Abstract
Skeletal muscle is dynamic, adapting to environmental needs, continuously maintained, and capable of extensive regeneration. These hallmarks diminish with age, resulting in a loss of muscle mass, reduced regenerative capacity, and decreased functionality. Although the mechanisms responsible for this decline are unclear, complex changes within the local and systemic environment that lead to a reduction in regenerative capacity of skeletal muscle stem cells, termed satellite cells, are believed to be responsible. We demonstrate that engraftment of myofiber-associated satellite cells, coupled with an induced muscle injury, markedly alters the environment of young adult host muscle, eliciting a near-lifelong enhancement in muscle mass, stem cell number, and force generation. The abrogation of age-related atrophy appears to arise from an increased regenerative capacity of the donor stem cells, which expand to occupy both myonuclei in myofibers and the satellite cell niche. Further, these cells have extensive self-renewal capabilities, as demonstrated by serial transplantation. These near-lifelong, physiological changes suggest an approach for the amelioration of muscle atrophy and diminished function that arise with aging through myofiber-associated satellite cell transplantation.
INTRODUCTION
Skeletal muscle is dynamic, adapting to changing needs, continuously maintained, and capable of extensive regeneration. These activities are attributed to a population of muscle progenitors termed satellite cells, which lie between the myofiber plasma membrane and the basal lamina (1, 2). This unique environment is responsible for maintaining an appropriate satellite cell pool to meet the demands of skeletal muscle function and repair; the loss of regenerative capacity arising from local environmental changes is thought to be responsible for loss of muscle function during aging (3–7). Although heterochronic transplantation of aged satellite cells into a young environment restores their regenerative capacity, the aged environment is refractory to the reverse transplantation (3, 6). We show that transplantation of myofiber-associated satellite cells, coupled with a simultaneous injury of the host muscle, profoundly affects the transplanted muscle and prevents the onset of age-associated muscle atrophy and weakness. These data establish a paradigm for satellite cell transplantation, providing evidence that adult stem cell therapies can ameliorate the deleterious effects of aging in skeletal muscle, and suggest an approach for the development of therapies to treat sarcopenia.
RESULTS
Myofiber-associated satellite cell transplantation increases muscle mass and myofiber number
Loss of muscle mass and regenerative capacity are inevitable consequences of aging that result in reduced mobility, increased frailty associated with a high incidence of injury, and a loss of quality of life (8). The inability of aged muscle to regenerate is thought to be due to an inhibitory effect of the local environment on regenerative capacity (3, 5–7). The satellite cell lies juxtaposed between the basement membrane and the myofiber and adjacent to capillaries, occupying a unique niche sensitive to both the systemic animal-wide environment and the local myofiber environment (1, 9). To test whether the systemic environment that injected donor cells encounter influences the efficiency and longevity of cell engraftment, we transplanted green fluorescent protein–positive (GFP+) myofibers with their associated cells into wild-type, injured (with BaCl2) tibialis anterior (TA) muscle. This procedure maintains the local myofiber environment surrounding the satellite cell before and during transplantation (fig. S1, A and B). In addition, concurrent muscle injury from BaCl2-induced muscle necrosis provides a systemic environment that promotes satellite cell proliferation and engraftment through the release of growth factors, chemokines, and cytokines from damaged myofibers, muscle interstitial cells, blood vessels, and invading inflammatory cells (7, 10–13). After injection, transplanted TA muscles were compared to the uninjured contralateral TA muscles, where we noted a 50% increase in mass and a 170% increase in size that persisted for the lifetime of the mouse (Fig. 1A). This increase in muscle mass required myofiber injection and concurrent injury; neither injury alone nor transplantation without injury resulted in similar mass increases (Fig. 1B). The increase in mass was independent of the injury agent used, because myofiber injection accompanied by either BaCl2 or cardiotoxin yielded similar results (fig. S1, C and D). Because BaCl2 depolarizes myofibers by interfering with ion transport and cardiotoxin injures muscle tissue via complex mechanisms that include phospholipase-mediated destruction of myofiber membranes (2, 14, 15), these data illustrate that an injured muscle environment appears supportive for transplantation, irrespective of the mechanism of injury.
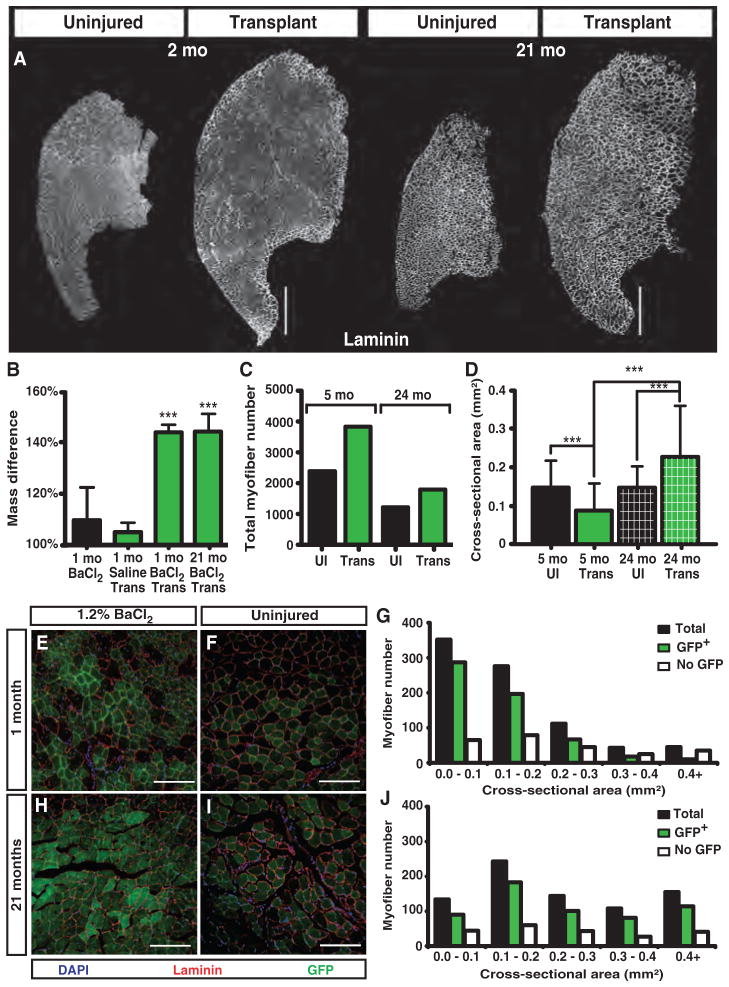
Fig. 1.
Myofiber-associated satellite cell engraftment increases muscle size and persists for 21 months (mo). (A to D) Increases of 170% in TA muscle size occur and persist for ~2 years upon transplantation of a single myofiber with ~15 associated satellite cells when compared to the contralateral controls (A). Mass and size increases require a concomitant injury and transplantation, because neither injury alone nor myofiber transplantation alone increases muscle mass (B). Accompanying the mass increase is a 38 and 25% increase in myofiber number 60 days and 21 months after transplant, respectively (C), and a successive increase in myofiber area in transplanted TA muscles compared to controls (D). UI, uninjured. (E to J) Donor GFP+ cells incorporate into 80% of the myofibers at 1 month after transplant (E) and persist for 21 months (H) compared to 1-month (F) and 21-month (I) uninjured control TA muscles, respectively. Myofiber area in GFP+ myofibers is smaller at 1 month (G) compared to 21 months after transplant (J). Scale bars, 500 μm (A), 150 μm (E, F, H, and I). n = 4 to 10 for (B) and (D). Number of myofibers transplanted is one (A, C, and D) and five (B and E to J). ***P < 0.001.
The mass increase after transplantation is not due to fibrosis, because transplanted TA muscles have similar wet and dry weights 30 days after transplantation (fig. S2A) and are indistinguishable in collagen VI deposition 30 or 60 days after transplantation when compared to uninjured TA muscles (fig. S2B). Associated with the increase in muscle mass is an increase in myofiber number, which reached a maximum at 2 months after transplantation and remained elevated when com pared to uninjured contralateral TA muscles for the lifetime of the transplanted muscle (Fig. 1C). The myofiber number, which is markedly increased 2 months after transplantation compared to contralateral controls, declines in number during aging, similar to the decline in the uninjured TA muscle (Fig. 1C). In contrast, the myofiber cross-sectional area 21 months after transplant is greater than the adult uninjured, the adult transplanted, or the 24-month-old uninjured TA muscles, indicating progressive myofiber hypertrophy (Fig. 1D and fig. S3, A and B).
A permanent increase in donor GFP+ myonuclei accompanies transplantation
The injected donor satellite cells that survive transplantation can adopt two distinct fates: commitment to myogenesis, eventually fusing and becoming a terminally differentiated myonucleus within a myofiber, or self-renewing, where the cells home to the satellite cell niche and become quiescent. We assessed the ability of donor cells to occupy the myonuclear compartment by scoring the percentage of GFP+ myofibers after transplantation. The capacity of donor cells to differentiate into myonuclei is extensive, where ≥80% of the myofibers are GFP+ by 30 days after transplant compared to contralateral controls (Fig. 1, E and F). Although most of the myofibers are GFP+, these myofibers are significantly smaller than the host myofibers that have no detectable donor GFP cell engraftment (Fig. 1G), suggesting that donor cells may preferentially fuse to form new myofibers in the injured host TA muscle.
We expected the donor GFP+ myofibers to gradually decline, reflecting myonuclear turnover (16, 17) and a gradual loss of donor satellite cells over time (7, 8). When the percentage of GFP+ myofibers as a function of mouse age was quantified for the transplanted TA muscle, we observed an unexpected result. The percentage of GFP+ myofibers appeared indistinguishable at 1 month (Fig. 1, E and F) and 21 months after transplantation when the myofiber number was normalized to the uninjured contralateral TA muscle controls (Fig. 1, H and I). Although morphometric analysis revealed that, 30 days after transplant, myofibers with donor myonuclei were on average smaller than those without detectable donor contribution (Fig. 1, D and G, and fig. S2A), GFP+ myofibers containing donor-derived myonuclei were much larger than myofibers with no detectable GFP in 2-year-old TA muscles 21 months after transplant (Fig. 1J and fig. S2B). These results are consistent with a continuous contribution of donor satellite cells to myonuclei. In support of this idea is the presence of centrally located nuclei in 58% of the 24-month-old transplanted TA myofibers compared to 3% in the contralateral control, which indicates persistent fusion of satellite cells into the myofibers (fig. S3C).
A permanent increase in satellite cell number accompanies transplantation
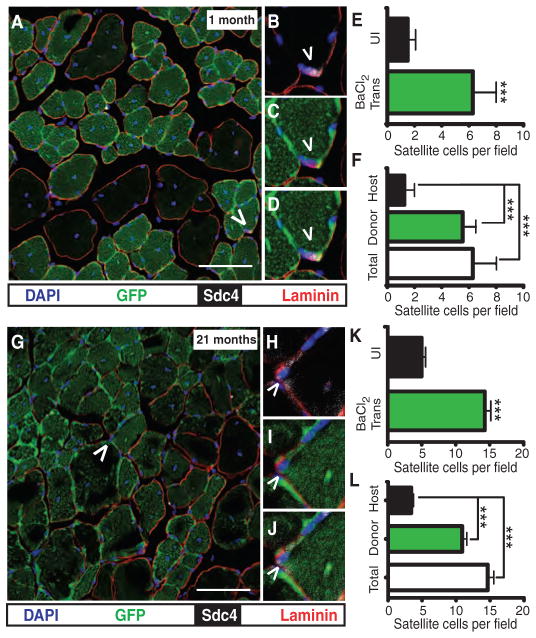
Changes in the regenerative capacity of satellite cells arising from an aged inhibitory environment are thought to play an important role in the loss of muscle mass with age (6, 18). The presence of large GFP+ myofibers in the transplanted TA muscles of aged mice suggests that the GFP+ donor-derived satellite cells persist for 21 months after transplantation. To determine whether the engrafted and endogenous satellite cells behaved differently in aged muscle, we examined cross sections of transplanted and uninjured contralateral muscles 1 and 21 months after transplant for donor GFP+ and endogenous satellite cells. First, we probed sections of 4-month-old TA muscles transplanted 1 month earlier for satellite cells with the satellite cell marker Syndecan-4 (19) (Fig. 2A). We observed Syndecan-4+ (Fig. 2, B and D, white) satellite cells located underneath the basal lamina (Fig. 2, B to D, red) that are GFP+ (Fig. 2, C and D, green) and thus are donor-derived. When scored, satellite cell numbers were three times higher in the transplanted TA muscle than in the contralateral TA muscle 1 month after transplant (Fig. 2E).
Fig. 2.
Donor satellite cells expand upon engraftment and persist for 21 months. (A to F) Donor satellite cells are present in transplanted, regenerated TA muscle (A, caret), located underneath the basal lamina (B, red); GFP+ (C, green); Syndecan-4+ (Sdc4) (D, white). Satellite cells in TA muscles 30 days after transplant are threefold higher than contralateral uninjured controls (E) arising from engrafted donor cells (F). (G to J) Similarly, donor satellite cells are present 21 months after transplant (G, caret), located underneath the basal lamina (H, red), and GFP+ (I, green) and Syndecan-4+ (J, white). (K and L) Transplanted TA muscles have threefold more satellite cells than contralateral controls (K), where the increase in satellite cell numbers arises from engrafted donor cells (L). Scale bars, 75 μm (A and G). n = 4 to 10 for (E), (F), (K), and (L). Number of myofibers transplanted is five (A to L). ***P < 0.001.
We determined the relative contribution of donor and host satellite cells to the total increase in satellite cells. The increase in satellite cell number appears to be exclusively a result of donor-derived cells, because the number of host satellite cells is similar in both control and transplanted muscles (Fig. 2F). At 21 months after transplantation, the 24-month-old TA muscle appears similar to the young muscle just 1 month after transplantation. In the 24-month-old mouse, 21 months after transplantation, we observed a high level of engraftment with readily identifiable donor satellite cells (Fig. 2, G, I, and J, green) that are Syndecan-4+ (Fig. 2, H and J, white) and located underneath the basal lamina (Fig. 2, H to J, red). The large increase in satellite cell number was maintained in transplanted 24-month-old TA muscles 21 months after transplantation (Fig. 2K). Similar to the increase in satellite cell numbers 1 month after transplantation, the increased satellite cell number 21 months after transplantation was due exclusively to donor-derived satellite cells (Fig. 2L). Because this large and persistent increase in donor satellite cells was unexpected, we independently quantified satellite cell numbers with an antibody to Syndecan-3 (19) by flow cytometry. The flow cytometry data agree with the scoring of satellite cells in sections, where there were three times more satellite cells in transplanted TA muscle than in the contralateral TA muscles (fig. S4, A to C, and table S1).
Functional donor satellite cells are required to increase muscle mass and satellite cell numbers in transplanted hosts
Most satellite cells are juxtaposed to capillaries, suggesting a functional relationship between satellite cells and the vasculature (7, 20). Because vessel-associated cells and muscle interstitial cells can contribute to muscle regeneration (21–25), we examined the cell types present on injected myofibers. When 20 random myofibers selected for transplantation and free of associated vessels, excess extracellular matrix, and myotendinous junction material were analyzed for the presence of pericytes with antibodies to NG2 (24, 25), endothelial cells with antibodies to CD31 (26), and satellite cells with antibodies to Pax7 (27), we found that 100% of the cells were Pax7+ (fig. S5). Thus, it is unlikely that cells other than satellite cells were present on the transplanted myofibers or that the transplants contributed to the host vasculature.
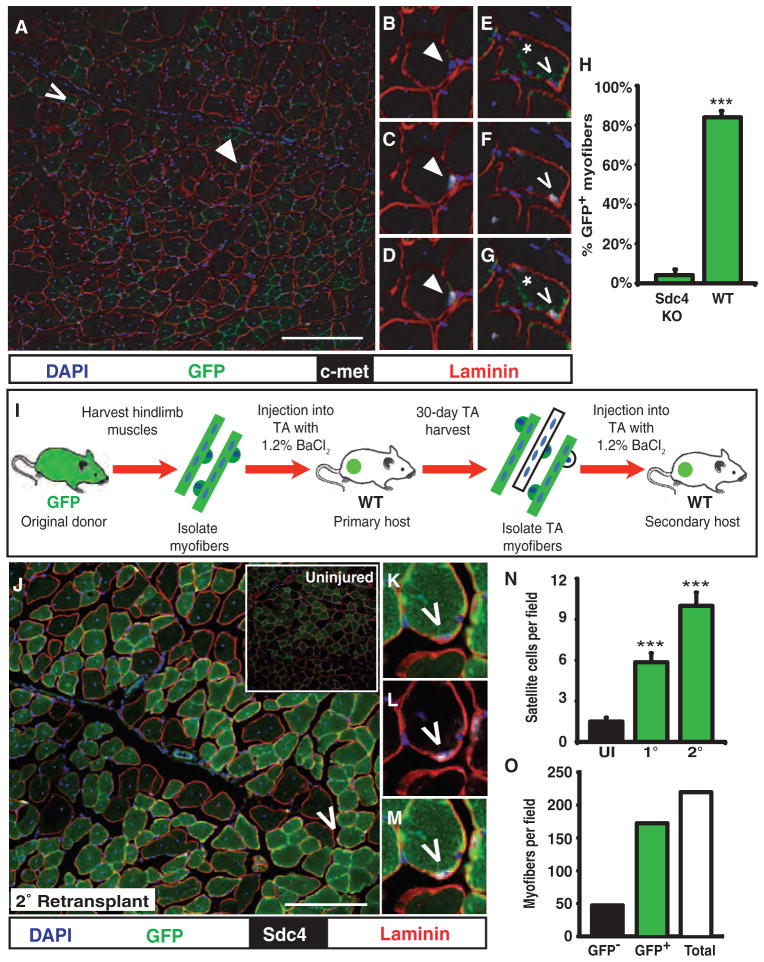
To further rule out a direct contribution by myofibers, myonuclei, or associated basement membrane to the observed increases in muscle mass and satellite cell number, we transplanted myofibers and their associated satellite cells from an sdc4−/−; βActGFP mouse into a 3-month-old wild-type host TA muscle. Syndecan-4 is expressed in satellite cells and is not expressed in the myonuclei of the myofiber (19). Moreover, sdc4 null muscle cannot regenerate muscle after a BaCl2-induced injury because of the inability of sdc4 null satellite cells to proliferate and differentiate (28). We transplanted sdc4−/−; βActGFP myofiber–associated satellite cells and analyzed sections from transplanted and contralateral control TA muscles for myofiber engraftment by donor-derived cells and for donor-derived satellite cells with GFP fluorescence and c-met, an established satellite cell marker (19). In sections of muscle transplanted with sdc4−/−; βActGFP myofibers 1 month previously, few donor-derived satellite cells were present (Fig. 3A). Almost all of the satellite cells identified appeared derived from the host, as they were GFP− (Fig. 3, B to D, green), c-met+ (Fig. 3, C and D, white), and located underneath the basal lamina (Fig. 3, B to D, red).
Fig. 3.
Functional donor satellite cells are required for engraftment and self-renew when serially transplanted. (A to H) Cross sections of TA muscles transplanted with sdc4 −/−; βActGFP myofibers are mostly devoid of donor GFP+ satellite cells (A), where host satellite cells (B to D, arrowhead) and donor satellite cells (E to G, caret) are located underneath the basal lamina (B and E, laminin, red), c-met+ (D and G, white), and either GFP− (B and D) or GFP+ (E and G), respectively. The sdc4−/−; βActGFP-transplanted muscle exhibits low levels of engraftment, where myofibers show limited GFP fluorescence (E to G, asterisk) and exhibit quantitatively low levels of engraftment compared to TA muscles transplanted with five myofibers from a βActGFP mouse (H). KO, knockout; WT, wild type. (I to M) Myofiber serial transplantation (I) demonstrates that myofiber-associated donor cells self-renew, because sections from serially transplanted TA muscles exhibit extensive GFP fluorescence (J) and contain primary donor GFP+ satellite cells (carets) that are GFP+ (K, green), located underneath the basal lamina (L, red), and Syndecan-4+ (M, white). (N) GFP+ satellite cells originating from the primary (1°) transplant outnumber the host cells 3:1 at 30 days after secondary (2°) transplantation. (O) Most of the myofibers in the secondary transplant contain GFP+ donor cells derived from the primary transplant. Scale bars, 150 μm [(A) and (K)]. n = 4 to 6 for (H) and (N). Number of myofibers transplanted is 5 (A to H) and 10 (J to O). **P < 0.01; ***P < 0.001.
Myofibers in cross sections of TA muscles transplanted with sdc4−/−; βActGFP myofibers show little, if any, GFP fluorescence (Fig. 3A), consistent with the limited differentiation of sdc4−/− satellite cells (28). When quantified, less than 5% of the myofibers were GFP+ (Fig. 3H) in TA muscles transplanted with sdc4−/−; βActGFP myofibers. Further, donor contribution to the host satellite cell niche was largely absent and in contrast to muscles transplanted with wild-type cells (Fig. 3A, compare with Fig. 2A). Few donor satellite cells were identified and were weakly GFP+ (Fig. 3, E and G), c-met+ (Fig. 3, F and G), and located underneath the basal lamina (Fig. 3, E to G). Because Syndecan-4 is not expressed on the myofiber or by resident myonuclei (28), these data support the idea that the myofiber-associated satellite cells, and not the myofiber per se, are responsible for the observed phenotypes.
Donor satellite cells self-renew in a serial transplantation assay
The increase in both mass and size of the transplanted TA muscle correlates with the presence of increased numbers of donor-derived satellite cells, suggesting that these cells provide an enhanced regenerative or self-renewal capacity. To test the self-renewal capacity of transplanted, myofiber-associated satellite cells, we developed a serial transplantation assay, successively retransplanting marked donor cells from a primary host TA muscle into a secondary host TA muscle (Fig. 3I). The extent of GFP engraftment in the secondary host after a serial transplantation is indistinguishable from the engraftment described for a primary transplantation (Fig. 3J, compare to Fig. 1, E and F), suggesting that the GFP+ donor satellite cells are capable of further expansion. Because it is highly unlikely that a satellite cell derived from the original transplant remained quiescent through two successive transplantations, the presence of original donor GFP+ cells (Fig. 3, J, K, and M, green), located underneath the basal lamina (Fig. 3, K to M, red) and expressing Syndecan-4 (Fig. 3, L and M, white), indicates that the primary donor satellite cell population has undergone self-renewal.
Further support for self-renewal of the original donor cells in a secondary transplantation was evident when the numbers of primary donor GFP+ satellite cells were quantified in the secondary transplant and found to outnumber the host satellite cells by 3:1 (Fig. 3N). Similar to primary transplants, the levels of myofiber engraftment by primary donor cells in the secondary transplanted TA muscles are ~80% (Fig. 3O). These data indicate that the self-renewing population of original donor cells maintains myogenic competency and self-renewal capability through two successive transplantations.
Retention of young muscle mass and youthful muscle physiology in aged, transplanted muscles
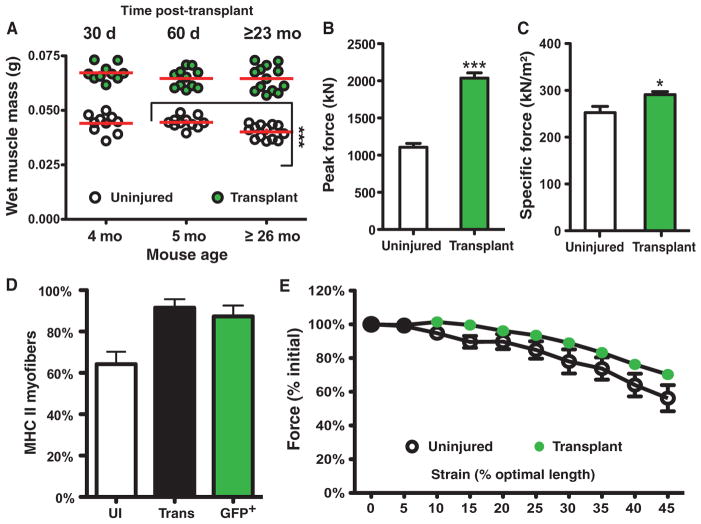
The capacity of a small number of introduced donor satellite cells to persist for the lifetime of the mouse prompted us to examine whether the transplantation affected either the composition or the physiologic function of the engrafted TA muscle in aged mice. Aged muscle loses strength, which is thought to be due to a loss of muscle mass and changes in myosin contractility (29, 30). Whereas we observed the expected loss of muscle mass in uninjured contralateral TA muscles between 5 months and 26 to 31 months of age (Fig. 4A), we noted no significant change in the mass of the transplanted TA muscle of 5-month-old mice, transplanted 60 days before analysis, when compared to ≥26-month-old mice that were transplanted ≥21 months before analysis (Fig. 4A).
Fig. 4.
Transplantation prevents age-related reductions in muscle mass, muscle function, and muscle strength. (A to D) Myofiber-associated cell transplantation maintains muscle mass and function in aged TA muscles, where the transplanted TA muscle does not atrophy (A) and generates twice the peak force (B), a significant increase in specific force (C), and retention of fast (type II) MHC (myosin heavy chain) (D) compared to the uninjured contralateral controls. (E) The susceptibility to injury in the engrafted and control TA muscles is indistinguishable, indicating that the increased number of myofibers established after transplantation is physiologically and functionally incorporated into the TA muscle. Mice, n = 5 to 12. Number of myofibers transplanted is five (A to E). *P < 0.05; ***P < 0.001.
The retention of muscle mass in aged, transplanted TA muscle suggests that the deleterious effects of muscle aging are mitigated by the transplant. We tested this by comparing the function of the transplanted and contralateral control TA muscles and measured twice the peak force generation in the 27-month-old transplanted as compared to the contralateral control TA muscles (Fig. 4B and table S2). The peak force in the 27-month-old transplanted female muscle is greater than that published for 4-month-old adult males (~1600 kN) (31) and is consistent with the mass increase and myofiber hypertrophy in the transplanted TA muscle. When normalized, the transplanted TA muscle exhibited an elevated specific force that was significantly greater than the contralateral control (Fig. 4C and table S3).
A preferential loss of fast twitch (glycolytic) myofibers is a hallmark of age-related muscle loss in the TA muscle, where a reduction of from 40 to 80% occurs between 5 and 20 months of age (32). Consistent with the increased force production in the transplanted muscle, fast glycolytic myofibers were retained in the transplanted aged TA muscle, whereas the contralateral uninjured TA muscle lost 35% of the fast twitch muscle (Fig. 4D and fig. S6, A to C). Using a series of ramped stretches, we then measured the susceptibility of the muscles to eccentric contraction-induced injury and showed no significant differences between the transplanted and uninjured contralateral TA muscles, demonstrating functional incorporation of the additional myofibers in the transplanted TA muscle (Fig. 4E and table S4). Together, our data show that transplantation of myofiber-associated satellite cells with a concurrent injury provides near-lifelong prevention of age-associated loss of muscle mass and regenerative capacity.
DISCUSSION
The mechanisms responsible for the age-related loss of muscle mass and function are not understood. However, a loss of regenerative capacity as a result of an environment that is inhibitory to satellite cell function plays an important role (3, 5, 6, 33–35). Current hypotheses for the loss of regenerative capacity in aged muscle suggest that the muscle environment inhibits satellite cell-mediated repair through production of soluble inhibitory factors (7, 36–38). Our data demonstrate that the inhibitory, aged environment can be permanently modified by transplanting myofiber-associated satellite cells into young host muscle. The transplantation coupled with an injury alters the environment of the host TA muscle and permits the retention of a robust, regenerative capacity in the donor satellite cells that increases muscle mass and maintains muscle function for nearly the lifetime of the mouse. This effect is localized to the injected and regenerated muscle; muscles in the contralateral limb are unaffected.
Studies of myofiber-associated stem cell or isolated stem cell transplantation in muscle have not addressed the longevity or long-term consequences of the injected cells beyond 5 weeks (2, 39) or 6 months (40). Here, we injected myofiber-associated cells into immunocompetent, adult hosts and examined the behavior of the transplanted muscles through the lifetime of the mouse. Unexpectedly, when the cells were delivered with a concurrent injury, we observed profound changes in the transplanted host TA muscle including (i) a time-dependent increase in muscle mass, (ii) progressive myofiber hypertrophy, (iii) increased force production, and (iv) retention of fast myofibers. Although we do not yet understand the mechanisms involved in this effect, the assumption that the aged environment is inhibitory and dominant is not supported by these data because the donor and the host satellite cells are the same age and coexist in the same environment for more than 21 months. Our data suggest that the donor satellite cells are resistant to the aged environment and have an enhanced regenerative capacity that maintains a functional young muscle phenotype within the aged mouse. Although we observed no significant differences in capillary density compared to contralateral controls, aged, transplanted muscle had large numbers of myofibers with centrally localized myonuclei compared to the uninjured contralateral TA muscles. These centrally located myonuclei are indicative of ongoing maintenance and repair, a probable result of the permanent changes in the donor satellite cell population (41, 42).
The prevention of age-related loss of muscle function in the transplanted TA muscle likely originates from a combination of effects acting on the transplanted cells when they are delivered to the host muscle. The donor cells are transplanted on intact myofibers, a procedure that maintains the local satellite cell niche during dissection and removal from the host mouse, during incubation in tissue culture, and during and after injection into the host muscle. This protection of the niche is important, as demonstrated by the fact that injection of isolated cells is much less efficient than injection of myofiber-associated cells (43–45). An important second factor is the host environment. Although previous publications have demonstrated that myofiber-associated cell transplantation is more efficient than transplantation of isolated cells, the host mice were dystrophic and immunocompromised, and the muscle was irradiated before transplantation (2, 35). These authors demonstrated short-term (3 to 4 weeks) engraftment of myofiber-associated satellite cells into myofibers and occupation of the satellite cell niche but did not examine long-term engraftment of myofibers or of the satellite cell niche by donor cells. If the host environment is important, the state of the host tissue would be expected to affect engraftment efficiency of the donor satellite cells. Thus, the diseased, irradiated muscle environment would differ from that of an uninjured or regenerating muscle. Consistent with this idea, we found that myofiber-associated cells injected in the absence of injury into the TA muscle yielded minimal engraftment and migration (fig. S4D). However, when the TA muscle is injured, engraftment and migration are enhanced such that donor cells can be found in most of the regenerated TA muscle through at least 31 months of age. Because injection of isolated cells into an injured muscle is much less efficient than injection of myofiber-associated satellite cells into injured muscle, we propose that unidentified factors in the injured muscle tissue can signal to the satellite cells only when the satellite cell niche is retained. These factors allow the donor cells to proliferate extensively and engraft with high efficiency into the host satellite cell niche, as well as commit to the muscle lineage, providing donor myonuclei. These putative factors that affect the behavior of the transplanted cells may be released from damaged muscle (10, 46) or from the surrounding microvasculature, interstitial cells, or inflammatory cells (11–13, 36, 37, 47, 48).
The loss of muscle mass, reduced regenerative capacity, and diminished function that occur upon aging, recently attributed to an inhibitory environment (20, 49), have been viewed as inevitable (5, 7, 8). In contrast to this view, we have demonstrated that engraftment of donor satellite cells coupled with injury elicits permanent changes in the transplanted muscle. Understanding the molecular mechanisms of this effect could accelerate the development of therapies to treat sarcopenia. It is likely that the changes induced in the donor cells arise from the signals received by these cells before injection and once transplanted in the injured muscle environment. Thus, it follows that treatment of an endogenous satellite cell population with the appropriate factors should elicit a response similar to that observed in the transplanted donor cells. Thus, a therapeutic approach predicated on enhancing the regenerative capacity of the endogenous cell population by enhancement or suppression of signaling pathways to mimic the environment of our transplanted satellite cells might ultimately provide therapeutic benefit in the absence of cell transplantation.
Major obstacles impeding the application of stem cell–based therapies for the treatment of muscle diseases or injuries include the potentially large number of cells required to elicit a functional response (50, 51), the absence of an established self-renewing donor cell population when transplanting vessel-associated cells (24, 51), and minimal physiological improvements when transplanting muscle-derived stem cells (52). The advantages of satellite cell transplantation include robust engraftment from small numbers of isolated cells (43, 45), from single myofibers (2), and from single-cell injections (44). Although improved muscle function has been documented for pericytes and mesoangioblasts, respectively (24, 51), similar functional improvements have not been shown for satellite cell transplants, nor has long-term engraftment been assessed for transplants of these cell types. Here, we have demonstrated a robust regenerative response in immunocompetent, wild-type mice with as few as three to five transplanted myofiber-associated Pax7+ cells. The ability to have a functional effect with only a single cell or a small number of cells markedly enhances the potential for the development of stem cell–based therapies. Our approach has other long-term advantages not previously reported, including engraftment of a self-renewing stem cell population, the lack of an immune response to the transplanted cells, and the amelioration of age-related loss of muscle function. Although we do not know whether transplantation into aged muscle will be directly effective at reducing or slowing sarcopenia, this procedure may be adaptable to both autologous and nonautologous stem cell transplants. Using these procedures for transplantation of low cell numbers, we envision the development of translational approaches for therapies aimed at muscle injury repair, muscle diseases, and the loss of muscle function associated with aging.
MATERIALS AND METHODS
Mice
Mice were housed and transplant experiments were performed in a pathogen-free facility at the University of Colorado. All protocols were approved and performed in accordance with the University of Colorado Internal Animal Use and Care Committee. Transplant recipients were C57Bl/6xDBA2 (B6D2F1; Jackson Labs). Donor tissue was obtained from β-actin GFP [FVB.Cg-Tg(CAG-EGFP)B5Nagy/J; Jackson Labs] referred to as βActGFP mice. Transplantations and donor cell and tissue isolation were performed with female adult mice between 3 and 5 months of age.
Myofiber transplantation
Donor mice were anesthetized with isoflurane and euthanized by cervical dislocation. Donor hindlimb muscles were isolated as described (45) and incubated in F12-C media containing 15% horse serum (HyClone) at ~6% O2 in the presence of 1.5 nM fibroblast growth factor–2 (FGF-2) for 4 to 5 hours. For transplantation, donor myofibers were transferred to a dish containing 50 ml of 1.2% BaCl2, 0.9% saline, or 100 μl of cardiotoxin (Sigma) where noted, drawn into a syringe of the number noted, and injected along the length of the TA muscle.
Immunofluorescence
Preparations of sections and immunofluorescence detection were performed as described (19, 28, 53). Tissue sections were preserved in 4% paraformaldehyde, with the exception of sections stained for pan–MHC II (myosin heavy chain II), preserved via isopentane. Primary antibodies used included chicken antibody to Syndecan-4 (1:500), rabbit antibody to Syndecan-3 (1:50), rat or rabbit antibody to α-laminin (1:200; Invitrogen), and mouse antibody to pan–MHC II (1:100; Abcam). Secondary antibodies used were antibodies to chicken, to mouse, to rat, or to rabbit labeled with Alexa Fluor 546, 555, 594, or 647 (1:500; Invitrogen).
Morphometric analysis
Myofiber cross-sectional area, number, and central nucleation, as well as donor and host satellite number in transplanted and uninjured TA muscle, were quantified via manual masking with SlideBook (Intelligent Imaging Innovations) based on laminin, 4′,6-diamidino-2-phenylindole (DAPI), and Syndecan-4 staining on mid-belly serial sections in 20× fields. A minimum of five fields and four sections were analyzed per biologic replicate to derive statistically significant cross-sectional areas.
Flow cytometry
Cells were prepared as described (45). Cells were labeled with rabbit antibody to Syndecan-3 (1:50) (19) for 1 hour at 4°C, and secondary antibody was Alexa Fluor 594 (1:500; Invitrogen). Profiles were obtained with a MoFlo cell sorter (Beckman Coulter) with background gates set based on secondary antibody–only controls yielding <0.5% possible events.
Muscle physiology
Experiments were performed as described (31). Briefly, mice were anesthetized and the peak isometric force of the TA muscle was analyzed in situ via nerve stimulation, and the maximum force-producing capacity of the muscle was determined at optimum length. Peak force was then divided by the unit area to determine specific force. The contraction-induced injury was measured immediately before increased length changes during maximal stimulation at 20-s intervals. Length changes were increased in 5% increments from 5 to 45% of muscle fiber length to produce injury.
Statistical analysis
Data shown are average values ± SD. P values were calculated by Student’s t test.
Supplementary Material
Acknowledgments
We thank G. Ackerman and A. Kelly for assistance with mouse colonies; K. Helm for flow cytometry; and K. Tanaka, A. Troy, and T. Antwine for technical assistance.
Funding: This work was supported by NIH grants AR039467, AR049446, AG028907 (to B.B.O.), and NS046788 (to J.S.C.) and NIH Training Grant GM06672801 (to J.K.H.).
Footnotes
Author contributions: J.K.H. designed and performed the experiments and wrote the manuscript, G.B. designed and performed the experiments and wrote the manuscript, J.S.C. designed the experiments and wrote the manuscript, and B.B.O. designed the experiments and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests. A patent has been filed by the University of Colorado, Boulder on the isolation, treatment, and transplantation of myofibers (US20100226898).
www.sciencetranslationalmedicine.org/cgi/content/full/2/57/57ra83/DC1
Fig. S1. Engraftment of donor cells is independent of injury agent.
Fig. S2. Fibrosis does not contribute to increased muscle mass after transplantation.
Fig. S3. Progressive hypertrophy in transplanted TA muscles.
Fig. S4. Quantification of satellite cells in transplanted TA muscles by FACS.
Fig. S5. Transplanted myofibers have only Pax7+ cells.
Fig. S6. Retention of MHC type II fibers 24 months after transplantation.
Table S1. FACS data for transplanted TA muscles.
Table S2. TA muscle peak force data at 24 months after transplantation.
Table S3. TA muscle–specific force data at 24 months after transplantation.
Table S4. TA muscle contraction–induced injury data at 24 months after transplantation. References
REFERENCES AND NOTES
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: Age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 4.Billington L, Carlson BM. The recovery of long-term denervated rat muscles after Marcaine treatment and grafting. J Neurol Sci. 1996;144:147–155. doi: 10.1016/s0022-510x(96)00219-5. [DOI] [PubMed] [Google Scholar]
- 5.Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann N Y Acad Sci. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- 6.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 7.Gopinath SD, Rando TA. Stem cell review series: Aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 8.Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9:213–228. doi: 10.1007/s10522-008-9131-0. [DOI] [PubMed] [Google Scholar]
- 9.Christov C, Chrétien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- 11.Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1173–R1187. doi: 10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol. 1990;16:225–238. doi: 10.1111/j.1365-2990.1990.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 15.Gayraud-Morel B, Chrétien F, Tajbakhsh S. Skeletal muscle as a paradigm for regenerative biology and medicine. Regen Med. 2009;4:293–319. doi: 10.2217/17460751.4.2.293. [DOI] [PubMed] [Google Scholar]
- 16.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.McLoon LK, Rowe J, Wirtschafter J, McCormick KM. Continuous myofiber remodeling in uninjured extraocular myofibers: Myonuclear turnover and evidence for apoptosis. Muscle Nerve. 2004;29:707–715. doi: 10.1002/mus.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson BM, Dedkov EI, Borisov AB, Faulkner JA. Skeletal muscle regeneration in very old rats. J Gerontol A Biol Sci Med Sci. 2001;56:B224–B233. doi: 10.1093/gerona/56.5.b224. [DOI] [PubMed] [Google Scholar]
- 19.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 20.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 21.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 22.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of α-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 24.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 25.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Ieronimakis N, Balasundaram G, Reyes M. Direct isolation, culture and transplant of mouse skeletal muscle derived endothelial cells with angiogenic potential. PLoS One. 2008;3:e0001753. doi: 10.1371/journal.pone.0001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 28.Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev. 2004;18:2231–2236. doi: 10.1101/gad.1214204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banks GB, Gregorevic P, Allen JM, Finn EE, Chamberlain JS. Functional capacity of dystrophins carrying deletions in the N-terminal actin-binding domain. Hum Mol Genet. 2007;16:2105–2113. doi: 10.1093/hmg/ddm158. [DOI] [PubMed] [Google Scholar]
- 32.Musarò A, Cusella De Angelis MG, Germani A, Ciccarelli C, Molinaro M, Zani BM. Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res. 1995;221:241–248. doi: 10.1006/excr.1995.1372. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan VK, Powers SK, Criswell DS, Tumer N, Larochelle JS, Lowenthal D. Myosin heavy chain composition in young and old rat skeletal muscle: Effects of endurance exercise. J Appl Physiol. 1995;78:2115–2120. doi: 10.1152/jappl.1995.78.6.2115. [DOI] [PubMed] [Google Scholar]
- 34.Schertzer JD, Plant DR, Ryall JG, Beitzel F, Stupka N, Lynch GS. β2-Agonist administration increases sarcoplasmic reticulum Ca2+-ATPase activity in aged rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E526–E533. doi: 10.1152/ajpendo.00399.2004. [DOI] [PubMed] [Google Scholar]
- 35.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 36.Ishai-Michaeli R, Eldor A, Vlodavsky I. Heparanase activity expressed by platelets, neutrophils, and lymphoma cells releases active fibroblast growth factor from extracellular matrix. Cell Regul. 1990;1:833–842. doi: 10.1091/mbc.1.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsmith KT, Gammon RB, Garver RI., Jr Modulation of bFGF in lung fibroblasts by TGF-β and PDGF. Am J Physiol. 1991;261:L378–L385. doi: 10.1152/ajplung.1991.261.6.L378. [DOI] [PubMed] [Google Scholar]
- 38.Robertson TA, Papadimitriou JM, Grounds MD. Fusion of myogenic cells to the newly sealed region of damaged myofibres in skeletal muscle regeneration. Neuropathol Appl Neurobiol. 1993;19:350–358. doi: 10.1111/j.1365-2990.1993.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 39.Boldrin L, Zammit PS, Muntoni F, Morgan JE. Mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal. Stem Cells. 2009;27:2478–2487. doi: 10.1002/stem.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace GQ, Lapidos KA, Kenik JS, McNally EM. Long-term survival of transplanted stem cells in immunocompetent mice with muscular dystrophy. Am J Pathol. 2008;173:792–802. doi: 10.2353/ajpath.2008.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher BD, Baracos VE, Shnitka TK, Mendryk SW, Reid DC. Ultrastructural events following acute muscle trauma. Med Sci Sports Exerc. 1990;22:185–193. [PubMed] [Google Scholar]
- 42.Winchester PK, Gonyea WJ. Regional injury and the terminal differentiation of satellite cells in stretched avian slow tonic muscle. Dev Biol. 1992;151:459–472. doi: 10.1016/0012-1606(92)90185-j. [DOI] [PubMed] [Google Scholar]
- 43.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 44.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4:217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 47.Li YP. TNF-α is a mitogen in skeletal muscle. Am J Physiol Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- 48.Sonnet C, Lafuste P, Arnold L, Brigitte M, Poron F, Authier FJ, Chrétien F, Gherardi RK, Chazaud B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci. 2006;119:2497–2507. doi: 10.1242/jcs.02988. [DOI] [PubMed] [Google Scholar]
- 49.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skuk D, Tremblay JP. Cell therapies for inherited myopathies. Curr Opin Rheumatol. 2003;15:723–729. doi: 10.1097/00002281-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 52.Cerletti M, Jurga S, Witczak CA, Hirshman MF, Shadrach JL, Goodyear LJ, Wagers AJ. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller JB, Crow MT, Stockdale FE. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol. 1985;101:1643–1650. doi: 10.1083/jcb.101.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.