Abstract
Background:
The basolateral amygdala plays a critical role in the etiology of anxiety disorders and addiction. Pyramidal neurons, the primary output cells of this region, display increased firing following exposure to stressors, and it is thought that this increase in excitability contributes to stress responsivity and the expression of anxiety-like behaviors. However, much remains unknown about the underlying mechanisms that regulate the intrinsic excitability of basolateral amygdala pyramidal neurons.
Methods:
Ex vivo gramicidin perforated patch recordings were conducted in current clamp mode where hyper- and depolarizing current steps were applied to basolateral amygdala pyramidal neurons to assess the effects of adenosine A2A receptor modulation on intrinsic excitability.
Results:
Activation of adenosine A2A receptors with the selective A2A receptor agonist CGS-21680 significantly increased the firing rate of basolateral amygdala pyramidal neurons in rat amygdala brain slices, likely via inhibition of the slow afterhyperpolarization potential. Both of these A2A receptor-mediated effects were blocked by preapplication of a selective A2A receptor antagonist (ZM-241385) or by intra-pipette infusion of a protein kinase A inhibitor, suggesting a postsynaptic locus of A2A receptors on basolateral amygdala pyramidal neurons. Interestingly, bath application of the A2A receptor antagonist alone significantly attenuated basolateral amygdala pyramidal cell firing, consistent with a role for tonic adenosine in the regulation of the intrinsic excitability of these neurons.
Conclusions:
Collectively, these data suggest that adenosine, via activation of A2A receptors, may directly facilitate basolateral amygdala pyramidal cell output, providing a possible balance for the recently described inhibitory effects of adenosine A1 receptor activation on glutamatergic excitation of basolateral amygdala pyramidal cells.
Keywords: basolateral amygdala, intrinsic excitability, adenosine, A2A, AHP
Introduction
The basolateral amygdala (BLA) is a key brain region wherein cortical and subcortical afferents relaying sensory information consolidate onto glutamatergic pyramidal neurons (Aggleton et al., 1980; LeDoux et al., 1991). BLA pyramidal neurons are thought to assign affective value to sensory information and relay this information on to downstream limbic and cortical structures known to play an integral role in the expression of emotional behaviors, including anxiety-like behaviors (Millan, 2003; Tye et al., 2011; Felix-Ortiz et al., 2013) and reward (Stuber et al., 2011). Not surprisingly, hyperexcitability of BLA pyramidal neurons plays a critical role in the manifestation of anxiety disorders (Millan, 2003; Etkin et al., 2009; Mahan and Ressler, 2012). For example, drugs that reduce BLA excitability often attenuate anxiety in humans (McEwen and Olie, 2005) and anxiety-associated behaviors in rodents (Silberman et al., 2010). Adding to this relationship, there is now compelling evidence that stress and anxiety play critical roles in alcohol abuse disorders (Silberman et al., 2009; Koob, 2013). For example, acute alcohol consumption reduces anxiety-like behaviors, while withdrawal from alcohol can increase anxiety measures (Valdez et al., 2002; Prediger et al., 2006; McCool et al., 2010; Koob, 2013). Moreover, human epidemiological studies suggest a high degree of comorbidity between anxiety and addiction (Grant et al., 2004; Balogun et al., 2014; Tsai et al., 2014), and this relationship can be effectively recapitulated in animal models (Ciccocioppo et al., 2006; Besheer et al., 2013; Chappell et al., 2013; Butler et al., 2014). Perhaps not surprisingly, pharmacological agents that reduce anxiety-like behaviors are often efficacious at attenuating ethanol consumption (Petrakis et al., 2012; Skelly and Weiner, 2014). As the BLA is an integral component of the circuitry governing many of these behaviors, understanding how its output (ie, BLA pyramidal cell firing) is modulated becomes critical to gaining insight into these disease states.
The output of BLA projection neurons is gated by the aforementioned excitatory synaptic inputs (Aggleton et al., 1980; LeDoux et al., 1991), feed forward and feedback GABAergic interneurons (Marowsky et al., 2005; Woodruff and Sah, 2007; Silberman et al., 2010), as well as the intrinsic excitability of the neurons themselves (Rosenkranz, 2011). BLA pyramidal neurons fire a burst of action potentials in response to stimuli with strong emotional valence (Nishijo et al., 1988; Tye and Janak, 2007; Motanis et al., 2014). Coincident elevations in intracellular calcium during bursts of action potentials activate calcium-gated potassium channels, resulting in potassium efflux and a pronounced afterhyperpolarization (AHP) (Faber and Sah, 2002; Bond et al., 2004; Power et al., 2011). AHPs consist of 3 separate components, each controlled by different calcium-gated potassium channels with distinguishable kinetics. The fast AHP (fAHP) is mediated by voltage-sensitive potassium channels as well as large conductance calcium-activated potassium channels (BK) (Poolos and Johnston, 1999). The fAHP follows single action potentials and decays within tens of milliseconds (Lancaster et al., 1991). Conversely, the medium (mAHP) and slow (sAHP) AHPs follow trains of action potentials, are mediated by small conductance potassium channels (SK), and have much slower decay kinetics (Blatz and Magleby, 1986; Faber, 2009; Power et al., 2011; Adelman et al., 2012).
AHPs play an integral role in the regulation of intrinsic neuronal excitability, and suppression of the AHP enhances neuronal firing (Hopf et al., 2010a, 2010b; Sanchez et al., 2011). Likewise, positive modulation of SK channels, and the subsequent enhancement of AHPs, reduces action potential frequency (Lujan et al., 2009; Hopf et al., 2010b). Importantly, disruption of SK channel activity and the ensuing dysregulation of neuronal output appear to play an important role in the neurobiological response to stressors such as fear conditioning (Santini et al., 2008; McKay et al., 2009; Motanis et al., 2014), restraint stress (Rosenkranz et al., 2010; Hetzel and Rosenkranz, 2014), and chronic exposure to ethanol (Hopf et al., 2010a, 2010b; Mulholland et al., 2011).
Over the years, the neuromodulator adenosine and its receptor system have gained appreciation as key regulators of neuronal excitability (Dunwiddie and Masino, 2001). Although 4 receptor subtypes have been identified (Daly et al., 1983), the primary action of adenosine within the central nervous system is mediated through the A1 and A2A subtypes (Fredholm and Dunwiddie, 1988). We recently demonstrated that adenosine A1 receptors reside at a presynaptic locus at glutamatergic synapses onto BLA pyramidal neurons and that activation of these receptors powerfully inhibits cortical and thalamic glutamate release (Rau et al., 2014). Notably, A1 receptor activation had no effect on the intrinsic excitability of these neurons. In contrast to the Gi/Go coupled A1 receptors (Londos et al., 1980; Fredholm and Dunwiddie, 1988), adenosine A2A receptors are G-protein-coupled receptors that upon activation liberate the Gs protein (Abbracchio et al., 2009). Gs proteins increase intracellular protein kinase A (PKA) via cAMP signaling (Castellucci et al., 1980; Hille, 1992) and can modulate intrinsic excitability (Faber and Sah, 2002). For example, activation of β-adrenoceptors enhances neuronal excitability in multiple brain regions, an effect attributed to PKA-dependent inhibition of sAHP amplitude (Dunwiddie et al., 1992; Pedarzani and Storm, 1993; Faber et al., 2008). Given the relatively high expression of A2A receptors in the rodent BLA (Braas et al., 1986; Jarvis et al., 1989), we developed the a priori hypothesis that activation of A2A receptors on BLA pyramidal neurons would inhibit AHPs and increase the intrinsic excitability of these cells.
To address this hypothesis, we employed the gramicidin perforated patch technique to evaluate the role of adenosine A2A receptors in the modulation of BLA pyramidal cell intrinsic excitability. Gramicidin is a polypeptide antibiotic that forms pores in the neuronal membrane selectively permeable to monovalent cations (Akaike and Harata, 1994). This technique allows for the study of neuronal firing without substantial cytosolic dialysis, thus leaving the intracellular signaling machinery more intact than traditional whole-cell recordings (Akaike, 1996). This is particularly relevant for studying AHPs, which are sensitive to G-protein regulation and are easily disrupted by patch clamp solutions (Kaczorowski et al., 2007). Our results suggest that A2A receptors are located postsynaptically on BLA pyramidal neurons and that activation of these receptors increases the intrinsic excitability of BLA pyramidal neurons through a PKA-dependent inhibition of the sAHP. Additionally, we present evidence that this mechanism is tonically active in the BLA, providing a possible excitatory balance to the recently described A1 receptor-dependent inhibitory effects of adenosine in this brain region.
Experimental Procedures
Male Long Evans rats between the ages of 4 and 7 weeks, an age typically associated with rodent adolescence, were used for all experiments. Animals arrived from a commercial supplier (Harlan Laboratories, Indianapolis, IN) at PD 21 and were allowed to acclimate for 1 week. Animals were pair housed in a vivarium with a 12-hour light-dark cycle and had ad libitum access to food and water. All experiments were performed in accordance with the Wake Forest University Animal Care and Use Committee and The Guide for the Care and Use of Laboratory Animals set forth by the National Institutes of Health.
Electrophysiological Recordings
Rats were anesthetized with halothane, decapitated, and their brains were removed and placed into ice-cold artificial cerebral spinal fluid (aCSF) consisting of (in mM): 124 NaCl, 3.3 KCl, 2.4 MgCl2, 1.2 KH2PO4, 10 d-glucose, and 25 NaHCO3 and bubbled with 95% O2 and 5% CO2. Then, 400 µm transverse slices containing the basolateral area of the amygdala were cut using a Leica VT1000S vibratome (Leica Microsystems Inc., Buffalo Grove, IL). Incubation of slices occurred for >1 hour at room temperature (22–25°C) in aCSF before experiments commenced.
Slices were transferred to a recording chamber and perfused with oxygenated, room temperature aCSF at 2mL/min. Filamented borosilicate glass capillary tubes (inner diameter 0.86 µm) were pulled using a horizontal pipette puller (P-97; Sutter Instruments, Novato, CA) to prepare recording electrodes. All recordings were acquired using an Axoclamp 2B amplifier, digitized (Digidata 1321 A; Axon Instruments, Union City, CA), and analyzed online and offline using an IBM-compatible computer and pClamp 10.4 software (Axon Instruments).
Whole cell patch clamp recordings were made from pyramidal neurons within the basolateral area of the amygdala (targeted at the basolateral nucleus). Spontaneous EPSCs were recorded using an internal solution containing (in mM) 140 gluconic acid, 140 CsOH, 10 CsCl, 10 HEPES, 1.1 EGTA, 0.1 CaCl2, 2 ATP, 0.3 GTP. pH was adjusted to 7.25 with 2M CsOH. Osmolarity was 270 to 280 mOsm. For recording of spontaneous IPSCs, gluconic acid and CsOH was replaced with 150mM CsCl. For both sEPSC and sIPSC experiments, 5mM N-(2,6-dimethyl-phenlcarbamoyl-methy)-triethylammonium chloride (QX-314) was added to the patch solution to block voltage-gated sodium channels. Cells were voltage-clamped at -65 to -70 mV.
For perforated patch experiments, gramicidin was diluted in dimethylsulfoxide (DMSO) to a stock concentration of 50mg/mL. The stock solution was further diluted to a final concentration of 200 µg/mL in a patch-pipette solution containing (in mM) KCl 135, HEPES 10, MgCl2 2, Na2-EGTA 5, CaCl2 0.5, adjusted to 7.2 pH with KOH. The KCl-gramicidin solution was sonicated for 5 minutes at the beginning of each day and vortexed for 15 to 30 seconds before filling each electrode. No filtering was applied. Each electrode was backfilled with gramicidin-free KCl to avoid interference of the antibiotic with seal formation, and the remainder of the electrode was filled with KCl-gramicidin. After forming a high-resistance seal (GOhm), the cell was held in current-clamp mode for 25 to 75 minutes until perforation occurred and access resistance stabilized. All intrinsic excitability recordings were conducted in current clamp mode where cells were maintained at a membrane potential of -60 mV with direct current injection. Occasionally, the perforated patch would rupture suddenly. This was associated with a rapid depolarizing shift in the membrane potential (approximately 6 mV) and a dramatic reduction in access resistance. These cells were excluded from analysis.
To examine the effect of adenosine A2A receptor modulation on neuronal excitability, BLA pyramidal neurons were injected with 600ms hyper- and depolarizing current pulses ranging from -300 pA to 550 pA in 50-pA increments. Current injections were separated by a 15-second interstimulus interval, resulting in a 270-second trial. The number of action potentials evoked by each current injection was counted and averaged across 5 trials for the baseline condition. Adenosinergic modulators were then bath applied for 7 trials (1890 seconds), and drug effects were quantified from an average of the last 5 of these trials. Input resistance was monitored throughout recordings by calculating the slope of the input/output relationship generated by the hyperpolarizing current injections. Resting membrane potential was assessed at the beginning of the recording period, following the stabilization of access resistance, and also was monitored periodically throughout the recordings. Any changes detected in resting membrane potential were offset with adjustments to the amount of injected current to maintain cells at -60 mV.
Picrotoxin (100 µM) picrotoxin was added to the aCSF to block GABAA receptors during spontaneous EPSC experiments. Conversely, 20 µM DNQX was bath applied during spontaneous IPSC experiments to block AMPA receptors. For all intrinsic excitability experiments, 100 µM picrotoxin and 20 µM DNQX were added to the superfusate to block GABAA and AMPA receptors, respectively.
Unless otherwise indicated, all drugs were purchased from Sigma (St. Louis, MO). Adenosinergic modulators were purchased from Tocris (Ellisville, MO) and prepared as 100- to 400-fold concentrates in water and DMSO and applied directly into the perfusion chamber via calibrated syringe pumps (Razel Scientific Instruments, Stanford, CT). Bath DMSO concentration did not exceed 0.05%, a concentration that had no effect on intrinsic excitability of BLA pyramidal neurons (data not shown).
Statistics
Data are expressed as mean ± SEM throughout the text and figures. Paired t tests were used when comparing drug effects to baseline. When multiple measures were compared between groups (eg, input-output relationships), a 2-way, repeated-measures ANOVA was used. Where noted, the Bonferroni multiple comparisons test was used as a posthoc analysis to compare group differences at individual current injections. The minimal level of significance was set as P<.05 for all analyses. All statistical analyses were conducted using Prism version 6.0 (GraphPad Software, La Jolla, CA).
Results
Effect of Adenosine A2A Receptor Activation on Spontaneous Synaptic Transmission
A2A Receptor Activation Did Not Modulate Spontaneous Excitatory Postsynaptic Currents onto BLA Pyramidal Neurons
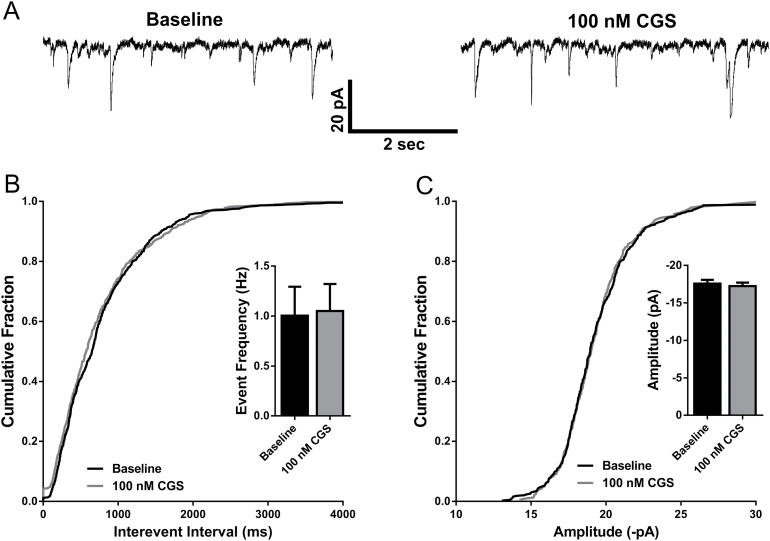
Our previous work has demonstrated that activation of adenosine A1 receptors inhibits glutamate release onto BLA pyramidal neurons (Rau et al., 2014). To investigate whether activation of A2A receptors modulates either pre- or postsynaptic excitatory transmission in the BLA, we bath applied 100nM CGS-21680, a potent and selective A2A receptor agonist, while recording spontaneous EPSCs onto BLA pyramidal neurons. Neurons were held at -70 mV in voltage clamp mode while monitoring spontaneous events in 3-minute epochs in the presence of 100 µM picrotoxin, both during a baseline period and during drug application. Consistent with our previous work (Rau et al., 2014), we did not observe a change in either the frequency (n=10, P=.6981, paired t test; Figure 1A-B) or amplitude (n=10; P=.1192, paired t test; Figure 1A,C) of sEPSCs.
Figure 1.
Adenosine A2A receptor activation does not modulate excitatory synaptic transmission onto basolateral amygdala (BLA) pyramidal neurons. (A) Representative current traces of sEPSCs recorded from a BLA pyramidal cell at baseline and in the presence of 100nM CGS-21680. (B) The distribution of cumulative interevent intervals of sEPSCs under baseline and 100nM CGS-21680 recording conditions. Inset: bar graph depicting mean frequency of sEPSCs at baseline and following bath application of 100nM CGS-21680 (NS, P>.05, paired t test; n=10). (C) The cumulative distribution of sEPSC amplitudes at baseline and following 100nM CGS-21680 application. Inset: bar graph depicting mean sEPSC amplitude at baseline and following 100nM CGS-21680 application (NS, P>.05, paired t test; n=10).
A2A Receptor Activation Did Not Modulate Spontaneous Inhibitory Postsynaptic Currents onto BLA Pyramidal Neurons
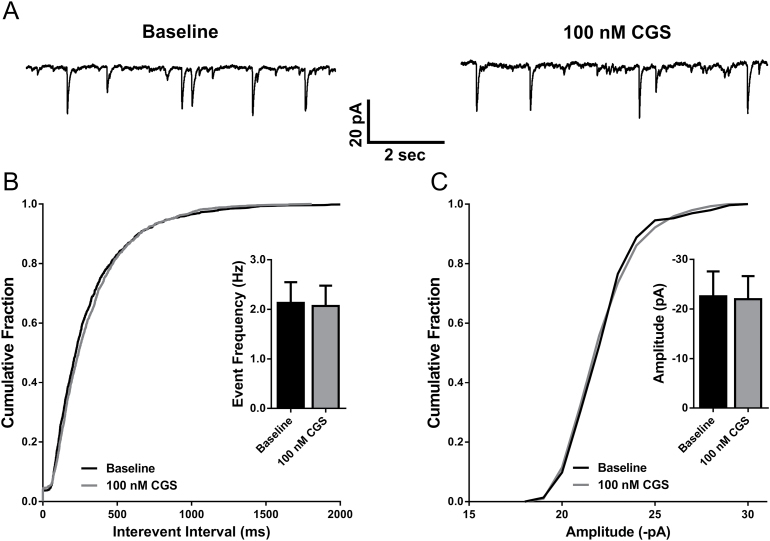
It was also of interest to evaluate whether activation of A2A receptors affects inhibitory synaptic transmission in the BLA. In other brain regions, A2A receptors located on GABAergic interneurons increase GABA release (Mayfield et al., 1993; Wirkner et al., 2004). However, under our recording conditions, we did not observe any changes in frequency (n=8; P=.7575, paired t test; Figure 2A-B) or amplitude (n=8; P=.2032, paired t test; Figure 2A,C) of sIPSCs.
Figure 2.
Adenosine A2A receptor activation does not modulate inhibitory synaptic transmission onto basolateral amygdala (BLA) pyramidal neurons. (A) Representative current traces of sIPSCs recorded from a BLA pyramidal cell at baseline and in the presence of 100nM CGS-21680. (B) The distribution of cumulative interevent intervals of sIPSCs under baseline and 100nM CGS-21680 recording conditions. Inset: bar graph depicting mean frequency of sIPSCs at baseline and following bath application of 100nM CGS-21680 (NS, P>.05, paired t test; n=8). (C) The cumulative distribution of sIPSC amplitudes at baseline and following 100nM CGS-21680 application. Inset: bar graph depicting mean sIPSC amplitude at baseline and following 100nM CGS-21680 application (NS, P>.05, paired t test; n=8).
Modulation of BLA Pyramidal Cell Intrinsic Excitability Following Activation of Adenosine A2A Receptors
CGS-21680 Increased Pyramidal Neuron Firing in Response to Depolarizing Current Steps
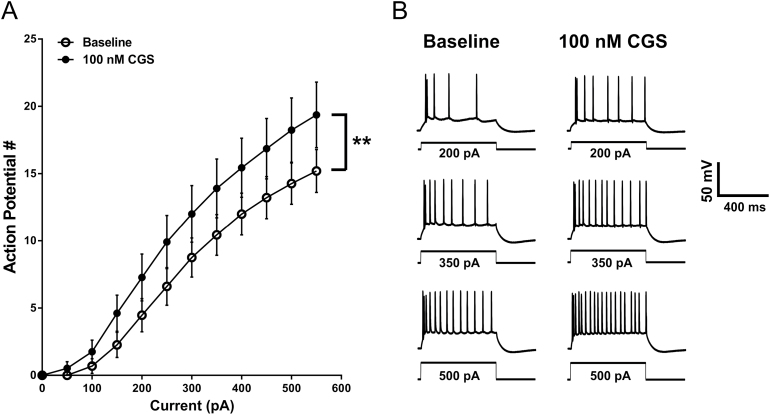
To evaluate intrinsic excitability, the frequency of action potentials in response to depolarizing currents steps was measured from BLA pyramidal neurons in the presence of GABAA and glutamate receptor antagonists (see Methods). Gramicidin perforated patch recordings were performed in current clamp mode where 600-ms hyperpolarizing and depolarizing current steps were applied (-300 pA to 550 pA in 50-pA increments; 15-second ISI). Between current pulses, neurons were held at -60 mV with direct current injection. Pyramidal neurons were characterized as those cells that displayed spike frequency adaptation, broad action potentials, and the presence of an AHP and lacked spontaneous discharge at resting membrane potential (Washburn and Moises, 1992). Neurons displayed a significant increase in firing in response to depolarizing current injections following bath application of 100nM CGS-21680 (n=11; F CGS (1, 10)=14.32, P=.0036; F interaction (11,110)=5.659, P<.0001, 2-way repeated-measures (RM) ANOVA; posthoc analysis indicated a significant difference from baseline at all currents ≥150 pA) (Figure 3A-B). We did not observe an increase in intrinsic excitability when we bath applied a lower (40nM) concentration of CGS-21680 (n=8 neurons; F CGS (1, 7)=0.05895, P=.8151; F interaction (11, 77)=1.045, P=.4163, 2-way RM ANOVA) (supplementary Figure 1A). However, CGS-21680 did significantly potentiate action potential firing at a concentration of 400nM (n=9; F CGS (1, 8)=7.169, P=.028; F interaction (11, 88)=7.058, P<.0001, 2-way RM ANOVA) (supplementary Figure 1B). The 100 nM concentration of CGS-21680 was used for the remainder of the study.
Figure 3.
Basolateral amygdala (BLA) pyramidal cell intrinsic excitability is enhanced by adenosine A2A receptor activation. (A) The relationship between depolarizing current injection and the number of action potentials elicited at baseline and following 100nM CGS-21680 application (**, F CGS (1, 10)=14.32, P=.0036, 2-way RM ANOVA, n=11). (B) Representative voltage responses to multiple depolarizing current injections recorded from BLA pyramidal neurons during baseline and in the presence of 100nM CGS-21680.
Passive and Active Membrane Properties Were Unaffected by A2A Receptor Activation
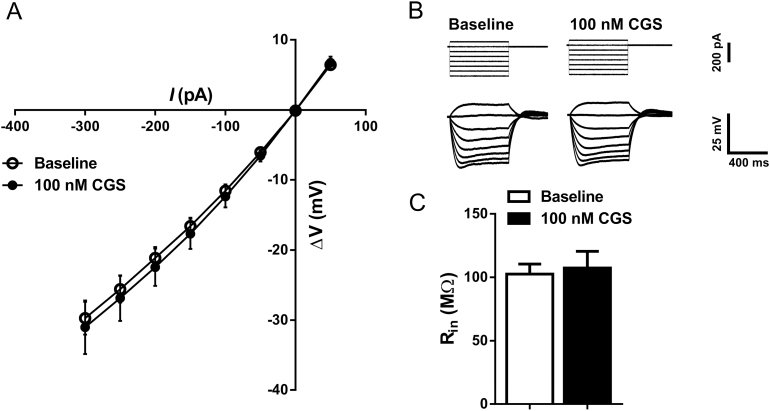
Activation of adenosine receptors can modulate G protein-coupled inwardly rectifying potassium channels (Dunwiddie and Masino, 2001; Takigawa and Alzheimer, 2002), and G protein-coupled inwardly rectifying potassium channel activity has been associated with changes in intrinsic excitability (Arora et al., 2010; Ma et al., 2012). To determine if this mechanism was involved in the CGS-mediated increase in intrinsic excitability, a current to voltage input-output slope was generated from hyperpolarizing current steps ranging from -300 to 50 pA. Two-way, RM ANOVA revealed no effect of CGS-21680 on this input-output relationship (n=11; F CGS (1,10)=0.2076, P=.6584; F interaction (7,70) =0.1552, P=.9927, 2-way RM ANOVA) (Figure 4A-B). Consistent with this observation, CGS-21680 did not modulate input resistance (P=.7172, paired t test) (Figure 4C; Table 1). Likewise, CGS-21680 did not alter rheobase (P=.3484, paired t test) or voltage sag (I h) (P=.814, paired t test) (Table 1). Neurons were held at -60 mV with direct current injection during the periods between hyper- and depolarizing current injections. However, this current was momentarily relieved at various points throughout the recording to monitor the resting membrane potential of the cell. CGS-21680 did not contribute to any appreciable changes in resting membrane potential (P=.2425, paired t test) (Table 1).
Figure 4.
A2A receptor activation has no effect on inward rectification or the input resistance of basolateral amygdala (BLA) pyramidal cells. (A) Current/voltage input-output plot generated from hyperpolarizing current injections applied to BLA pyramidal neurons at baseline and after bath application of 100nM CGS-21680 (NS, F CGS (1, 10)=0.2076, P=.6584, 2-way RM ANOVA; n=11). (B) Sample traces recorded from BLA pyramidal neurons showing voltage responses (bottom) to hyperpolarizing current injections (top) at baseline and following application of 100nM CGS-21680. (C) Bar graph summarizing the effect of 100nM CGS-21680 on BLA pyramidal cell input resistance (NS, P=.7172, paired t test; n=11).
Table 1.
Passive and Active Membrane Properties of BLA Pyramidal Neurons at Baseline and Following Bath Application of 100nM CGS-21680
| Baseline | 100nM CGS | P | |
|---|---|---|---|
| Vm (mV) | -62.36±1.42 | -61.45±1.43 | .2425 |
| Rin (MΩ) | 102.6±7.88 | 107.4±13.08 | .7172 |
| Rheobase (pA) | 301.0±33.97 | 276.0±45.95 | .3484 |
| fAHP (mV) | -6.11±1.32 | -5.28±1.41 | .1488 |
| mAHP (mV) | -3.82±0.49 | -3.25±0.46 | .1042 |
| I h (mV) | -5.58±0.76 | -5.50±0.66 | .8141 |
| AP peak | 70.86±3.67 | 71.72±2.59 | .7384 |
| AP threshold | -36.17±2.08 | -38.98±1.67 | .1717 |
Values represent mean ± SEM (P values were derived using a paired t test; n = 11).
A2A-Mediated Increase in Intrinsic Excitability Was Associated with a Decreased Slow AHP
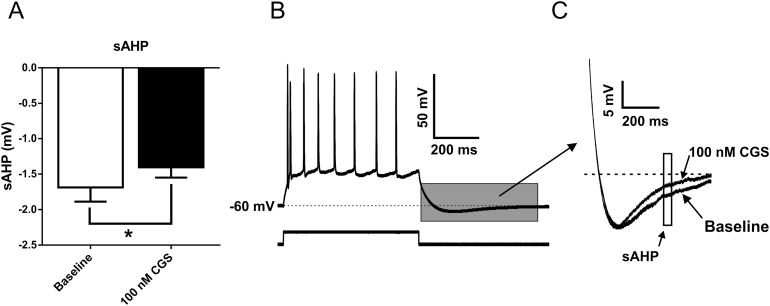
Potassium channel activity plays a critical role in the repolarization of neurons following action potentials (Faber and Sah, 2002; Power and Sah, 2008; Adelman et al., 2012). For example, enhancement of calcium-gated potassium channels results in decreased action potential frequency (Hopf et al., 2010a; Atchley et al., 2012). Potassium efflux through these channels generates an AHP that can be separated into 3 distinct components: fast, medium, and slow (Faber and Sah, 2002). For this study, fAHPs were quantified as the peak negative potential following the first action potential at the current step that elicited the least number of action potentials. No CGS-21680-dependent differences were detected in the fAHP (P=.1488, paired t test) (Table 1). The amplitude of sAHPs and mAHPs were measured from traces with similar action potential frequency, as the amplitude of these potentials is dependent upon the number of spikes (Abel et al., 2004). mAHPs were measured as the peak negative potential following the cessation of the current step, while sAHPs were quantified as the average voltage during a 50-ms window, 280ms after the end of the current step (Santini et al., 2008). mAHPs were reduced following CGS-21680 application; however, this effect was not statistically significant (P=.1042; paired t test) (Table 1). In contrast, the increase in intrinsic excitability observed following bath application of CGS-21680 was associated with a significant reduction in the amplitude of the sAHP (P=.0384, paired t test) (Figure 5).
Figure 5.
Activation of A2A receptors reduces slow afterhyperpolarizations (sAHPs) recorded from basolateral amygdala (BLA) pyramidal neurons. (A) Bar graph quantifying mean amplitude of the sAHP recorded from BLA pyramidal neurons at baseline and following activation of adenosine A2A receptors with 100nM CGS-21680 (*, P=.0384, paired t test; n=11). (B) Sample voltage response to a depolarizing current step recorded from a representative BLA pyramidal cell illustrating the portion of the recoding (grey box) that is magnified in C. (C) Representative voltage traces depicting a reduction in sAHP amplitude following bath application of 100nM CGS-21680. The sAHP was quantified as the mean voltage during a 50-ms window starting 280ms after the cessation of the current step.
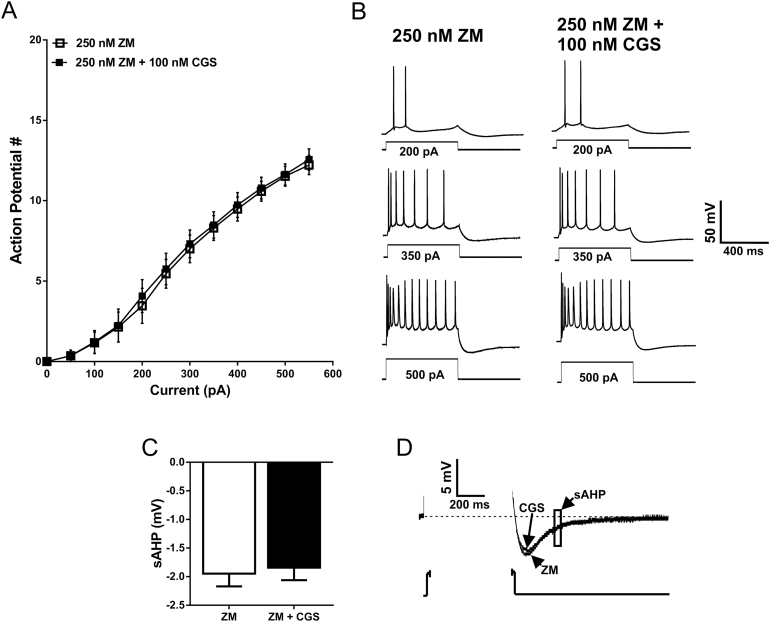
Preapplication of the Adenosine A2A Receptor Antagonist ZM-241385 Blocked the Potentiating Effect of CGS-21680 on Intrinsic Excitability
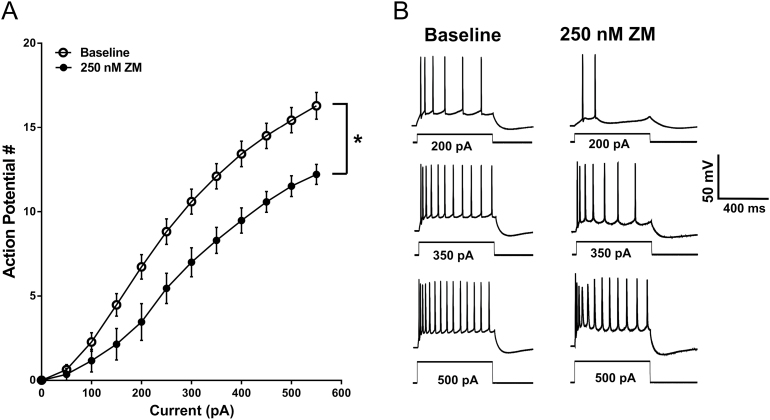
To exclude off-target effects of CGS-21680 application on neuronal firing, we bath applied the adenosine A2A receptor antagonist ZM-241385 (250nM) (Poucher et al., 1995) prior to application of 100nM CGS-21680. Baseline firing in the presence of ZM-241385 was measured with the same protocol as before using a range of hyper- and depolarizing current steps. Following a preapplication of 250nM ZM-241385, 100nM CGS-21680 was no longer effective at modulating the firing rate of BLA pyramidal neurons (n=6; F CGS (1, 6)=0.6153, P=.4626; F interaction (11, 66)=0.7056, P=.7288, 2-way RM ANOVA) (Figure 6A-B). Furthermore, preapplication of ZM-241385 also blocked the CGS-associated reduction in sAHP amplitude (P=.7459, paired t test) (Figure 6C-D). Interestingly, a significant difference was observed when baseline firing frequency from all neurons recorded under standard conditions was compared with the firing frequency in the presence of 250nM ZM-241385 (n=30 neurons for baseline, 7 neurons for ZM-241385; F ZM (1, 35)=4.840 P=.0345; F interaction (11,385)=4.457 P<.0001, 2-way RM ANOVA) (Figure 7A-B). This finding suggests that adenosinergic tone in BLA slices may be sufficient to engage A2A receptors and modulate firing frequency of BLA pyramidal neurons.
Figure 6.
Preapplication of an A2A receptor antagonist blocks the potentiating effect of 100nM CGS-21680 on basolateral amygdala (BLA) pyramidal cell intrinsic excitability and prevents the CGS-associated reduction in slow afterhyperpolarization (sAHP) amplitude. (A) Action potential responses to depolarizing current steps in the presence of the A2A receptor antagonist, ZM-241385, and in the presence of ZM-241385 and CGS-21680 (NS, F CGS (1, 6)=0.6153, P=.4626, 2-way RM ANOVA; n=7). (B) Representative voltage traces demonstrating that when ZM-241385 is preapplied (left), CGS-21680 no longer modulates action potential firing (right). (C) Group data summarizing that CGS-21680 no longer modulates sAHPs when A2A receptors are blocked by ZM-241385 (NS, P=.7459, paired t test; n=7). (D) Representative voltage traces highlighting that CGS-21680 no longer modulates sAHP amplitude when A2A receptors are blocked by ZM-241385.
Figure 7.
A2A receptor blockade is associated with a reduction in basolateral amygdala (BLA) pyramidal cell intrinsic excitability. (A) Relationship of action potential responses to depolarizing current steps under standard recording conditions (open circles) and in the presence of the A2A receptor antagonist ZM-241385 (250nM, closed circles) (*, F ZM (1, 35)=4.840, P=.0345, 2-way RM ANOVA; n=30 for baseline, 7 for ZM-241385). (B) Representative voltage responses to current steps of increasing magnitude recorded from BLA pyramidal neurons under standard recording conditions and in the presence of the A2A receptor antagonist, ZM-241385. Traces in the presence of ZM-241385 are the same as those used in Figure 6.
A2A-Mediated Enhancement of Intrinsic Excitibilty Is Dependent upon PKA Signaling
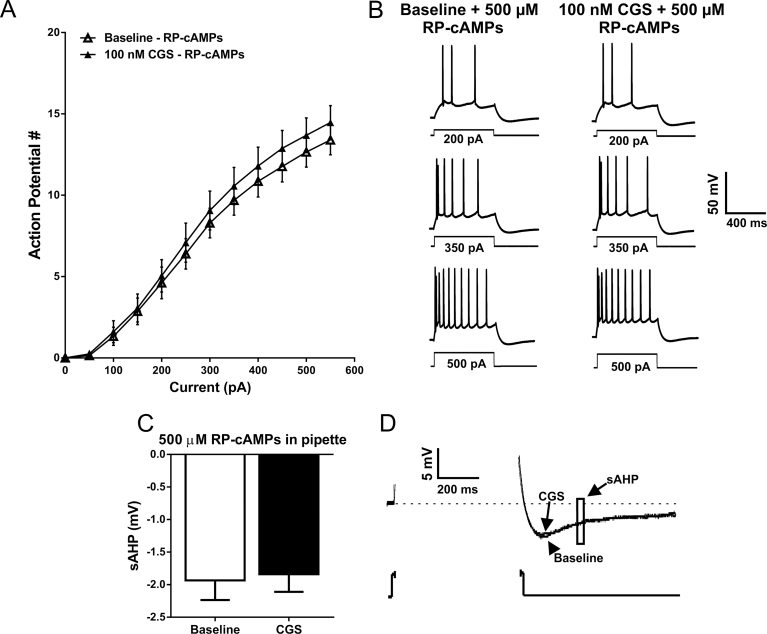
To evaluate whether activation of A2A receptors requires PKA signaling to modulate the intrinsic excitability of BLA pyramidal neurons, we included 500 µM RP-cAMPS, a PKA inhibitor, in the patch pipette. With intracellular PKA selectively blocked in the cell being recorded, bath application of CGS-2680 no longer modulated action potential firing rate in response to depolarizing current injections (n=9; F CGS (1, 8)=2.951, P=.1241; F interaction (11, 88)=1.702 P=.0859, 2-way RM ANOVA) (Figure 8A-B). Moreover, CGS-21680 did not modulate the sAHP when RP-cAMPs was included in the patch pipette (P=.2287, paired t test) (Figure 8C-D).
Figure 8.
Inclusion of a protein kinase A (PKA) inhibitor in the patch pipette blocks the CGS-associated increase in basolateral amygdala (BLA) pyramidal cell intrinsic excitability and the reduction in slow afterhyperpolarization (sAHP) amplitude. (A) The relationship between depolarizing current steps and the firing rate of action potentials elicited at baseline with the inclusion of the PKA inhibitor RP-cAMPs included in the patch pipette (open triangles) and with RP-cAMPs in the pipette following bath application of CGS-21680 (closed triangles) (NS, F CGS (1, 8)=2.951, P=.1241; F interaction (11, 88)=1.702 P=.0859, 2-way RM ANOVA; n=9). (B) Representative voltage responses to depolarizing current steps recorded from BLA pyramidal neurons with RP-cAMPs included in the patch pipette at baseline and following the bath application of 100nM CGS-21680. (C) Bar graph indicating that the CGS-associated reduction in sAHP amplitude is block when the protein kinase A (PKA) inhibitor is included in the patch pipette (NS, P=.2287, paired t test; n=9). (D) Representative voltage responses to a depolarizing current step illustrating that when RP-cAMPs is included in the patch pipette, CGS-21680 no longer modulates the amplitude of the sAHP.
Discussion
Recently, intrinsic excitability of BLA pyramidal cells has emerged as an important component of neurobiological stress responsivity. For example, firing rate and AHP amplitudes are modulated in BLA pyramidal cells following fear conditioning (McKay et al., 2009; Motanis et al., 2014) as well as acute (Guo et al., 2012) and chronic stress (Rosenkranz et al., 2010; Hetzel and Rosenkranz, 2014). Further, other regions implicated in the etiology of anxiety and addiction also display disrupted firing frequency following behavioral stressors, or drug exposure [eg, prefrontal cortex (Santini et al., 2008), ventral tegmental area (Hopf et al., 2007), hippocampus (Mulholland et al., 2011), putamen (Cuzon Carlson et al., 2011), bed nucleus of the stria terminalis (Marcinkiewcz et al., 2014), and nucleus accumbens (Hopf et al., 2010b)]. Thus, it is crucial to identify the neuromodulatory mechanisms that govern intrinsic excitability, as these neurobiological substrates would likely represent promising targets for the development of novel pharmacotherapies for the treatment of anxiety disorders. (Mitra et al., 2009; Atchley et al., 2012) and addiction (Hopf et al., 2011a, 2011b).
The results of these studies indicate that activation of adenosine A2A receptors significantly increases the intrinsic excitability of BLA pyramidal neurons without any effects on glutamatergic or GABAergic synaptic transmission. This effect was blocked by an A2A receptor antagonist as well as an intrapipette inhibitor of PKA. The increased intrinsic excitability of BLA pyramidal neurons following A2A receptor activation was associated with a reduction in sAHP amplitude, an effect that was also blocked by the A2A antagonist and the PKA inhibitor. Interestingly, the firing rate in the presence of the A2A antagonist was significantly reduced compared with firing under standard recording conditions. These findings reveal a novel role of adenosine signaling in the BLA and suggest that tonic adenosine levels may influence BLA output through nonsynaptic mechanisms. Additionally, these findings provide support for the hypothesis that postsynaptic modulation of PKA signaling plays an integral role in the shaping of AHPs and that this modulation plays a critical role in the regulation of pyramidal cell firing. Perhaps most intriguing is that these results reveal a surprising, excitatory role of adenosine in the BLA. This is in opposition to the powerful inhibitory role that A1 receptors play in this (Rau et al., 2014) and other brain regions (Dunwiddie and Masino, 2001). These findings raise the intriguing possibility that chronic stress or drug exposure may potentially shift this A1/A2A receptor balance in the BLA in favor of A2A receptors, an effect that could contribute to the increase in BLA excitability associated with anxiety disorders and addiction.
Drugs that alter intrinsic excitability represent powerful modulators of regional output. Here, we used the gramicidin perforated patch technique to probe A2A receptor effects on intrinsic excitability of BLA pyramidal neurons, as this technique allows for electrical access to the cell with minimal disruption of intracellular signaling mechanisms (Akaike and Harata, 1994; Akaike, 1996). Bath application of 100 or 400nM CGS-21680 was associated with a significant increase in action potential number across a range of depolarizing current injections, an effect blocked by preapplication of the A2A receptor antagonist ZM-241385. To our knowledge, this represents the first evidence implicating adenosine A2A receptors in the enhancement of intrinsic excitability within the BLA. Surprisingly, 40nM CGS-21680, a concentration that activates A2A receptors in other brain regions (Mayfield et al., 1993; Cunha et al., 1996), did not modulate action potential firing at any of the depolarizing current steps. As these recordings were conducted in the presence on GABAA and AMPA receptor antagonists, we can infer that the A2A receptors governing the modulation of BLA intrinsic excitability likely reside at a postsynaptic locus on pyramidal neurons. Although outside the scope of this current investigation, it will be of interest to determine if BLA A2A receptors are located somatically or on the dendrites of BLA pyramidal cells. Applicably, the recent findings of Power et al. (2011) suggest that the currents underlying the sAHP are primarily located within the dendritic tree of BLA pyramidal neurons.
Action potential frequency is mediated in part by both voltage- and calcium-activated potassium channels (Lancaster et al., 1991; Hille, 1992; Faber and Sah, 2002). K+ efflux through calcium-activated BK and SK channels particularly contributes to neuronal repolarization and mitigates excessive neuronal firing (Faber, 2009; Hopf et al., 2010a; Adelman et al., 2012). Importantly, K+ efflux through BK and SK channels overshoots the resting membrane potential, resulting in a quantifiable AHP consisting of 3 components. As a functional correlate of BK and SK channel activity, we measured AHP amplitudes following bath application of CGS-21680. Although CGS application resulted in a blunting of all AHPs measured, only the amplitude of the sAHP was significantly reduced. When A2A receptors were blocked by preapplication of ZM-241385, CGS-21680 no longer modulated sAHPs. BLA SK channels are trafficked from the membrane following activation of β-adrenergic receptors through a PKA-dependent mechanism, which can affect the AHP (Faber et al., 2008). It is possible that A2A receptors modulate sAHPs through a similar mechanism, although the direct role of SK channels in mediating the sAHP is not fully understood. Alternatively, activation of A2A receptors may modulate the AHP indirectly by decreasing calcium entry, thus reducing activation of SK channels by intracellular calcium. However, this interpretation seems unlikely as previous reports suggest that A2A activation does not alter voltage-gated calcium channel activity in BLA pyramidal neurons (McCool and Farroni, 2001).
Modulation of neuronal excitability by G-protein-coupled receptors can be mediated through multiple second messenger systems, including PKC (Melyan et al., 2004; Cohen-Matsliah et al., 2007), PKG (Gonzalez-Forero et al., 2007; Artinian et al., 2012), and PKA (Castellucci et al., 1980; Oh et al., 2009; van Welie and du Lac, 2011). Activation of A2A receptors liberates the Gs protein, which increases cAMP-dependent PKA (Hille, 1992). Inclusion of 500 µM RP-cAMPs, a potent PKA inhibitor, in the patch pipette blocked the effect of CGS-21680 on intrinsic excitability, suggesting that indeed, PKA signaling is required for A2A-associated increases in intrinsic excitability. Further, the CGS-associated decrease in sAHP amplitude was blocked by inclusion of RP-cAMPs, further supporting the hypothesis that the A2A activation-dependent increases in intrinsic excitability were driven by a reduced sAHP. Importantly, although we used the gramicidin perforated patch technique for this study, RP-cAMPs is membrane permeable (Bell and McDermott, 1994; Baudonnat et al., 2011; Arguello et al., 2014), and the duration of time in which cells are held in current clamp prior to achieving full perforation of the patch (25–75 minutes) is likely sufficient for RP-cAMPs to travel down its concentration gradient and enter the cell. Additionally, baseline firing in neurons recorded with RP-cAMPs in the patch pipette was significantly lower than baseline firing in neurons with no RP-cAMPs present (F interaction (11,407)=2.225, P=.0126; 2-way RM ANOVA), consistent with a block of intracellular PKA.
Interestingly, preapplication of ZM-241385 was associated with an attenuation of intrinsic excitability. This finding suggests that under basal conditions, tonic adenosine concentrations are high enough in the BLA to activate A2A receptors on pyramidal neurons and regulate their action potential output. Extracellular adenosine concentrations are thought to fluctuate between 20 and 300nM (Dunwiddie and Masino, 2001), sufficient to be tonically active at high affinity A2A receptors (Daly et al., 1983).
These data were collected in ex vivo brain slices maintained at ambient room temperature (22–25°C). The ex vivo recording environment may be representative of a posttraumatic/ischemic state that could influence adenosine signaling. However, recent studies suggest that adenine nucleotide levels are stable in the slice for at least 5 hours (zur Nedden et al., 2011). Moreover, adenosine and adenine nucleotide levels are thought to be similar in the slice to that of the intact brain (Fredholm et al., 1984). Ambient temperature experiments were conducted to promote the stability of the gramicidin perforated patch recordings. However, maintaining slices at subphysiological temperatures can occlude adenosine transport through equilibrative transporters, reducing extracellular levels (Masino and Dunwiddie, 1999). Therefore, it is possible that our data underestimate the extent of tonic adenosine regulation of intrinsic excitability.
These experiments raise compelling questions about the role that A2A receptors may play in the modulation of anxiety and other behaviors known to involve BLA signaling. Extensive work has been carried out investigating the role of A2A receptors in the striatum where these receptors are highly colocalized with D2 receptors on enkaphalin-positive neurons, influencing addiction-like behaviors and Parkisonian symptomology (Svenningsson et al., 1999). However, systemic administration of adenosine A2A modulators has produced inconsistent findings pertaining to ethanol self-administration. Blockade of adenosine A2A receptors reduces ethanol self-administration at high doses in Long Evans (Arolfo et al., 2004) and Wistar (Thorsell et al., 2007) rats while increasing ethanol intake in rats bred to prefer ethanol (Micioni Di Bonaventura et al., 2012). Meanwhile, systemic administration of A2A receptor agonists can reduce operant ethanol self-administration in both nondependent and dependent Wistar rats (Houchi et al., 2013). Given our findings presented here and the incongruence of systemic studies, microinjection studies wherein adenosine A2A receptor modulators are delivered into the BLA are warranted to ascertain the behavioral outcomes of increased and decreased BLA intrinsic excitability mediated by adenosine A2A receptors.
Although endogenous adenosine is often thought of as an inhibitory neuromodulator (primarily through actions at the A1 receptor), these findings suggest that activation of A2A receptors in the BLA can actually increase BLA excitability. Indeed, a couple of prior studies have demonstrated a facilitatory role of A2A receptors on neuronal excitability (Sebastiao and Ribeiro, 1992) and long-term potentiation in the hippocampus (de Mendonca and Ribeiro, 1994; Rebola et al., 2008). Further, mice lacking striatal A2A receptors show impaired LTP induction (d’Alcantara et al., 2001). If indeed endogenous adenosine plays a biphasic role in regulating BLA excitability, it will be of interest to determine if environmental and/or behavioral factors can influence the balance between A1 vs A2A receptor effects. For example, it is possible that under normal conditions, the inhibitory actions of A1 receptors are dominant and adenosine acts primarily to inhibit BLA output. However, exposure to drugs of abuse or other chronic stressors may potentially shift this balance to favor A2A receptor activation. This shift would be expected to lead to an increase in BLA excitability, a phenotype commonly observed in animal models of anxiety disorders and addiction.
Collectively, these results provide new insight into the complex neuroregulatory mechanisms governing the intrinsic excitability of BLA pyramidal neurons and identify A2A receptors as novel regulators of BLA excitability. Given the integral role of the BLA in mood disorders and addiction, these results provide the impetus for future studies directed at elucidating the behavioral consequences associated with the modulation of BLA A2A receptors. It will also be of interest to investigate whether exposure to stressors differentially alters the functional activity of adenosine receptor subtypes, potentially shifting the balance from the predominantly inhibitory actions of A1 receptors to the excitatory effects associated with A2A receptor activation.
Statement of Interest
None.
Supplementary Material
Acknowledgements
The research for this study was funded by NIAAA grants F31 AA022046 (A.R.R.), PO1 AA021099 (J.L.W.), R37AA017531 (J.L.W.), and R37AA010422 (J.L.W.).
References
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. (2009). Purinergic signalling in the nervous system: an overview. Trends Neurosci 32:19–29. [DOI] [PubMed] [Google Scholar]
- Abel HJ, Lee JC, Callaway JC, Foehring RC. (2004). Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol 91:324–335. [DOI] [PubMed] [Google Scholar]
- Adelman JP, Maylie J, Sah P. (2012). Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74:245–269. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. (1980). Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta). Brain Research 190:347–368. [DOI] [PubMed] [Google Scholar]
- Akaike N. (1996). Gramicidin perforated patch recording and intracellular chloride activity in excitable cells. Progress Biophysics Molecular Biol 65:251–264. [DOI] [PubMed] [Google Scholar]
- Akaike N, Harata N. (1994). Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol 44:433–473. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Hodges MA, Wells AM, Lara H III, Xie X, Fuchs RA. (2014). Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology (Berl) 231:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. (2004). Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res 28:1308–1316. [DOI] [PubMed] [Google Scholar]
- Arora D, Haluk DM, Kourrich S, Pravetoni M, Fernandez-Alacid L, Nicolau JC, Lujan R, Wickman K. (2010). Altered neurotransmission in the mesolimbic reward system of Girk mice. J Neurochem 114:1487–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinian L, Zhong L, Yang H, Rehder V. (2012). Nitric oxide as intracellular modulator: internal production of NO increases neuronal excitability via modulation of several ionic conductances. Eur J Neurosci 36:3333–3343. [DOI] [PubMed] [Google Scholar]
- Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA. (2012). Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res 232:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogun O, Koyanagi A, Stickley A, Gilmour S, Shibuya K. (2014). Alcohol consumption and psychological distress in adolescents: a multi-country study. J Adolesc Health 54:228–234. [DOI] [PubMed] [Google Scholar]
- Baudonnat M, Guillou JL, Husson M, Vandesquille M, Corio M, Decorte L, Faugere A, Porte Y, Mons N, David V. (2011). Disrupting effect of drug-induced reward on spatial but not cue-guided learning: implication of the striatal protein kinase A/cAMP response element-binding protein pathway. J Neurosci 31:16517–16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D, McDermott BJ. (1994). Use of the cyclic AMP antagonist, Rp-cAMPS, to distinguish between cyclic AMP-dependent and cyclic AMP-independent contractile responses in rat ventricular cardiomyocytes. J Mol Cell Cardiol 26:1439–1448. [DOI] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Lindsay TG, Cannady R. (2013). Transient increase in alcohol self-administration following a period of chronic exposure to corticosterone. Neuropharmacology 72:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. (1986). Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature 323:718–720. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. (2004). Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci 24:5301–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, Newby AC, Wilson VS, Snyder SH. (1986). Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci 6:1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Ariwodola OJ, Weiner JL. (2014). The impact of social isolation on HPA axis function, anxiety-like behaviors, and ethanol drinking. Front Integr Neurosci 7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci VF, Kandel ER, Schwartz JH, Wilson FD, Nairn AC, Greengard P. (1980). Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A 77:7492–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. (2013). Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res 37:E394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. (2006). Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol 11:339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Matsliah SI, Brosh I, Rosenblum K, Barkai E. (2007). A novel role for extracellular signal-regulated kinase in maintaining long-term memory-relevant excitability changes. J Neurosci 27:12584–12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Johansson B, Constantino MD, Sebastiao AM, Fredholm BB. (1996). Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 353:261–271. [DOI] [PubMed] [Google Scholar]
- Cuzon Carlson VC, Seabold GK, Helms CM, Garg N, Odagiri M, Rau AR, Daunais J, Alvarez VA, Lovinger DM, Grant KA. (2011). Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36:2513–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Alcantara P, Ledent C, Swillens S, Schiffmann SN. (2001). Inactivation of adenosine A2A receptor impairs long term potentiation in the accumbens nucleus without altering basal synaptic transmission. Neuroscience 107:455–464. [DOI] [PubMed] [Google Scholar]
- Daly JW, Butts-Lamb P, Padgett W. (1983). Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol 3:69–80. [DOI] [PubMed] [Google Scholar]
- de Mendonca A, Ribeiro JA. (1994). Endogenous adenosine modulates long-term potentiation in the hippocampus. Neuroscience 62:385–390. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. (1992). Long-term increases in excitability in the CA1 region of rat hippocampus induced by beta-adrenergic stimulation: possible mediation by cAMP. J Neurosci 12:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. (2001). The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 24:31–55. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66:1361–1372. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. (2002). Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22:1618–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. (2008). Modulation of SK channel trafficking by beta adrenoceptors enhances excitatory synaptic transmission and plasticity in the amygdala. J Neurosci 28:10803–10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES. (2009). Functions and modulation of neuronal SK channels. Cell Biochem Biophys 55:127–139. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79:658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV, Bergman B, Lindstrom K. (1984). Levels of adenosine and adenine nucleotides in slices of rat hippocampus. Brain Res 295:127–136. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. (1988). How does adenosine inhibit transmitter release? Trends Pharmacological Sci 9:130–134. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Forero D, Portillo F, Gomez L, Montero F, Kasparov S, Moreno-Lopez B. (2007). Inhibition of resting potassium conductances by long-term activation of the NO/cGMP/protein kinase G pathway: a new mechanism regulating neuronal excitability. J Neurosci 27:6302–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 61:807–816. [DOI] [PubMed] [Google Scholar]
- Guo YY, Liu SB, Cui GB, Ma L, Feng B, Xing JH, Yang Q, Li XQ, Wu YM, Xiong LZ, Zhang W, Zhao MG. (2012). Acute stress induces down-regulation of large-conductance Ca2+-activated potassium channels in the lateral amygdala. J Physiol 590:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzel A, Rosenkranz JA. (2014). Distinct effects of repeated restraint stress on basolateral amygdala neuronal membrane properties in resilient adolescent and adult rats. Neuropsychopharmacology 39:2114–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. (1992). G protein-coupled mechanisms and nervous signaling. Neuron 9:187–195. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. (2007). Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol 98:2297–2310. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Bonci A. (2011a) The SK channel as a novel target for treating alcohol use disorders. Channels (Austin) 5:289–292. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. (2011b) Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry 69:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Mohamedi ML, Chen BT, Bonci A. (2010a) The small-conductance calcium-activated potassium channel is a key modulator of firing and long-term depression in the dorsal striatum. Eur J Neurosci 31:1946–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, Cho SL, Tye K, Bonci A. (2010b) Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron 65:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchi H, Persyn W, Legastelois R, Naassila M. (2013). The adenosine A2A receptor agonist CGS 21680 decreases ethanol self-administration in both non-dependent and dependent animals. Addict Biol 18:812–825. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Jackson RH, Williams M. (1989). Autoradiographic characterization of high-affinity adenosine A2 receptors in the rat brain. Brain Res 484:111–118. [DOI] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. (2007). Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol 578:799–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. (2013). Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel DJ. (1991). Calcium activates two types of potassium channels in rat hippocampal neurons in culture. J Neurosci 11:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. (1991). Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neuroscience Letters 134:139–144. [DOI] [PubMed] [Google Scholar]
- Londos C, Cooper DM, Wolff J. (1980). Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A 77:2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Maylie J, Adelman JP. (2009). New sites of action for GIRK and SK channels. Nat Rev Neurosci 10:475–480. [DOI] [PubMed] [Google Scholar]
- Ma YY, Cepeda C, Chatta P, Franklin L, Evans CJ, Levine MS. (2012). Regional and cell-type-specific effects of DAMGO on striatal D1 and D2 dopamine receptor-expressing medium-sized spiny neurons. ASN Neuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. (2012). Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL. (2015). Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology 89:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. (2005). A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron 48:1025–1037. [DOI] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. (1999). Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci 19:1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD, Suzuki F, Zahniser NR. (1993). Adenosine A2a receptor modulation of electrically evoked endogenous GABA release from slices of rat globus pallidus. J Neurochem 60:2334–2337. [DOI] [PubMed] [Google Scholar]
- McCool BA, Farroni JS. (2001). A1 adenosine receptors inhibit multiple voltage-gated Ca2+ channel subtypes in acutely isolated rat basolateral amygdala neurons. Br J Pharmacol 132:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Christian DT, Diaz MR, Lack AK. (2010). Glutamate plasticity in the drunken amygdala: the making of an anxious synapse. Int Rev Neurobiol 91:205–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Olie JP. (2005). Neurobiology of mood, anxiety, and emotions as revealed by studies of a unique antidepressant: tianeptine. Mol Psychiatry 10:525–537. [DOI] [PubMed] [Google Scholar]
- McKay BM, Matthews EA, Oliveira FA, Disterhoft JF. (2009). Intrinsic neuronal excitability is reversibly altered by a single experience in fear conditioning. J Neurophysiol 102:2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Lancaster B, Wheal HV. (2004). Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J Neurosci 24:4530–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micioni Di Bonaventura MV, Cifani C, Lambertucci C, Volpini R, Cristalli G, Froldi R, Massi M. (2012). Effects of A(2)A adenosine receptor blockade or stimulation on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 219:945–957. [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2003). The neurobiology and control of anxious states. Prog Neurobiol 70:83–244. [DOI] [PubMed] [Google Scholar]
- Mitra R, Ferguson D, Sapolsky RM. (2009). SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry 14:847–855, 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motanis H, Maroun M, Barkai E. (2014). Learning-induced bidirectional plasticity of intrinsic neuronal excitability reflects the valence of the outcome. Cereb Cortex 24:1075–1087. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. (2011). Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry 69:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo H, Ono T, Nishino H. (1988). Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance. J Neurosci 8:3570–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MM, McKay BM, Power JM, Disterhoft JF. (2009). Learning-related postburst afterhyperpolarization reduction in CA1 pyramidal neurons is mediated by protein kinase A. Proc Natl Acad Sci U S A 106:1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. (1993). PKA mediates the effects of monoamine transmitters on the K+ current underlying the slow spike frequency adaptation in hippocampal neurons. Neuron 11:1023–1035. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. (2012). Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology 37:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. (1999). Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 19:5205–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PW, Jones G, Coll MG. (1995). The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br J Pharmacol 115:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P. (2008). Competition between calcium-activated K+ channels determines cholinergic action on firing properties of basolateral amygdala projection neurons. J Neurosci 28:3209–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Bocklisch C, Curby P, Sah P. (2011). Location and function of the slow afterhyperpolarization channels in the basolateral amygdala. J Neurosci 31:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, da Silva GE, Batista LC, Bittencourt AL, Takahashi RN. (2006). Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (hangover) in mice. Neuropsychopharmacology 31:2210–2220. [DOI] [PubMed] [Google Scholar]
- Rau AR, Ariwodola OJ, Weiner JL. (2014). Presynaptic adenosine A1 receptors modulate excitatory transmission in the rat basolateral amygdala. Neuropharmacology 77:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Lujan R, Cunha RA, Mulle C. (2008). Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron 57:121–134. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. (2010). Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 67:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA. (2011). Neuronal activity causes rapid changes of lateral amygdala neuronal membrane properties and reduction of synaptic integration and synaptic plasticity in vivo. J Neurosci 31:6108–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez G, Rodriguez MJ, Pomata P, Rela L, Murer MG. (2011). Reduction of an afterhyperpolarization current increases excitability in striatal cholinergic interneurons in rat parkinsonism. J Neurosci 31:6553–6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. (2008). Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci 28:4028–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA. (1992). Evidence for the presence of excitatory A2 adenosine receptors in the rat hippocampus. Neurosci Lett 138:41–44. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, Kash T, Lack AK, Messing RO, Siggins GR, Winder D, Roberto M, McCool BA, Weiner JL. (2009). Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol 43:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. (2010). Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of beta 3 adrenoceptor activation. Neuropsychopharmacology 35:1886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Weiner JL. (2014). Chronic treatment with prazosin or duloxetine lessens concurrent anxiety-like behavior and alcohol intake: evidence of disrupted noradrenergic signaling in anxiety-related alcohol use. Brain Behav 4:468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. (2011). Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. (1999). Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 59:355–396. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C. (2002). Phasic and tonic attenuation of EPSPs by inward rectifier K+ channels in rat hippocampal pyramidal cells. J Physiol 539:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Johnson J, Heilig M. (2007). Effect of the adenosine A2a receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res 31:1302–1307. [DOI] [PubMed] [Google Scholar]
- Tsai J, Harpaz-Rotem I, Pilver CE, Wolf EJ, Hoff RA, Levy KN, Sareen J, Pietrzak RH. (2014). Latent class analysis of personality disorders in adults with posttraumatic stress disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 75:276–284. [DOI] [PubMed] [Google Scholar]
- Tye KM, Janak PH. (2007). Amygdala neurons differentially encode motivation and reinforcement. J Neurosci 27:3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. (2002). Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res 26:1494–1501. [DOI] [PubMed] [Google Scholar]
- van Welie I, du Lac S. (2011). Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J Neurophysiol 105:1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. (1992). Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12:4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Gerevich Z, Krause T, Gunther A, Koles L, Schneider D, Norenberg W, Illes P. (2004). Adenosine A2A receptor-induced inhibition of NMDA and GABAA receptor-mediated synaptic currents in a subpopulation of rat striatal neurons. Neuropharmacology 46:994–1007. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. (2007). Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci 27:553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nedden S, Hawley S, Pentland N, Hardie DG, Doney AS, Frenguelli BG. (2011). Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors. J Neurosci 31:6221–6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.