (graph box) represents plasma levels of TNF-α measured by ELISA. Different from control group: *P < .05, **P < .01; different from vehicle + LPS rats: # P < .05 (2-way ANOVA followed by Bonferroni posthoc test).
To test whether the CNS effects of acylethanolamides may be affected by peripheral modulation of circulating cytokines, we measured TNF-α and IL-1β in plasma after treatments. Figure 1D (graph box) shows that both OEA and PEA modified the increase in plasma TNF-α observed after LPS injection (interaction effect F(2,67) P=4.968; P=.0097). Levels of plasma IL-1β were not affected by the treatments (data not shown).
Effect of OEA and PEA in the Activation of Proinflammatory NF-κB
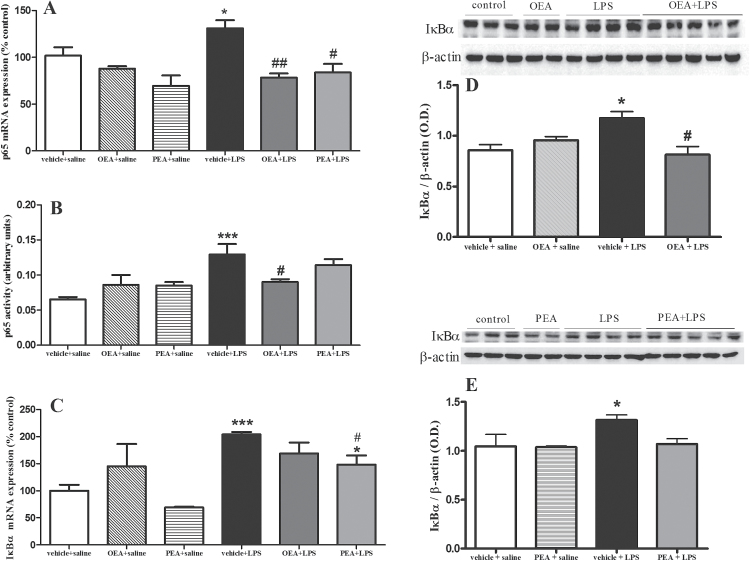
The release of proinflammatory cytokines TNF-α, IL-1β, and IL-6 after LPS may account for NF-κB activation, so we studied the mRNA expression and activity of NF-κB proinflammatory subunit p65 (Figure 2A-B) and its inhibitory protein IκBα (Figure 2C-E). LPS increased p65 subunit (F(1,18) P=13.37, P=.0011) in nuclear extracts of frontal cortex, which is inhibited by OEA pretreatment at the level of mRNA (Figure 2A; F(2,16) P=12.54, P=.0009) and activity (Figure 2B; interaction effect F(2,15) P=5.313, P=.0180). PEA administration reduced the p65 mRNA expression (Figure 2A; F(2,15) P=5.313, P=.018) but had no significant effect in the activity assay (Figure 2B; F(2,18) P=.7028, P=.5108). LPS also induced an upregulation of IκBα mRNA (Figure 2C; F(1,20) P=25.60, P < .0001) and protein expression (F(1,13) P=22.79, P=.0008) in cytosolic extracts that was prevented by PEA (F(2,20) P=4.943, P=.0187) at the level of mRNA. Pretreatment with OEA reduced IκBα protein expression in LPS-treated animals (interaction effect (F(1,13) P=39.48, P < .0001), whereas PEA had no significant effect at protein level.
Figure 2.
Nuclear factor (NF)-κB proinflammatory subunit p65 and its inhibitory protein IκBα in frontal cortex. A) Real time-polymerase chain reaction (RT-PCR) analysis of the proinflammatory p65 subunit of NF-κB in nuclear extracts. (B) Activity of NF-κB p65 subunit in nuclear extracts. (C) RT-PCR analysis of the NF-κB inhibitory protein IκBα in cytosolic extracts. (D) Western-blot and densitometric analysis of IκBα after oleoylethanolamide (OEA) pretreatment. (E) Western-blot and densitometric analysis of IκBα after palmitoylethanolamide (PEA) pretreatment. Data (n=3–5) on RT-PCR and Western blot are normalized by tubulin and β-actin. Different from control group: *P < .05, ***P < .001; different from vehicle + lipopolysaccharide (LPS) rats: # P < .05, ## P < .01 (2-way ANOVA followed by Bonferroni posthoc test).
Proinflammatory Enzymes (COX-2 and iNOS): Effect of OEA and PEA
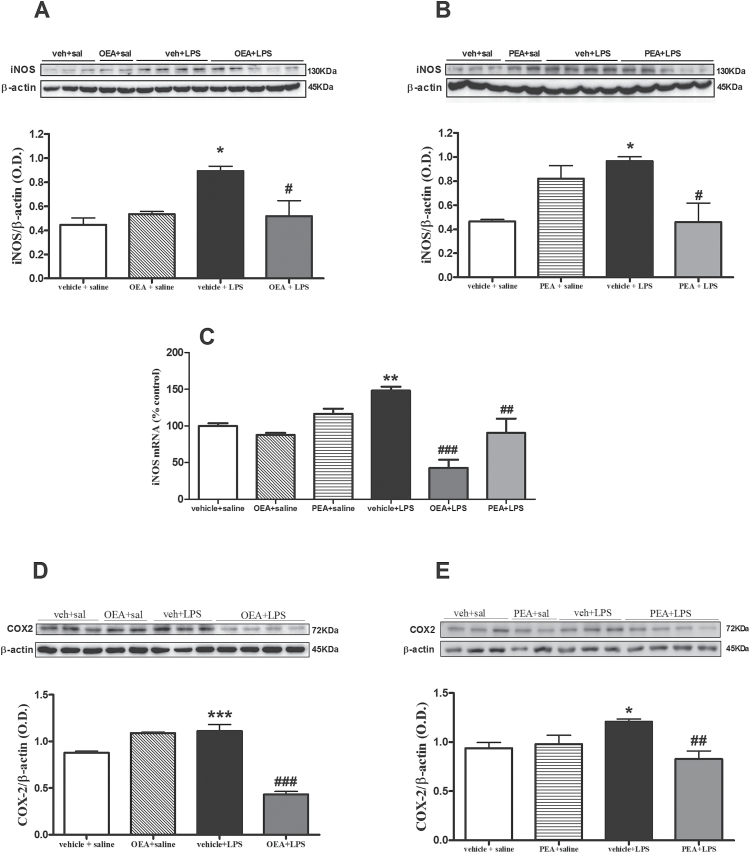
NF-κB regulates the expression of genes involved in the accumulation of oxidative/nitrosative and inflammatory mediators after LPS exposure. Among others, 2 main sources of these mediators dependent on NF-κB are iNOS and COX-2. LPS induced an increase in iNOS protein expression (F(1,13) P=5.221, P=.0482) that was prevented by OEA (Figure 3A; interaction effect: F(1,13) P=6.112, P=.0354) and by PEA (Figure 3B; interaction effect: F(1,13) P =14.68, P=.004). Both acylethanolamides reduced iNOS mRNA expression in the LPS-treated condition (Figure 3C; interaction effect (F(2,20) P=10.44, P=.0013). Similarly, LPS-induced COX-2 upregulation (F(1, 10) P=13.97, P=.0057) was blocked by the respective preadministration of OEA and PEA (Figure 3D-E, interaction effects: F(1, 10) P=61.66, P < .0001 and F(1,11) P=9.388, P=.0135). COX-2 mRNA levels remain unchanged in all treatments at this time point (data not shown).
Figure 3.
Effect of acylethanolamides in lipopolysaccharide (LPS)-induced inducible isoforms of nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expression. (A) Western-blot and densitometric analysis of iNOS after oleoylethanolamide (OEA) pretreatment. (B) Western-blot and densitometric analysis of iNOS after palmitoylethanolamide pretreatment. (C) Real time-polymerase chain reaction (RT-PCR) analysis of iNOS after OEA and PEA pretreatments. (D) Western- blot and densitometric analysis of COX-2 after OEA pretreatment. (E) Western-blot and densitometric analysis of COX-2 after PEA pretreatment. Data (n=3–5) are represented as means ± SEM. Different from control group: *P < .05, **P < .01, ***P < .001; different from vehicle + lipopolysaccharide (LPS): # P < .05, ## P < .01, ### P < .001 (2-way ANOVA followed by Bonferroni posthoc test).
Brain COX-2 and iNOS Main Products: PGE2 Synthesis and NO2 - Accumulation. Effect of OEA and PEA
The presumed major iNOS and COX-2 brain products, NO and PGE2, respectively, are potent oxidant/proinflammatory molecules that have been directly related to cellular damage/death in multiple CNS pathologies.
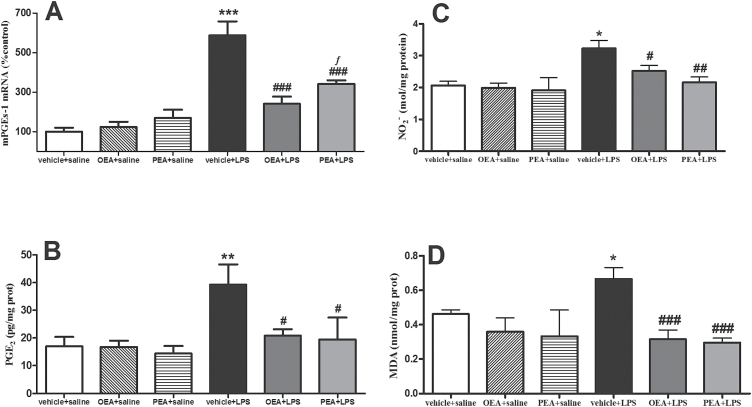
PGE2 is synthesized by a multienzymatic pathway in which the specific enzyme microsomal prostaglandin E2 synthase (mPGES-1) is the last step (Ivanov and Romanovsky, 2004). LPS increased mPGES-1 mRNA (Figure 4A; F(1,20) P=70.19, P < .0001) and PGE2 production (Figure 4B; F(1,22) P=9.574, P=.063) in cortical tissue. As can be observed in Figure 4A-B, both OEA and PEA prevented the LPS-induced upregulation of mPGES-1 and PGE2 (interaction effects: F(2, 20) P=13.20, P=.0004 and (F(2, 20)P=3.074, P=.0711).
Figure 4.
Prostaglandin (PG)E2 synthesis and release, nitrite accumulation, and lipid peroxidation in frontal cortex. (A) Real time-polymerase chain reaction (RT-PCR) analysis of the PGE2 synthesis enzyme microsomal prostaglandin E2 synthase (mPGES-1). (B) PGE2 levels measured by enzyme immunoassay. (C) NO2 - accumulation. (D) Malondialdehide accumulation as marker of lipid peroxidation. Data (n=3–6) are represented as means ± SEM. Different from control group: *P < .05, **P < .01, ***P < .001; different from vehicle + lipopolysaccharide (LPS): # P < .05, ## P < .01, ### P < .001; different from palmitoylethanolamide (PEA) + saline: ƒ P < .05 (2-way ANOVA followed by Bonferroni posthoc test).
Figure 4C shows the accumulation of the main NO metabolite, NO2 -, after LPS (F(1, 19) P=5.692, P=.0276) that was prevented by pretreatment with both OEA and PEA (main effect of pretreatment (F(2, 19) P=6.203, P=.0084; interaction F(2, 19) P=.4733, P=.63).
Lipid Peroxidation: Effect of OEA and PEA
As a marker of cellular damage elicited by oxidative/nitrosative stress, lipid peroxidation was assessed by measuring MDA accumulation. Figure 4D shows that OEA and PEA pretreatments prevented the LPS-induced overaccumulation of MDA in frontal cortex (effect of pretreatment F(2, 19) P=10.57, P=.0008 and interaction F(2, 19) P=4.009, P=.0353).
Effects of OEA and PEA on Plasma Corticosterone Levels
The quantification of plasma corticosterone levels at the time of blood extraction (1:00-3:00 pm) revealed an expected corticosterone increase in LPS-injected animals (F(1, 24) P=18.29, P=.0003). The LPS-induced increase in corticosterone levels rose in 42% over control values (control: 244.10±13.8ng/mL). Interestingly, at that time point, neither OEA (51.3% over controls) nor PEA (57.57% over controls) prevented the increase in corticosterone induced by LPS, suggesting that the mechanism of these compounds modulating neuroinflammation is independent of systemic corticosterone levels (Table 2).
Table 2.
Plasma Corticosterone Levels.
The values of corticosterone obtained in basal conditions (244.10±13.8ng/mL) were in accordance with the kit manufacturer’s expected values in adult male Wistar rats. Lipopolysaccharide (LPS) increased plasma levels of corticosterone compared with control animals. Pretreatment with oleoylethanolamide (OEA) or palmitoylethanolamide (PEA) did not modify the LPS-induced increase in corticosterone. The time of blood extraction oscillated between 1:00 and 3:00 pm. Data are presented as means ± SEM with n=5 for each group of treatment.
| Treatment | Corticosterone (ng/mL) |
|---|---|
| Vehicle + saline | 244.10±13.80 |
| OEA + saline | 259.17±32.94 |
| PEA + saline | 272.96±25.72 |
| Vehicle + LPS | 346.64±11.39 |
| OEA + LPS | 369.34±35.22 |
| PEA + LPS | 384.64±28.50 |
Hypothalamic Markers of Thermoregulation: Effects of OEA and PEA
As another acute-phase response after LPS administration, we studied the expression of molecular markers related with temperature regulation in the hypothalamus.
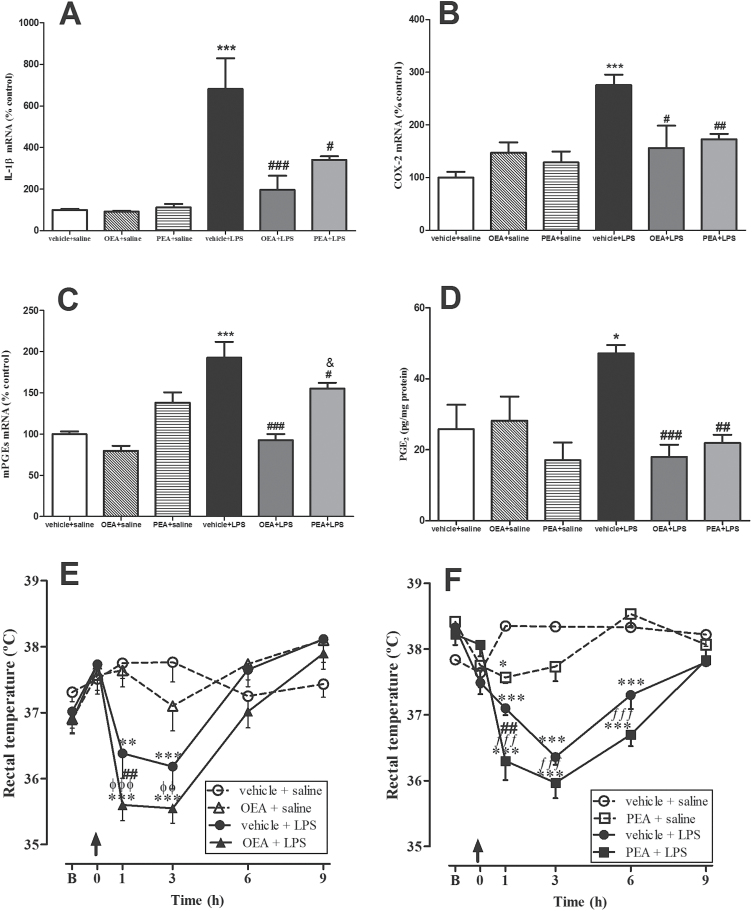
The pyrogenic and proinflammatory cytokine IL-1β increased its mRNA up to 6 times in hypothalamus after LPS administration (F(1, 26) P=14.62, P=.0007), and this increase was blocked by OEA and PEA pretreatments (Figure 5A; interaction effect: F(2, 26) P= 4.982, P=.0147). Similarly, LPS induced an upregulation of COX-2 mRNA in hypothalamus (Figure 5B; F(1, 25) P=12.60, P=.0016) that was prevented by OEA and PEA (interaction effect: F(2, 25) P=8.285, P=.0017).
Figure 5.
Thermoregulation and its hypothalamic markers. Real time-polymerase chain reaction (RT-PCR) analysis of interleukin (IL)-1β (A), cyclooxygenase (COX)-2 (B), and microsomal prostaglandin E2 synthase (mPGES-1) (C), and protein levels of prostaglandin (PG)E2 (D) in the hypothalamus. Rectal temperatures of rats pretreated with oleoylethanolamide (OEA) (E) and palmitoylethanolamide (PEA) (F). Biochemical data are means ± SEM (n=3–5). Rectal temperature data for OEA (E) and PEA (F) are represented as means ± SEM (n=5–6). Arrows in temperature graphs indicate time of lipopolysaccharide (LPS) injection, “B” represents the mean of 2 first basal measures previous to LPS injection, and t=0 represents the third basal measure right before treatment administration. Different from control group: *P < .05, **P < .01, ***P < .001; different from vehicle + LPS: # P < .05, ## P < .01, ### P < .001; different from OEA + saline group: øø P < .01, øøø P < .001; different from PEA + saline: ƒƒ P< .01,ƒƒƒ P < .001 (biochemical data: 2-way ANOVA followed by Bonferroni posthoc test; behavioral data: repeated-measures 2-way ANOVA with Bonferroni posthoc test).
PGE2, one of the major COX-2 products, is presumably a mediator of temperature deregulation after LPS (Ivanov and Romanovsky, 2004). As represented in Figure 5C-D, OEA and PEA prevented the mRNA upregulation of its synthesis enzyme mPGES-1 (interaction effect: F(2,28) P=5.950, P=.0070) and the PGE2 accumulation (interaction: F(2,20) P=6.132, P=.0113) induced by LPS in hypothalamus, suggesting an involvement of both acylethanolamides in the acute-phase responses of LPS related with body temperature regulation.
Thermic Response
Figure 5E and F show the temperature deregulation after LPS injection. Three basal temperatures were recorded every 30 minutes before LPS administration. The media of the 2 first basal temperatures (t=-1.0 hour and -0.5 hour) was represented as “B” in the figures. Arrow indicates time of LPS injection. Basal temperatures immediately before LPS injection (t=0) did not differ significantly between groups of treatments (F(3,23) P=.58, P=.65, n.s., and F(3,20) P =1.15, P=.037, n.s., for OEA (Figure 5E) and PEA (Figure 5F) experiments, respectively). Analysis of the temperature temporal curves by repeated measures 2-way ANOVA showed interactions between time and treatment (F(15,95) P=6.696, P < .0001 and F(15,60) P=14.60, P <. 0001) and main effects of time (F(5,95) P=19.0, P < .0001 and F(15,60) P=27.12, P <. 0001) and treatment (F(3,95) P=4.91, P=.0108 and F(3,60) P=51.35, P < .0001). Additional analyses revealed that LPS induced a hypothermic response immediately after the injection and up to 3 to 6 hours posttreatment. Two-way ANOVA at specific time points revealed that pretreatments with OEA and PEA potentiated the hypothermic response 60 minutes after LPS (F(1,20) P=40.25, P < .0001 and (F(1,12) P=33.31, P < .0001) (Figure 5E-F).
Saccharine Preference Test
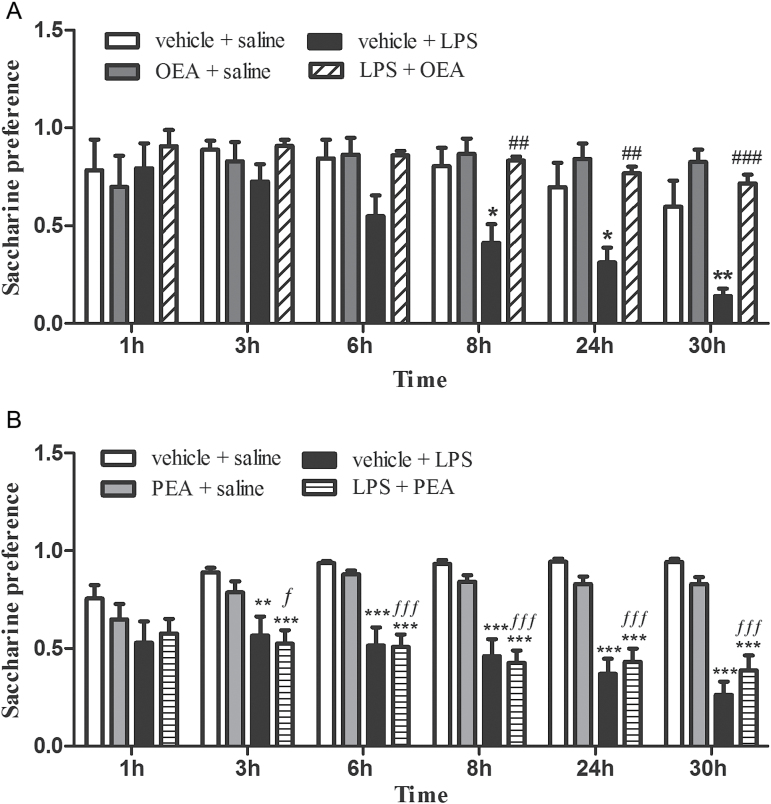
The saccharine preference test was used to evaluate motivational behavior. A decrease in the preference for a natural reward (sucrose or saccharine) is reflective of anhedonia, which is a core symptom of a depressive-like state (Willner et al., 1987) and considered part of the sickness behavior after LPS administration (Yirmiya, 1996).
Repeated-measures 2-way ANOVA (Figure 6A and B, respectively) found an overall interaction between time and treatments (F(15,100) P=3.625, P < .0001 and F(15,220) P=5.011, P <.0 001) and main effects of time (F(5,100) P=9.904, P < .0001 and F(5,220) P=3.83, P=.0024) and treatment (F(3,100) P=7.443, P=.0077 and F(3,220) P=19.17, P < .0001). Subsequent analyses revealed that, as reflected in Figure 6, LPS injection induced a gradual decrease in the preference for a saccharine solution (from 3–8 hours up to 30 hours postadministration). Pretreatment with OEA (Figure 6A) to LPS-injected rats restored the preference for saccharine to the level of controls at any time point, whereas PEA pretreatment (Figure 6B) had no effect in this motivational test. The comparison between the factors inflammation and pretreatment by 2-way ANOVA at the time point of 30 hours posttreatment (see statistical methods) revealed a main effect of OEA pretreatment in LPS conditions (F(1,20) P=25.92, P < .0001) and no effect of PEA in the same condition (F(1,44) P=.012, P=.92, n.s.).
Figure 6.
Saccharine preference test. (A) Rats pretreated with oleoylethanolamide (OEA). (B) Rats pretreated with palmitoylethanolamide (PEA). Saccharine preference was calculated as quantity of saccharine solution drunk/total fluid intake and is an index of the motivational state of the animal. The gradual decrease in the preference for a saccharine solution observed in LPS-injected rats reflects an anhedonic state, which is totally prevented by pretreatment with OEA (A) but not by PEA (B). Data (n=6–12) are means ± SEM. Repeated-measures 2-way ANOVA with Bonferroni posthoc test: different from control: *P < .05, **P < .01, ***P < .001; different from vehicle + lipopolysaccharide (LPS): ## P < .01, ### P < .001; different from PEA+saline group: ƒ P < .05; ƒƒƒ P < .001.
The total amount of liquid (water + saccharine solution) drunk by the animals in this test differs significantly between control and LPS-treated animals. OEA or PEA did not modify this LPS-induced effect (Table 3).
Table 3.
Total Fluid Intake at Different Time Points in the Saccharine Preference Test
(A) Pretreatment with oleoylethanolamide (OEA) in lipopolysaccharide (LPS) or vehicle-injected rats. (B) Pretreatment with palmitoylethanolamide (PEA) in LPS or vehicle-injected rats. Animals injected with LPS decreased the total fluid intake (water + saccharine solution) during the saccharine preference test (6–30 hours) independently of the pretreatment with OEA (9–30 hours) (A) or PEA (6–30 hours) (B). Data are means ± SEM. Repeated-measures 2-way ANOVA with Bonferroni posthoc test for Table 3A and B, respectively: overall interactions between time and treatment (F(15,100) P=8.399, P < .0001 and F(15,220) P=31.14, P < .0001) and main effects of time (F(5,100) P=48.88, P < .0001 and F(5,220) P=135.0, P < .0001) and treatment (F(3,100) P =8.503, P=.0008 and F(3,220) P=32.40, P < .0001). Different from control group: *P < .05, **P < .01, ***P < .001; different from OEA + saline group: ø P < .05, øø P < .01, øøø P < .001; different from PEA + saline group: ƒƒƒ P < .001.
| Total Fluid Intake | ||||||
|---|---|---|---|---|---|---|
| A | ||||||
| Treatment/time | 1 h | 3 h | 6 h | 9 h | 24 h | 30 h |
| Vehicle + saline | 5.08±1.30 | 14.47±3.55 | 22.33±6.30 | 27.77±8.20 | 34.02±8.80 | 48.42±12.30 |
| OEA + saline | 1.34±0.55 | 15.2±3.58 | 26.68±5.29 | 33.45±6.79 | 45.82±9.66 | 57.12±10.21 |
| Vehicle + LPS | 0.95±0.53 | 1.27±0.51 | 2.46±0.75* | 3.82±0.78** | 7.58±2.29** | 14.883±1.94*** |
| OEA + LPS | 3.23±1.12 | 4.35±1.14 | 4.98±1.18ø | 5.58±1.29*øø | 6.90±1.69*øøø | 8.35±2.02**øøø |
| Total Fluid Intake | ||||||
| B | ||||||
| Treatment/time | 1 h | 3 h | 6 h | 9 h | 24 h | 30 h |
| Vehicle + saline | 8.68±1.75 | 19.10±3.04 | 40.71±4.51 | 63.86±6.12 | 87.65±8.98 | 109.70±11.08 |
| PEA + saline | 7.69±3.58 | 14.96±4.12 | 33.82±5.34 | 49.93±7.84 | 62.80±9.99 | 83.81±12.69 |
| Vehicle + LPS | 2.58±0.67 | 2.83±0.65 | 3.31±0.71*** | 4.37±0.95*** | 6.92±1.79*** | 11.45±2.98*** |
| PEA + LPS | 2.33±0.61 | 4.37±1.47 | 3.31±0.74***ƒ ƒ ƒ | 4.26±0.86***ƒ ƒ ƒ | 7.79±1.74***ƒ ƒ ƒ | 15.24±3.32***ƒ ƒ ƒ |
Discussion
Recent studies have demonstrated that PEA and OEA endogenous levels are regulated in several CNS pathologies (Baker et al., 2001; Hansen et al., 2001; Schabitz et al., 2002; Berger et al., 2004; Degn et al., 2007; Bisogno et al., 2008; Hill et al., 2009; Hauer et al., 2013) and in acute inflammatory conditions induced by LPS (Balvers et al., 2012). Because of the proposed homeostatic protective role for both bioactive lipids, this acute response could be considered as part of an antiinflammatory protective homeostatic response regulating cell survival and damage (Fidaleo et al., 2014). Herewith, to further investigate the role of both acylethanolamides as a possible homeostatic mechanism in the brain, we decided to explore whether their exogenous administration might serve as a new neuroprotective pharmacologic manoeuvre.
Our study provides new evidence of the brain antiinflammatory properties of OEA and PEA in a model of neuroinflammation in vivo. Our previous data indicate that OEA crosses the blood-brain barrier and reaches the brain rapidly after i.p. administration. Specifically, peripheral administration of OEA (20mg/kg, i.p.) induced an increase in the OEA dialysate concentration in the dorsal striatum 20 minutes after injection (Gonzalez-Aparicio et al., 2014). Other authors detected a sustained 2-fold increase in OEA striatal levels over baseline for more than 2 hours after a single i.p. administration of OEA (20mg/kg), reaching the maximum peak concentration around 15 minutes postinjection (Plaza-Zabala et al., 2010). In both studies, the OEA concentration is within the range reported to produce stimulation of PPAR-α receptor-dependent transcription (120nM) (Fu et al, 2003). PEA has been reported to cross modestly the blood brain barrier after an oral dose (Artamonov et al., 2005). Nevertheless, in the present study we observed that OEA and PEA prevented the LPS-induced increase in plasma TNF-α levels. These results, together with the studies mentioned above, indicate that the antiinflammatory effects of OEA and PEA observed in the brain may be a consequence of the modulation of peripheral inflammation (ie, modulation of innate immune TLR4 receptors) by these acylethanolamides and/or the direct action in the CNS. Disregarding the mechanisms involved, the brain is deeply affected by OEA and PEA pretreatments.
Here, we observed that OEA prevented LPS-induced increase in cortical TNF-α mRNA levels and both acylethanolamides reduced NF-κB activation, the expression of iNOS and COX-2, accumulation of NO2 -, and lipid peroxidation in frontal cortex. We supply further confirmation of this antiinflammatory mechanism by showing OEA and PEA reductions in LPS-induced increases in mPGES-1 and PGE2 levels.
We also provide the first evidence to our knowledge supporting a differential role for OEA and PEA influencing the acute-phase responses after LPS. Thus, OEA and PEA did not modify the increase in plasma corticosterone levels elicited by LPS. In the hypothalamus, OEA and PEA potently altered the expression of IL-1β, COX-2, and PGE2, which are presumably mediators of body temperature regulation, and they enhanced the hypothermic response 60 minutes after LPS administration. Interestingly, at a behavioral level, only OEA affected the motivational state of the animals by inhibition of LPS-induced anhedonia, demonstrating that OEA might exert important roles in controlling motivational processes (hedonic responses) as described for fat-containing food (Rodriguez de Fonseca et al., 2001; Tellez et al., 2013).
These selective effects of both acylethanolamides on LPS-induced acute-phase responses might reflect differential mechanisms of action that need to be further explored. OEA/PEA binding to PPAR-α receptor may mediate these effects (Fidaleo et al., 2014), but PPAR-α independent actions of these acylethanolamides cannot be ruled out.
The antiinflammatory profile of both acylethanolamides has been previously described in vitro, where OEA was shown to reduce iNOS, COX-2, and the cytokines TNF-α and IL-6 in blood vessels after LPS-induced LDL modification and inflammation (Fan et al., 2014) and in animal models of inflammatory and neurophatic pain (Lo Verme et al., 2005; Suardiaz et al, 2007; Di Cesare Mannelli et al., 2013).
OEA and PEA blocked the expression and/or activity of the p65 subunit in cortical nuclear extracts, which mediates most of the NF-κB transcriptional activity. LPS also increased the expression of the NF-κB inhibitory protein IκBα in cytosolic extracts, which can be considered an autoregulatory mechanism switched on by NF-κB to block its stimulation, and was similarly prevented by OEA and PEA pretreatments. Our results are in agreement with other studies where PEA and OEA prevented IκBα degradation and p65 NF-κB nuclear translocation in peripheral hyperalgesia (D’Agostino et al., 2009) and stroke injury (Sun et al., 2007; Ahmad et al., 2012b).
Sickness behavior after LPS was evaluated by measurement of the following acute-phase responses: activation of HPA axis, body temperature regulation, and anhedonia. Activation of HPA axis was checked by measurement of plasma corticosterone levels. LPS induced an increase in plasma corticosterone that has been previously reported (Pérez-Nievas et al., 2010). However, neither OEA nor PEA prevented the rise in corticosterone induced by LPS. Our results are in agreement with a previous study in which the administration of URB597, a selective inhibitor of FAAH that enhances the levels of AEA, OEA, and PEA, did not alter an LPS-induced increase in plasma corticosterone (Kerr et al., 2012). However, the bidirectional relationship between endocannabinoids and plasma glucocorticoids released in the stress response is well documented (Gorzalka et al., 2008; Hill et al., 2010). It is necessary to develop more detailed neuroendocrine studies regarding the time course of synthesis and release of corticosterone and other stress hormones after LPS to completely discard a role of noncannabinoid acylethanolamines in the regulation of HPA axis activation.
Regarding temperature regulation, we observed a marked hypothermia induced by LPS immediately after its administration and lasting between 3 and 6 hours. Our results are in agreement with other studies reporting dose- and serotype-specific effects of LPS: high doses (0.25 -0.5 mg/kg, i.p.) of E.coli O111:B4 induced a monophasic hypothermic response in rodents (Akarsu and Mamuk, 2007). It is important to note that, although fever is a most predicted response, hypothermia occurs in the most severe cases of sepsis (Clemmer et al., 1992; Arons et al., 1999). It has been suggested that the hypothermia in response to LPS is caused by reduced thermogenesis, involves antipyretic products released from peripheral macrophages, and is mediated by prostaglandins (Derijk RH et al., 1994). In our study, the onset of this hypothermic response caused by LPS is around the time of sacrifice of the animals (150 minutes). Biochemical determinations revealed that, at this time point, LPS induced a marked increase in pyretic molecules, such as IL-1β, COX-2, and PGE2 in the hypothalamus, probably as a homeostatic mechanism to recover normal temperature. Interestingly, OEA and PEA pretreatments potentiated the hypothermic response 60 minutes after LPS. Body temperature regulation is a highly preserved homeostatic response that is probably difficult to maintain altered by a single dose of these endogenous components. We observed robust effects of both acylethanolamides preventing the LPS-induced high increases in IL-1β, COX-2, mPGES-1 mRNAs, and PGE2 levels, which strengthens our hypothesis of OEA and PEA attempting to maintain the hypothermia induced by LPS. Hypothermia can be understood as an adaptive response that enhances recovery by conserving energy to combat acute inflammation and enhance survival (Leon, 2004; Maes et al., 2012). Recently, another N-ethanolamide derived from fatty acids, commonly known as the endocannabinoid AEA, has been involved in the LPS-induced thermic response through action on CB1 receptors (Steiner et al., 2011), and a role for COX-1 and not COX-2 has been suggested for LPS-induced hypothermia (Steiner et al., 2009). Interestingly, peripheral and brain AEA levels are elevated during the systemic inflammatory response to LPS (Liu et al., 2003; Fernandez-Solari et al., 2006). However, Kerr and colleagues (2012) reported that LPS failed to alter AEA, OEA, and PEA levels in the hypothalamus.
The sickness behavior is also characterized by a behavioral inhibition, physio-somatic disturbances such as fatigue and malaise, and an inability to feel pleasure or anhedonia (Maes et al., 2012). In our study, the influence of OEA and PEA in motivational behavior was tested by checking anhedonia in a saccharine preference test. LPS-injected animals pretreated with OEA, but not PEA, showed a preference for the natural reward saccharine similar to control animals, which is interpreted as a disruption of LPS-induced anhedonia. Anhedonia is a prolonged effect of LPS that persists beyond the acute sickness response, and this behavioral change is thought to reflect a depressive-like phenotype (Willner et al., 1987). Modulation of LPS acute neuroinflammatory responses by OEA can therefore elicit long-lasting motivational behavioral effects and possibly antidepressant-like effects. Total amount of liquid (water plus saccharine solution) was, however, reduced in LPS-injected rats independently of any pretreatment. A decrease in total drinking could be indicative of behavioral inhibition or fatigue during LPS-induced sickness behavior. Despite the fact it is a satiety factor (Romano et al., 2014), OEA at a single dose did not modify the preference for fluids or the total drinking in control animals. However, interestingly, OEA affected the saccharine preference in LPS rats and modified the anhedonic state after LPS, inducing a positive motivational state similar to control animals. Elimination of motivational deficits by OEA could be linked with a role of this lipid mediator on the control of dopamine release in the reward system. This effect has been clearly demonstrated for high fat-containing foods (Tellez et al., 2013) or nicotine-mediated reward (Mascia et al., 2011; Buczynski et al., 2012). Alternatively, anhedonia has been directly related with lasting lipid peroxidation alterations in the prefrontal cortex in a chronic stress depression paradigm (Cline et al., 2014). In our study, OEA prevented both lipid peroxidation in frontal cortex and anhedonia after LPS. Further studies will be necessary to ascertain whether inhibition of LPS-induced lipid peroxidation by OEA is long lasting and may be related with the OEA antianhedonic effect.
The proposed neuroprotective effects of OEA and PEA may derive in part from their antiinflammatory and antioxidative functions, as well as their modulation of neuronal activity (Melis et al., 2013). Given the importance of neuroinflammation in the physiopathology of neuropsychiatric diseases, our results suggest that OEA and PEA might help delay the onset of neurodegenerative and neuropsychiatric diseases by reducing the insults to brain functions. Finally, from a translational point of view, OEA might also have a beneficial profile as a therapeutic agent, since it may ameliorate the motivational state of individuals with neuroinflammatory or immune related neuropsychiatric conditions.
Acknowledgments
This research was supported by The Spanish Ministry of Health and Social Policy (PNSD, PR29/11-18295 to L.O.), the Regional Government of Madrid (S2011/BMD-2308. CANNAB to JC.L.), Universidad Complutense-Santander (2878–920140 to J.C.L.), and Consejería de Salud y Bienestar Social, Junta Andalucía (PI0228-2013). B.G.-B. is a Ramón y Cajal postdoctoral fellow (Spanish Ministry of Education and Science).
Interest Statement: None.
References
- Ahmad A, Genovese T, Impellizzeri D, Crupi R, Velardi E, Marino A, Esposito E, Cuzzocrea S.(2012a) Reduction of ischemic brain injury by administration of palmitoylethanolamide after transient middle cerebral artery occlusion in rats. Brain Res 1477:45–58. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Crupi R, Impellizzeri D, Campolo M, Marino A, Esposito E, Cuzzocrea S.(2012b) Administration of palmitoylethanolamide (PEA) protects the neurovascular unit and reduces secondary injury after traumatic brain injury in mice. Brain, behavior, and immunity 26:1310–1321. [DOI] [PubMed] [Google Scholar]
- Akarsu ES, Mamuk S. (2007). Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol Regul Integr Comp Physiol 292:R1846–R1850. [DOI] [PubMed] [Google Scholar]
- Almasi R, Szoke E, Bolcskei K, Varga A, Riedl Z, Sandor Z, Szolcsanyi J, Petho G. (2008). Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci. 82:644–651. [DOI] [PubMed] [Google Scholar]
- Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. (1999). Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med 27:699–707. [DOI] [PubMed] [Google Scholar]
- Artamonov M, Zhukov O, Shuba I, Storozhuk L, Khmel T, Klimashevsky V, Mikosha A, Gula N. (2005). Incorporation of labelled N-acylethanolamine (NAE) into rat brain regions in vivo and adaptive properties of saturated NAE under x-ray irradiation. Ukr Biokhim Zh. 77(6):51–62. [PubMed] [Google Scholar]
- Bachur NR, Masek K, Melmon KL, Udenfriend S. (1965). Fatty acid amides of ethanolamine in mammalian tissues. J Biol Chem 240:1019–1024. [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. (2001). Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J 15:300–302. [DOI] [PubMed] [Google Scholar]
- Balvers MG, Verhoeckx KC, Meijerink J, Bijlsma S, Rubingh CM, Wortelboer HM, Witkamp RF. (2012). Time-dependent effect of in vivo inflammation on eicosanoid and endocannabinoid levels in plasma, liver, ileum and adipose tissue in C57BL/6 mice fed a fish-oil diet. Int Immunopharmacol. 13:204–214. [DOI] [PubMed] [Google Scholar]
- Berger C, Schmid PC, Schabitz WR, Wolf M, Schwab S, Schmid HH. (2004). Massive accumulation of N-acylethanolamines after stroke. Cell signalling in acute cerebral ischemia? J Neurochem 88(5):1159–1167. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Blanco E, Luque-Rojas MJ, Suarez J, Palomino A, Vida M, Araos P, Bermudez-Silva FJ, Fernandez-Espejo E, Spanagel R, Rodriguez de Fonseca F. (2013). Oleoylethanolamide dose-dependently attenuates cocaine-induced behaviours through a PPARalpha receptor-independent mechanism. Addict Biol 18:78–87. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Martire A, Petrosino S, Popoli P, Di Marzo V. (2008). Symptom-related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington’s disease. Neurochem Int. 52:307–13. [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Polis IY, Parsons LH. (2013). The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology 38:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmer TP, Fisher CJ, Bone RC, Slotman GJ, Metz CA, Thomas FO. (1992). Hypothermia in the sepsis syndrome and clinical outcome. Crit Care Med 20:1395–1401. [DOI] [PubMed] [Google Scholar]
- Cline BH, Anthony DC, Lysko A, Dolgov O, Anokhin K, Schroeter C, Malin D, Kubatiev A, Steinbusch HW, Lesch KP, Strekalova T. (2014). Lasting downregulation of the lipid peroxidation enzymes in the prefrontal cortex of mice susceptible to stress-induced anhedonia. Behav Brain Res pii:S0166-4328(14)00255-1 doi:10.1016/j.bbr.2014.04.037. [DOI] [PubMed] [Google Scholar]
- Coppola M, Mondola R. (2013). Palmitoylethanolamide: from endogenous cannabimimetic substance to innovative medicine for the treatment of cannabis dependence. Med Hypotheses 81:619–622. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. (1996). Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 384:83–87. [DOI] [PubMed] [Google Scholar]
- D’Agostino G, La Rana G, Russo R, Sasso O, Lacono A, Esposito E, Mattace Raso G, Cuzzocrea S, Loverme J, Piomelli D, Meli R, Calignano A. (2009). Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur J Pharmacol 613:54–9. [DOI] [PubMed] [Google Scholar]
- Das NP, Ratty AK. (1987). Studies on the effects of the narcotic alkaloids, cocaine, morphine, and codeine on nonenzymatic lipid peroxidation in rat brain mitochondria. Biochem Med Metab Biol 37:258–264. [DOI] [PubMed] [Google Scholar]
- Degn M, Lambertsen KL, Petersen G, Meldgaard M, Artmann A, Clausen BH, Hansen SH, Finsen B, Hansen HS, Lund TM. (2007). Changes in brain levels of N-acylethanolamines and 2-arachidonoylglycerol in focal cerebral ischemia in mice. J Neurochem 103:1907–1916. [DOI] [PubMed] [Google Scholar]
- Derijk RH, Van Kampen M, Van Rooijen N, Berkenbosch F. (1994). Hypothermia to endotoxin involves reduced thermogenesis, macrophage-dependent mechanisms, and prostaglandins. Am J Physiol 266:R1–8. [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, D’Agostino G, Pacini A, Russo R, Zanardelli M, Ghelardini C, Calignano A. (2013). Palmitoylethanolamide is a disease-modifying agent in peripheral neuropathy: pain relief and neuroprotection share a PPAR-alpha-mediated mechanism. Mediators Inflamm 2013:328797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan A, Wu X, Wu H, Li L, Huang R, Zhu Y, Qiu Y, Fu J, Ren J, Zhu C. (2014). Atheroprotective effect of oleoylethanolamide (OEA) targeting oxidized LDL. PLoS One 9:e85337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Solari J, Prestifilippo JP, Bornstein SR, McCann SM, Rettori V. (2006). Participation of the endocannabinoid system in the effect of TNF-alpha on hypothalamic release of gonadotropin-releasing hormone. Ann N Y Acad Sci 1088:238–250. [DOI] [PubMed] [Google Scholar]
- Fidaleo M, Fanelli F, Ceru MP, Moreno S. (2014). Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands. Curr Med Chem. In press (PMID:24606520). [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. (2003). Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature 425:90–93. [DOI] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. (2011). A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci 15:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan-Rodriguez B, Suarez J, Gonzalez-Aparicio R, Bermudez-Silva FJ, Maldonado R, Robledo P, Rodriguez de Fonseca F, Fernandez-Espejo E. (2009). Oleoylethanolamide exerts partial and dose-dependent neuroprotection of substantia nigra dopamine neurons. Neuropharmacology 56(3):653–664. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Leza JC. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32:1136–1151. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. (2000). Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem 280:87–93. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Wagner JA, Kunos G. (2009). Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat 89:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Blanco E, Serrano A, Pavon FJ, Parsons LH, Maldonado R, Robledo P, Fernandez-Espejo E, de Fonseca FR. (2013). The systemic administration of oleoylethanolamide exerts neuroprotection of the nigrostriatal system in experimental Parkinsonism. Int J Neuropsychopharmacol 17:455–468. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Aparicio R, Moratalla R. (2013). Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinsons disease. Neurobiol Dis 62:416–425 [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. (2008). Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci Biobehav Rev 32:1152–1160. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Ikonomidou C, Bittigau P, Hansen SH, Hansen HS. (2001). Accumulation of the anandamide precursor and other N-acylethanolamine phospholipids in infant rat models of in vivo necrotic and apoptotic neuronal death. J Neurochem 76:39–46. [DOI] [PubMed] [Google Scholar]
- Hart BL. (1988). Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137. [DOI] [PubMed] [Google Scholar]
- Hauer D, Schelling G, Gola H, Campolongo P, Morath J, Roozendaal B, Hamuni G, Karabatsiakis A, Atsak P, Vogeser M, Kolassa IT. (2013). Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One 8:e62741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. (2009). Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 34:1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. (2010). Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. J Neurosci 30:14980–14986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Romanovsky AA. (2004). Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 9:1977–1993. [DOI] [PubMed] [Google Scholar]
- Kilaru A, Isaac G, Tamura P, Baxter D, Duncan SR, Venables BJ, Welti R, Koulen P, Chapman KD. (2010). Lipid profiling reveals tissue-specific differences for ethanolamide lipids in mice lacking fatty acid amide hydrolase. Lipids 45:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr DM, Burke NN, Ford GK, Connor TJ, Harhen B, Egan LJ, Finn DP, Roche M. (2012). Pharmacological inhibition of endocannabinoid degradation modulates the expression of inflammatory mediators in the hypothalamus following an immunological stressor. Neuroscience 204:53–63. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. (2002). Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci 25:154–159. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki DL. (1997). The acute phase response. In: Mackowiak PA. Fever: basic mechanisms and management. Lippincott-Raven: Philadelphia. [Google Scholar]
- Leon LR. (2004). Hypothermia in systemic inflammation: role of cytokines. Front Biosci 9:1877–1888 [DOI] [PubMed] [Google Scholar]
- Liu J, Batkai S, Pacher P, Harvey-White J, Wagner JA, Cravatt BF, Gao B, Kunos G. (2003). Lipopolysaccharide induces anandamide synthesis in macrophages via CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of platelet-activating factor. J Biol Chem 278:45034–45039. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. (2005). The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the antiinflammatory actions of palmitoylethanolamide. Mol Pharmacol 67:15–19. [DOI] [PubMed] [Google Scholar]
- LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Mattace-Raso G, Meli R, Hohmann A, Calignano A, Piomelli D. (2006). Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. 319:1051–1061. [DOI] [PubMed] [Google Scholar]
- MacDowell KS, Garcia-Bueno B, Madrigal JL, Parellada M, Arango C, Mico JA, Leza JC. (2013). Risperidone normalizes increased inflammatory parameters and restores antiinflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol 16:121–135. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, Leza JC. (2001). Inducible nitric oxide synthase expression in brain cortex after acute restraint stress is regulated by nuclear factor kappaB-mediated mechanisms. J Neurochem 76:532–538. [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Gałecki P, Leonard B. (2012). Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Medicine 10:66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le Foll B, Tanda G, Piomelli D, Goldberg SR. (2011). Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry 69:633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M. (2008). Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci 28:13985–13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Carta G, Pistis M, Banni S. (2013). Physiological role of peroxisome proliferator-activated receptors type alpha on dopamine systems. CNS Neurol Disord Drug Targets 12:70–77. [DOI] [PubMed] [Google Scholar]
- Orio L, Pavon FJ, Blanco E, Serrano A, Araos P, Pedraz M, Rivera P, Calado M, Suarez J, de Fonseca FR. (2013). Lipid transmitter signaling as a new target for treatment of cocaine addiction: new roles for acylethanolamides and lysophosphatidic acid. Curr Pharm Des 19:7036–7049. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C. (2006). Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3:167–175. [DOI] [PubMed] [Google Scholar]
- Paterniti I, Impellizzeri D, Crupi R, Morabito R, Campolo M, Esposito E, Cuzzocrea S. (2013). Molecular evidence for the involvement of PPAR-δ and PPAR-γ in antiinflammatory and neuroprotective activities of palmitoylethanolamide after spinal cord trauma. J Neuroinflammation 10:20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nievas BG, Madrigal JL, Garcia-Bueno B, Zoppi S, Leza JC. (2010). Corticosterone basal levels and vulnerability to LPS-induced neuroinflammation in the rat brain. Brain Res 1315:159–168. [DOI] [PubMed] [Google Scholar]
- Piomelli D. (2003). The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884. [DOI] [PubMed] [Google Scholar]
- Plaza-Zabala A, Berrendero F, Suarez J, Bermudez-Silva FJ, Fernandez-Espejo E, Serrano A, Pavon FJ, Parsons LH, De Fonseca FR, Maldonado R, Robledo P. (2010). Effects of the endogenous PPAR-alpha agonist, oleoylethanolamide on MDMA-induced cognitive deficits in mice. Synapse 64:379–389. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. (2006). Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci 26:12967–12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. (2001). An anorexic lipid mediator regulated by feeding. Nature 414:209–212. [DOI] [PubMed] [Google Scholar]
- Romano A, Karimian Azari E, Tempesta B, Mansouri A, Micioni Di Bonaventura MV, Ramachandran D, Lutz TA, Bedse G, Langhans W, Gaetani S. (2014). High dietary fat intake influences the activation of specific hindbrain and hypothalamic nuclei by the satiety factor oleoylethanolamide. Physiol Behav pii:S0031-9384(14)00230-3 doi:10.1016/j.physbeh.2014.04.039. [DOI] [PubMed] [Google Scholar]
- Saper CB. (1998). Neurobiological basis of fever. Ann NY Acad Sci 856:90–94. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Giuffrida A, Berger C, Aschoff A, Schwaninger M, Schwab S, Piomelli D. (2002). Release of fatty acid amides in a patient with hemispheric stroke: a microdialysis study. Stroke 33:2112–2114. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Zuzarte-Augustin ML, Schmid HH. (1985). Properties of rat liver N-acylethanolamine amidohydrolase. J Biol Chem 260:14145–14149. [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. (1989). Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. (2009). Cyclooxygenase-1 or -2--which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol 297:R485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner AA, Molchanova AY, Dogan MD, Patel S, Pétervári E, Balaskó M, Wanner SP, Eales J, Oliveira DL, Gavva NR, Almeida MC, Székely M, Romanovsky AA. (2011). The hypothermic response to bacterial lipopolysaccharide critically depends on brain CB1, but not CB2 or TRPV1, receptors. J Physiol 589:2415–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suardíaz M, Estivill-Torrús G, Goicoechea C, Bilbao A, Rodríguez de Fonseca F. (2007). Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 133:99–110. [DOI] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. (2007). Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol 152:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limón P, Ren X, Lam TT, Schwartz GJ, de Araujo IE. (2013). A gut lipid messenger links excess dietary fat to dopamine deficiency. Science 341:800–802. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. (1996). Endotoxin produces a depressive-like episode in rats. Brain Res 711:163–174. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang L, Ma A, Zhang X, Li W, Yang W, Chen C, Jin X. (2012). Orally administered oleoylethanolamide protects mice from focal cerebral ischemic injury by activating peroxisome proliferator-activated receptor alpha. Neuropharmacology 63:242–249. [DOI] [PubMed] [Google Scholar]