Abstract
Background
The reported effects of bariatric surgery on food cravings have been inconsistent. Moreover, research has been largely limited to sweet cravings, and no study has examined whether surgery patients’ cravings differ from those of normal weight (NW) controls. Our objective was to use an empirically validated instrument to examine changes in bariatric surgery patients’ frequency of food cravings and consumption of craved foods from before to 3 and 6 months after surgery and to compare surgery patients’ frequency of food cravings to those of NW controls. The setting was private hospitals and research center in the United States.
Methods
Bariatric surgery patients (n = 32) and NW controls (n = 20) completed the Food Cravings Inventory and had their height and weight measured.
Results
Before surgery, the patients reported more overall cravings and cravings for high fat and fast foods and a greater consumption of craved high-fat foods than the NW controls. From before to 3 and 6 months after surgery, the patients had significant reductions in overall cravings for, and consumption of, craved foods, with specific effects for sweets and fast food; however, surgery had virtually no effect on the cravings for high-fat foods. Moreover, high-fat and fast food cravings did not reduce to normative levels. The postoperative patients were less likely to consume craved sweets than NW controls, and the patients’ postoperative weight loss was largely unrelated to food cravings.
Conclusion
Bariatric surgery is associated with significant reductions in food cravings and consumption of craved foods, with the exception of high-fat foods. Despite these decreases, patients’ cravings do not fully reduce to “normative” levels and are not associated with postoperative weight loss.
Keywords: Food craving, Bariatric surgery, Normal weight
Food cravings are intense urges to consume a particular food or a particular type of food [1]. A variety of factors have been shown to influence food cravings. Obese individuals (body mass index [BMI] ≥30 kg/m2) have more cravings for high-fat foods compared with normal weight (NW) individuals [1]. Similarly, Burton et al. [2] showed that a greater BMI is associated with greater levels of food cravings, including cravings for high-fat foods, fast food, and carbohydrates and starches. Behavioral weight loss programs involving very low calorie diets (e.g., 400 kcal/d) and low calorie diets (e.g., 1000 kcal/d) have been shown to decrease food cravings [3,4]. Likewise, prescribed energy restriction is associated with a decreased consumption of craved food, and these decreases are associated with greater weight loss in lifestyle programs [5].
Although the effects of dietary restriction on food cravings have been examined among overweight/obese individuals enrolled in behavioral weight loss programs, aside from sweet cravings, no one has ever investigated the changes in food cravings and consumption of craved foods associated with surgical approaches to weight loss. Moreover, those studies that have examined the effects of bariatric surgery on sweet cravings have not used empirically validated measures. For example, using an unpublished interview, Busetto et al. [6] reported that preoperative sweet cravings are not associated with postoperative weight loss. However, using an unvalidated questionnaire, Burgmer et al. [7] concluded that sweet cravings increase after bariatric surgery and that both pre- and postoperative sweet cravings are associated with poorer weight outcomes. The inconsistencies in the bariatric surgery literature and between some of the surgery findings and those reported from behavioral weight loss programs might be real. However, they could also be an artifact of the untested and unvalidated assessment instruments that were used. Thus, more research is needed that not only uses empirically validated instruments to determine the effects of bariatric surgery on food cravings but also assesses different types of food cravings in surgery patients (e.g., high fat, fast food), not just sweet cravings.
The purpose of the present prospective study was to examine the frequency of food cravings and the consumption of craved foods in bariatric surgery patients using an empirically validated measure and to compare the surgery patients’ food cravings to those of NW controls. From previous findings [3–5], we hypothesized that, (1) given their greater BMI, bariatric surgery patients would report more food cravings and a greater consumption of craved foods compared with NW controls before and 3 and 6 months after surgery; (2) bariatric surgery patients’ food cravings and consumption of craved foods would decrease from before to after surgery; and (3) the decreases in food cravings and consumption of craved foods would be associated with postoperative weight loss.
Methods
Procedure
The surgery patients were recruited from 3 bariatric surgery clinics from January 2008 to April 2009. During a preoperative visit, prospective patients were informed of the study. Those who agreed to be interviewed were screened by telephone to ensure eligibility. The inclusion criteria included age 21–70 years and a plan to undergo bariatric surgery. A total of 52 participants were invited to attend an orientation session, agreed to participate, and completed the baseline assessment session within 6 weeks before surgery. Of these 52 patients, 32 completed the 3- and 6-month postoperative follow-up assessments. No significant differences were present between the completers and noncompleters in demographic characteristics or baseline measures of food cravings or consumption of craved foods. The reasons for not completing included relocation (n = 2), death (n = 1), serious illness (n = 5), and lost to follow-up (n = 12). Patient compensation included $15 for the preoperative and 3-month postoperative assessments and $20 for the 6-month postoperative assessment.
The NW participants were recruited by placing an advertisement on a closed online network only available to hospital employees. Those interested were screened by telephone to determine eligibility, which included a BMI of 18.5–24.9 kg/m2 and age 21–70 years. A total of 23 individuals were invited to attend an orientation session; 20 provided informed consent and completed the 1-time assessment session. The NW participants were compensated $20 for assessment completion.
The institutional review board at The Miriam Hospital approved all procedures.
Measures
The participants reported basic demographic information. The participants’ weight was measured to the nearest .1 kg, and their height was measured to the nearest millimeter. The BMI was calculated using the following formula: weight in kilograms/height in square meters. This information was used to confirm the weight status of the NW controls and to calculate the percentage of excess weight loss (%EWL) in the surgery patients ([%EWL = weight change/(preoperative weight − ideal body weight) × 100], the ideal body weight was determined from the Metropolitan Height and Weight Tables for a person of a medium frame; American Society for Bariatric Surgery Standards Committee, 2005).
The Food Craving Inventory (FCI) [1] was used to assess food cravings and consumption of craved food. The FCI consists of 28 food items. The respondents are given the definition of a food craving and asked to indicate how often they have craved each food item during the past 30 days and how often they have eaten the craved food. The response options range from 1 (“never”) to 5 (“always”). To assess food consumption as it relates to craving, if a food item is never craved, the respondents indicate that they never ate the food. In addition to an overall score (the mean of all 28 items), the FCI produces 4 subscale scores that represent the mean ratings for specific food types, including high-fat foods (e.g., gravy), sweets (e.g., ice cream), carbohydrates/starches (e.g., rolls), and fast foods (e.g., hamburger). The overall scale assessing the frequency of food cravings and the associated subscales have demonstrated good to excellent internal consistency (α = .76–.93) and test–retest reliability (r = .79–.91) [8]. With regard to validity, the FCI is associated with other empirically validated measures of food cravings, discriminates between overweight and NW individuals, and is responsive to changes in dietary intake and weight loss [4]. Validity data are available for the consumption of craved food scale [8]; however, additional assessment of the psychometric properties of this subscale is warranted.
Statistical analysis
Differences in the demographic characteristics between the surgery patients and NW controls were examined using analysis of variance or chi-square tests for continuous or categorical variables, respectively. Significant differences were controlled for when comparing the 2 groups on the frequency of food cravings and consumption of craved foods. For example, using the procedures of Cohen and Cohen [9], significant categorical variables were dichotomized and entered as covariates (i.e., having a college education was dichotomized as 0 or 1 and included as a covariate). The 1-time measurement of food cravings in the NW controls was compared with the pre- and postoperative food cravings in the surgery patients using separate between-subjects multivariate analyses of variance.
A within-subject repeated-measures multivariate analyses of variance was used to examine the changes in food cravings from preoperatively to 3 and 6 months postoperatively in the bariatric surgery patients. Significant differences were followed by post hoc paired samples t tests. The surgery type (laparoscopic adjustable gastric banding [LAGB] versus Rouxen-Y gastric bypass [RYGB]) was not included as a covariate, because the surgery type was not associated with differential changes in food cravings or the consumption of craved foods (P ≥.39 for all). Correlations were conducted to determine whether food cravings and the consumption of craved foods were associated with the %EWL in surgery patients. Given that RYGB was associated with significantly greater weight loss outcomes than LAGB (P ≤ .003 for all; see Table 1), we conducted a partial correlation between food cravings and the %EWL, including surgery type as a covariate (0 = RYGB; 1 = LAGB). The Statistical Package for Social Sciences (SPSS, Chicago, IL) was used for all analyses.
Table 1.
Participant characteristics
| Variable | Bariatric surgery patients (n = 32) | NW controls (n = 20) |
|---|---|---|
| Demographic data | ||
| Women (n) | 28 (88) | 18 (90) |
| Age (yr) | 47.9 ± 10.6 | 47.9 ± 9.2 |
| Race/ethnicity | ||
| White | 27 (84) | 20 (100) |
| Hispanic | 3 (9) | 0 (0) |
| Black | 2 (6) | 0 (0) |
| Marital status (n) | ||
| Never married | 11 (34) | 3 (15) |
| Married | 17 (53) | 13 (65) |
| Divorced | 4 (13) | 4 (20) |
| Education (n) | ||
| High school | 8 (25) | 0 (0) |
| Vocational training | 1 (3) | 0 (0) |
| Some college | 8 (25) | 4 (20) |
| College | 10 (31) | 5 (25) |
| Graduate/professional | 5 (16) | 11 (55) |
| LAGB (n = 20) | RYGB (n = 12) | ||
|---|---|---|---|
| Anthropometric data | |||
| Weight (kg) | 60.1 ± 8.3 | ||
| Preoperatively | 122.4 ± 19.3 | 136.6 ± 38.1 | |
| 3-mo Postoperatively | 106.9 ± 15.1 | 109.6 ± 31.8 | |
| 6-mo Postoperatively | 101.5 ± 13.5 | 97.1 ± 28.2 | |
| BMI (kg/m2) | 22.5 ± 1.3 | ||
| Preoperatively | 47.6 ± 8.6 | 50.5 ± 9.6 | |
| 3-mo Postoperatively | 40.7 ± 7.0 | 40.3 ± 8.7 | |
| 6-mo Postoperatively | 38.5 ± 6.4 | 35.9 ± 7.7 | |
| %EWL | NA | ||
| Preoperatively | NA | NA | |
| 3-mo Postoperatively | 27.8 ± 6.4 | 41.2 ± 14.5 | |
| 6-mo Postoperatively | 35.6 ± 10.6 | 58.1 ± 15.7 | |
NW = normal weight; LAGB = laparoscopic adjustable gastric banding; BMI = body mass index; %EWL = percentage of excess weight loss; NA = not applicable.
Results
The surgery patients (n = 32) were predominantly women and white, with a mean age of 47.9 ± 10.6 years. Of the 32 patients, 20 underwent LAGB and 12 underwent RYGB. The typical band adjustment schedule for the LAGB patients was 4–4.5 mL 6 weeks after surgery followed by 4 adjustments each month of 1.5, 1.0, .5, and .25 mL. The 20 NW controls were mostly women and white, with a mean age of 47.9 ± 9.2 years. Education was the only demographic characteristic that differed between the 2 groups; the surgery patients were less likely to have a college education than were the NW controls (47% versus 80%; P = .02). Details of the demographics and anthropometrics are listed in Table 1.
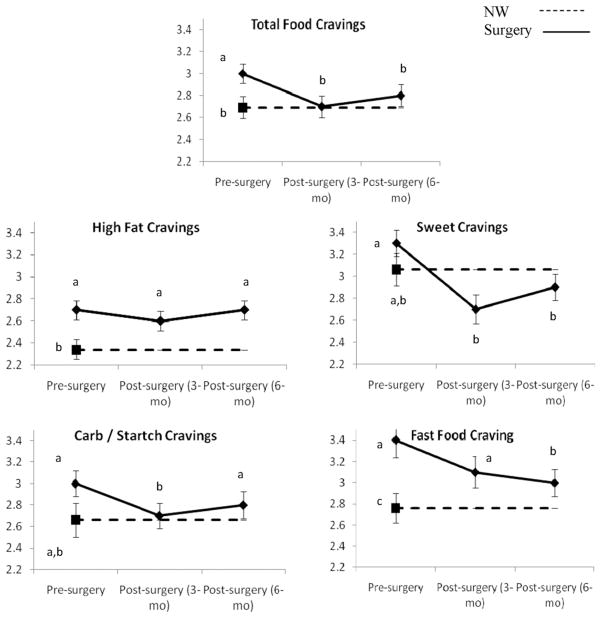
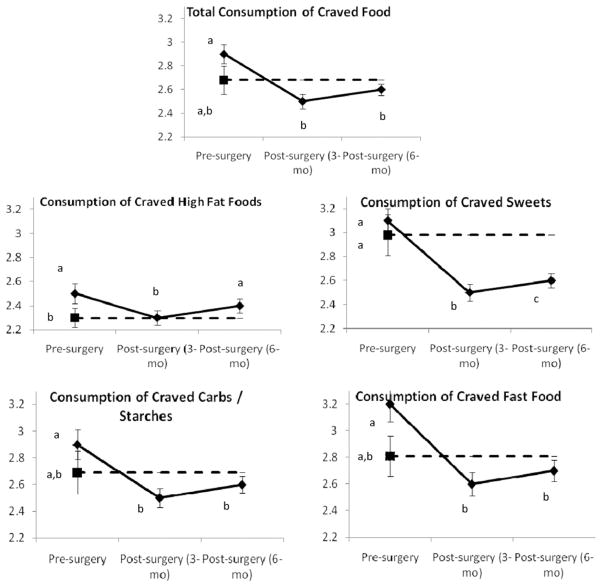
Before surgery, the patients reported more overall food cravings and more cravings for high-fat and fast foods than the NW controls (P < .02 for all). Similarly, the surgery candidates were more likely to consume the craved high-fat foods than were the NW controls (P = .04; Fig. 1).
Fig. 1.
Frequency of food cravings and consumption of craved foods in bariatric surgery patients (solid line) measured preoperatively and 3 and 6 months postoperatively and NW controls (dashed line) measured at preoperative point. These data represent results from separate between-subject and within-subject analyses. For each dependent variable, data points with different labels (a, b, c) differ significantly from each other (P < .05). For example, in total food cravings, bariatric surgery patients had more frequent food cravings before surgery than at either postoperative point and compared with NW controls (a versus b). However, after surgery, patients did not differ from NW controls (shared b). Data points represent mean; error bars, standard error of mean.
After surgery, the patients had a significant decrease in total food cravings (P < .05), such that their total cravings after surgery were similar to those of the NW controls (P > .28; Fig. 1). Surgery type (RYGB versus LAGB) did not differentially affect the changes in food cravings (P ≥ .39). Compared with the preoperative levels, the patients reported a significant reduction in their cravings for sweets, carbohydrates/starches, and fast food (P < .05 for all). Despite the decrease in fast food cravings, the postoperative patients continued to report greater cravings for fast food than did the NW participants. Bariatric surgery had virtually no effect on the high-fat food cravings at either point (P = .23 and P = .96). Thus, the surgery patients’ high-fat food cravings were consistently greater than those of the NW controls.
Patients reported a significant reduction in the total consumption of craved foods, including sweets, carbohydrates/starches, and fast foods (P < .05 for all). However, 6 months after surgery, the patients’ consumption of craved high-fat foods was not significantly different from the pre-operative levels (P = .16). Thus, postoperatively, the patients’ consumption of craved fats remained greater than that of the NW controls (P = .048). In contrast, the bariatric surgery patients were less likely to consume craved sweets than were the NW controls at both 3 and 6 months after surgery (P = .016 and P = .036, respectively; Fig. 1). Surgery type did not differentially affect the changes in the consumption of craved foods (P ≥ .46 for all).
Neither the preoperative food cravings nor the consumption of craved foods was associated with postoperative weight loss (P > .14 for all). However, greater reductions in the cravings for sweets and the consumption of craved sweets from baseline to 3 months after surgery were associated with a greater %EWL during the same period (r = −.35, P = .048 and r = −.35, P = .049, respectively), but this relationship was no longer significant at 6 months after surgery (r =−.27, P =.14 and r = −.18, P = .33, respectively). The remaining changes in food cravings and consumption were not associated with the %EWL at either postoperative period.
Discussion
The present study is the first to examine the different types of food cravings, not just sweet cravings, and the consumption of craved foods in bariatric surgery patients using an empirically validated measure. Moreover, this is the first investigation of the differences in food cravings between morbidly obese bariatric surgery patients and NW individuals. Our results have shown that bariatric surgery is associated with significant decreases in overall food cravings and the overall consumption of craved foods at 3 and 6 months postoperatively. Despite these decreases, surgery patients’ cravings did not fully reduce to a level comparable to that of NW controls. Moreover, the surgery patients’ changes in cravings and consumption were largely unrelated to weight loss.
Several mechanisms could account for the decreases in food cravings during the 3–6-month postoperative period. Immediately after surgery, many patients are unable to consume certain types of food (e.g., bread, pasta) without experiencing physical discomfort and vomiting. This postoperative phenomenon might not only reduce consumption of various foods, but also might result in a conditioned taste aversion to specific food types, explaining why the reduction in cravings and the consumption of craved foods was most evident from baseline to 3 months postoperatively, after which the cravings and consumption either plateaued or significantly increased to preoperative levels. Moreover, after surgery, the patients report dramatic improvements in mood [10], showed increases in the neurotransmitter serotonin [11], and demonstrated decreases in ghrelin [12], all of which have been implicated in the reduction of food cravings [13–17]. Other mechanisms that could explain the decreases in cravings include changes in dietary intake and the significant weight loss associated with surgery [18,19], as these factors have been shown to reduce food cravings in behavioral weight loss programs [3,5]. In addition, although nutritional counseling might not affect one’s food cravings per se, it could affect the consumption of craved foods, especially during the immediate postoperative period. Thus, the particular nutritional counseling received by patients in the present study (e.g., avoid high-sugar foods) might have decreased the consumption of such foods. However, bariatric surgery had virtually no effect on the high-fat food cravings or the consumption of the craved high-fat foods. It is possible that given their moisture content, many of these foods (e.g., gravy, fried fish) are more easily tolerated after surgery compared with other, drier foods (e.g., bread, cookies, biscuits), thereby making these foods more palatable and desirable. Future research might consider examining which mechanisms are responsible for the immediate and differential reduction of food cravings in bariatric surgery patients, with a particular focus on the dietary, biologic, and affective changes. Moreover, given that RYGB and LAGB procedures might lead to differential changes in the mechanisms associated with appetite regulation (e.g., ghrelin, polypeptide YY) [20], future research could examine whether the mechanisms associated with changes in food cravings differ according to surgery type.
Our findings are inconsistent with those from a previous surgery study on the changes in sweet cravings. Instead of significant increases in sweet cravings from before to after surgery [7], we demonstrated significant decreases. These findings might have resulted from the differences in the assessment timelines. Burgmer et al. [7] examined sweet cravings before surgery and at 1 year after surgery. However, we assessed the cravings before surgery and 3 and 6 months after surgery. Given that sweet cravings were increasing from the 3-month point to the 6-month point in the present study, it is possible that longer term follow-up would have revealed a significant increase in sweet cravings from preoperatively to 1 year postoperatively. Another plausible explanation for the discrepancy in the findings is the difference in the assessment instruments used. Burgmer et al. [7] used an unvalidated instrument to assess sweet cravings, whereas the present study used a psychometrically sound measure of food cravings, which might have resulted in a more accurate assessment of sweet cravings.
We found that the changes in food cravings did not differ significantly between the RYGB and LAGB patients. For example, the total food cravings decreased from baseline to 3 months by .3 ± .5 in the RYGB patients and by .2 ± .6 in the LAGB patients, and the total sweet cravings decreased by .8 ± .5 and .6 ± .6 in the RYGB and LAGB patients, respectively. We recognize that our ability to compare the 2 groups was limited by the sample size. However, we examined the sample size necessary to detect such a small difference and found that >200 participants would be required. Moreover, an examination of the effect sizes (all part. η2 ≤ .03) showed that neither these nor any other differences between surgery type appeared to be clinically meaningful. However, given that these data were from a relatively small sample size, additional examination of the differences in food cravings by surgery type is definitely warranted.
Environmental and biologic differences between obese and NW individuals might account for the greater food cravings and consumption of craved foods in surgery patients both before and after surgery. Increased weight has been associated with decreased sensitivity of the dopamine-reward system [21], increased responsiveness to food cues [22], and deficiencies in the brain chemical serotonin [23], all of which have been speculated to increase food cravings [24,25]. However, we also found that postoperative patients were less likely to consume craved sweets than were the NW controls. This effect might have resulted from the “dumping syndrome”; gastric bypass patients frequently experience nausea and vomiting after consuming foods high in sugar, which could make these foods aversive and therefore avoided.
In contrast to some findings from behavioral weight loss programs [5], our results showed that the level of preoperative food cravings and consumption of craved foods were not associated with postoperative weight loss. However, the reductions in sweet cravings and the consumption of craved sweets from before to 3 months after surgery were associated with a greater %EWL during the same period. This is the first study to capture the changes in cravings during the immediate postoperative period (i.e., 3 mo) and to show that these changes are associated with concurrent weight loss. However, our 6-month results are largely consistent with published surgical studies [6,7,26], demonstrating that food cravings, including sweet cravings, are not associated with longer term weight loss outcomes.
The limitations of the present study included the small sample size, relatively short-term follow-up period, and predominantly white sample. Given that we only investigated food cravings in patients who underwent LAGB or RYGB, additional research on food cravings in patients who undergo other types of surgical procedures is needed. Moreover, the surgery patients self-selected to participate in the study and a large number of participants who completed baseline assessments did not complete the follow-up assessments; thus, the surgery sample might not represent the larger population of patients undergoing bariatric surgery. Also, validity data have only been reported for the FCI consumption scale. However, this questionnaire is the only food cravings consumption survey with any published psychometric data and, therefore, was the best available self-report measure of the consumption of craved food. Future research should continue to develop psychometrically sound measures of the consumption of craved foods and examine craved food consumption in surgery patients. The lack of a dietary measure did not permit us to assess the relationship between the changes in food cravings and changes in macro- and micronutrient food consumption. However, in an analogue study, Martin et al. [27] showed that cravings for specific foods (e.g., sweets, fats) are associated with the consumption of those foods. Also, we did not formally measure smoking status, a factor that might be linked to food cravings [28]. However, the surgery patients were required to stop smoking for ≥1 month before surgery (measured by a urine test). The NW individuals were assessed once and the surgery patients were assessed 3 times; thus, it is possible that time effects might account for the differences in food cravings between the 2 groups. Although the menstrual cycle is associated with food cravings, the FCI assesses food cravings “during the past month”; thus, given that a typical menstrual cycle is 1 month long, the cravings associated with the menstrual cycle should not affect the stability of FCI if assessed at 3-month intervals. Moreover, 3 independent studies (White et al. [1], Cepeda-Benito et al. [29], and Nijs et al. [30]) have all shown that in the absence of intervention or manipulation, the frequency of food cravings appears to be stable. In contrast, no studies, to our knowledge, have shown that the frequency of food cravings or the consumption of craved foods changes without some sort of intervention or disruption in appetite.
The present study had several important strengths. Previous research on surgery patients’ food cravings focused exclusively on sweet or carbohydrate cravings and did not use empirically validated instruments. The present study is the first to examine a variety of food cravings (e.g., sweets, fast foods, high-fat foods) and the consumption of craved foods in bariatric surgery patients using a psychometrically sound measure. In addition, the weight data were objective, not self-reported. Furthermore, the longitudinal nature of these data allowed us to examine the effects of surgery on food cravings and the relationship between the changes in the cravings and consumption of craved food and postoperative weight loss. Finally, this is the only study to compare the frequency of food cravings and the consumption of craved food between NW individuals and morbidly obese bariatric surgery patients.
Taken together, these results suggest that although bariatric surgery is associated with significant reductions in overall food cravings and the consumption of craved foods, the cravings do not “normalize” after surgery. Instead, surgery patients continue to experience relatively high levels of food cravings, suggesting that the surgical procedure and associated dietary restrictions might help patients to resist these cravings. Also of note is that the food cravings and consumption of craved foods tended to plateau or increase during the postoperative period. Thus, future research might consider a longer follow-up period to determine the durability of the effects of bariatric surgery on food cravings and the mechanisms responsible for the changes in food cravings during the postoperative period.
Footnotes
Disclosures
The authors have no commercial associations that might be a conflict of interest in relation to this article.
References
- 1.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obes Res. 2002;10:107–14. doi: 10.1038/oby.2002.17. [DOI] [PubMed] [Google Scholar]
- 2.Burton P, Smit HJ, Lightowler HJ. The influence of restrained and external eating patterns on overeating. Appetite. 2007;49:191–7. doi: 10.1016/j.appet.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Harvey J, Wing RR, Mullen M. Effects on food cravings of a very low calorie diet or a balanced, low calorie diet. Appetite. 1993;21:105–15. doi: 10.1016/0195-6663(93)90003-3. [DOI] [PubMed] [Google Scholar]
- 4.Martin CK, O’Neil PM, Pawlow L. Changes in food cravings during low-calorie and very-low-calorie diets. Obesity. 2006;14:115–21. doi: 10.1038/oby.2006.14. [DOI] [PubMed] [Google Scholar]
- 5.Gilhooly CH, Das SK, Golden JK, et al. Food cravings and energy regulation: the characteristics of craved foods and their relationship with eating behaviors and weight change during 6 months of dietary energy restriction. Int J Obes. 2007;31:1849–58. doi: 10.1038/sj.ijo.0803672. [DOI] [PubMed] [Google Scholar]
- 6.Busetto L, Segato G, De Marchi F, et al. Outcome predictors in morbidly obese recipients of an adjustable gastric band. Obes Surg. 2002;12:83–92. doi: 10.1381/096089202321144649. [DOI] [PubMed] [Google Scholar]
- 7.Burgmer R, Grigutsch K, Zipfel S, et al. The influence of eating behavior and eating pathology on weight loss after gastric restriction operations. Obes Surg. 2005;15:684–91. doi: 10.1381/0960892053923798. [DOI] [PubMed] [Google Scholar]
- 8.ASBSS Committee. Oria HE, Carrasquilla C, et al. Guidelines for weight calculations and follow-up in bariatric surgery. Surg Obes Relat Dis. 2005;1:67–8. doi: 10.1016/j.soard.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Cohen JC, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale: Erlbaum; 1983. [Google Scholar]
- 10.Hayden MJ, Dixon JB, Dixon ME, Shea TL, O’Brien PE. Characterization of the improvement in depressive symptoms following bariatric surgery. Obes Surg. 2011;21:328–35. doi: 10.1007/s11695-010-0215-y. [DOI] [PubMed] [Google Scholar]
- 11.Romanova IV, Ramos EJ, Xu Y, et al. Neurobiologic changes in the hypothalamus associated with weight loss after gastric bypass. J Am Coll Surg. 2004;199:887–95. doi: 10.1016/j.jamcollsurg.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Beckman LM, Beckman TR, Earthman CP. Changes in gastrointestinal hormones and leptin after Rouxen-Y gastric bypass procedure: a review. J Am Diet Assoc. 2010;110:571–84. doi: 10.1016/j.jada.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelchat ML. Food addiction in humans. J Nutr. 2009;139:620–2. doi: 10.3945/jn.108.097816. [DOI] [PubMed] [Google Scholar]
- 14.Rogers PJ, Smit HJ. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000;66:3–14. doi: 10.1016/s0091-3057(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 15.Tuomisto T, Hetherington MM, Morris MF, Tuomisto MT, Turjanmaa V, Lappalainen R. Psychological and physiological characteristics of sweet food “addiction”. Int J Eat Disord. 1999;25:169–75. doi: 10.1002/(sici)1098-108x(199903)25:2<169::aid-eat6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Kalra SP, Kalra PS. Overlapping and interactive pathways regulating appetite and craving. J Addict Dis. 2004;23:5–21. doi: 10.1300/J069v23n03_02. [DOI] [PubMed] [Google Scholar]
- 17.Wurtman JJ. Carbohydrate cravings: a disorder of food intake and mood. Clin Neuropharmacol. 1988;11(Suppl 1):S139–45. [PubMed] [Google Scholar]
- 18.Bavaresco M, Paganini S, Lima TP, et al. Nutritional course of patients submitted to bariatric surgery. Obes Surg. 2010;20:716–21. doi: 10.1007/s11695-008-9721-6. [DOI] [PubMed] [Google Scholar]
- 19.Brolin RL, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Rouxen-Y gastric bypass. Ann Surg. 1994;220:782–90. doi: 10.1097/00000658-199412000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Rouxen-Y gastric bypass. Int J Obes. 2009;33:786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet. 2001;357:354–7. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 22.Mela DJ. Determinants of food choice: relationships with obesity and weight control. Obes Res. 2001;9(Suppl 4):249S–55S. doi: 10.1038/oby.2001.127. [DOI] [PubMed] [Google Scholar]
- 23.Erritzoe D, Frokjaer VG, Haugbol S, et al. Brain serotonin 2A receptor binding: relations to body mass index, tobacco and alcohol use. Neuroimage. 2009;46:23–30. doi: 10.1016/j.neuroimage.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Leibowitz SF, Hoebel BG. Behavioral neuroscience of obesity. In: Bray GA, editor. Handbook of obesity. New York: Marcel Dekker; 1998. pp. 313–58. [Google Scholar]
- 25.Gendall KA, Sullivan PF, Joyce PR, Bulik CM. Food cravings in women with a history of anorexia nervosa. Int J Eat Disord. 1997;22:403–9. doi: 10.1002/(sici)1098-108x(199712)22:4<403::aid-eat5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Madan AK, Orth WS, Ternovits CA, Tichansky DS. Preoperative carbohydrate “addiction” does not predict weight loss after laparoscopic gastric bypass. Obes Surg. 2006;16:879–82. doi: 10.1381/096089206777822304. [DOI] [PubMed] [Google Scholar]
- 27.Martin CK, O’Neill PM, Tollefson G, Greenway FL, White MA. The association between food cravings and consumption of specific foods in a laboratory taste test. Appetite. 2008;51:324–6. doi: 10.1016/j.appet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepino MY, Finkbeiner S, Mennella JA, Mennella JA. Similarities in food cravings and mood states between obese women and women who smoke tobacco. Obesity. 2009;17:1158–63. doi: 10.1038/oby.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cepeda-Benito A, Gleaves DH, Williams TL, Erath SA. The development and validation of the state and trait food cravings questionnaires. Behav Ther. 2000;31:151–73. doi: 10.1016/s0005-7967(99)00141-2. [DOI] [PubMed] [Google Scholar]
- 30.Nijs IMT, Franken IHA, Muris P. The modified trait and state food-cravings questionnaires: development and validation of a general index of food craving. Appetite. 2007;49:38–46. doi: 10.1016/j.appet.2006.11.001. [DOI] [PubMed] [Google Scholar]