Abstract
Background
Colonic microbiota digest resistant starches producing short chain fatty acids (SCFAs). The main SCFAs produced are acetate, propionate, and butyrate. Both excitatory and inhibitory effects of SCFA on motility have been reported. We hypothesized that the effect of SCFAs on colonic motility varies with chain length and aimed to determine the effects of SCFAs on propagating and non-propagating contractions of guinea pig proximal and distal colon.
Methods
In isolated proximal colonic segments, Krebs solution alone or containing 10–100 mM acetate, propionate, or butyrate was injected into the lumen, motility was videorecorded over 10 min, and spaciotemporal maps created. In distal colon, the lumen was perfused with the same solutions of SCFAs at 0.1 mL min−1, the movement of artificial fecal pellets videorecorded, and velocity of propulsion calculated.
Key Results
In proximal colon butyrate increased the frequency of full length propagations, decreased short propagations and had a biphasic effect on non-propagating contractions. Propionate blocked full and short propagations and had a biphasic effect on non-propagating contractions. Acetate decreased short and total propagations In distal colon, butyrate increased and propionate decreased velocity of propulsion.
Conclusions & Inferences
The data suggest that luminal SCFAs have differing effects on proximal and distal colonic motility depending on chain length. Thus the net effect of SCFAs on colonic motility would depend on the balance of SCFAs produced by microbial digestion of resistant starches.
Keywords: Gastrointestinal tract, intestinal motility, colonic propulsion, peristalsis, spaciotemporal maps
Introduction
Short chain fatty acids (SCFAs) are a product of fermentation of indigestible carbohydrates by commensal bacteria that reside in the gastrointestinal (GI) tract, with the highest populations occurring in the proximal large intestine(1). The most commonly occurring SCFAs are acetate, propionate, and butyrate, which are used as a nutrient source by colonic epithelial cells. These SCFAs also play a role in electrolyte and water transport by the colon, cell differentiation and growth, and have been shown to be protective against diseases such as colon cancer(2).
Absolute concentrations of individual and total SCFAs vary depending on organism, diet, and microflora diversity and concentration present in the colon (1,3). Individual concentrations of SCFAs tend to occur in ratios of 3:2:1 or 3:1:1 with acetate being the most prevalent, followed by propionate and butyrate respectively, with a total concentration of SCFAs around 100mM (4). These ratios are location dependent, occurring at highest levels in the proximal colon and decreasing caudally (5). This pattern correlates with SCFA production by bacteria and absorption by colonic epithelial cells.
SCFAs also activate two receptors, FFA2 (GPR43) and FFA3 (GPR41), which are expressed in various tissues and cells including adipose tissue, inflammatory cells, and enteroendocrine cells in the GI tract (4,6–8). Recent pharmacological studies have shown that SCFAs bind to both receptors but have varied potency (8–10). Propionate and acetate are approximately equipotent at FFA2, and butyrate is less potent. Propionate and butyrate are equipotent at FFA3, whereas acetate is less potent at FFA3. Both receptors are able to couple with Gαi/o, but only FFA2 is able to couple to Gαq and increase intracellular calcium levels (10).
Many previous studies have been conducted to elucidate the effect of SCFAs on the smooth muscle and neurons of the GI tract, and how these components might regulate GI motility(11,12). It is generally accepted that mucosal stimulation by SCFAs results in a release of paracrine or hormonal agents from enteroendocrine cells. The nature of the agent released and the effect of SCFA varies based on the species used, the type of preparation, and method of analysis used. In the rat colon, acetate, propionate and butyrate release 5-HT, which initiates or augments the peristaltic reflex (13). Other studies have shown that individual SCFAs can have differential effects (14,15). Propionate, for example, has been shown to cause the release of PYY and slow intestinal transit(16).
Within the past two decades, it has become possible to digitally videorecord and analyze the complete motility pattern of isolated intact intestinal tissue in real time(17–23). This technique of spaciotemporal mapping in the gut was first described by Hennig and colleagues in 1999 (24). In the present study we have used this technique to evaluate the effect of SCFAs on motility in the proximal and distal colon of the guinea pig. Using video recording and computer analysis, spatiotemporal maps of proximal colon were used to visualize motility patterns and determine the effects of intraluminal addition of SCFAs. Velocity of pellet propulsion was used to measure distal colonic motility. The results show that butyrate initiates or augments propulsive motility whereas propionate has the opposite effect causing a decrease in colonic motility at most concentrations. Acetate is less effective than either butyrate or propionate and tends to decrease propulsive activity. The effects were similar in proximal colon where fluid movement was measured and in distal colon where propulsion of fecal pellets was measured.
Methods
Hartley guinea pigs of either sex (150–300g) were purchased from Charles River Laboratories International and euthanized by asphyxiation with carbon dioxide. The animals were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were approved and conducted in accordance with the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Following asphyxiation, the proximal and distal colon were removed by dissection and placed in warmed, oxygenated (95% oxygen/5% carbon dioxide) Krebs buffer (pH 7.4, 37 °C) composed of (in mM): 118 NaCl, 4.75 KCl, 1.19 KH2PO4, 1.2 MgSO4, 2.54 CaCl2, 25 NaHCO3, 11 glucose. The proximal colon was cut to approximately 8 cm long and flushed with warmed Krebs buffer before being cannulated with 3 mm glass rods in the orad and caudad openings. Using molder’s clay, the cannulated segment was secured in a transilluminated organ bath positioned under a videorecording system (Catamount, St Albans VT) (Figure 1A) and allowed to equilibrate for 15 minutes and expel any remaining Krebs buffer from the lumen. Using a clamp to occlude the caudad cannula and a syringe to inject fluid through the orad cannula, the proximal colon was minimally distended in order to visualize the baseline motility. The distension volume was 0.5 – 1.0 ml and contained either Krebs buffer alone as control or Krebs buffer with 10, 30, or 100 mM of butyrate, propionate, or acetate as experimental condition. In each colonic segment the same volume was used for each condition and different concentrations were tested in random order. A different segment was used for each SCFA. Each condition was videorecorded for 600 seconds (10 minutes) and the preparation was washed with ~5 ml of Krebs buffer for ten minutes with no distension between test periods. Spatiotemporal maps of the proximal colon motility were then generated using commercially available software (Gastrointestinal Motility Monitoring System, Catamount, St. Albans VT.) for the latter 300 seconds (5 minutes) of a recording period and the motility quantified based on three patterns: (1) full propagations consisting of propagations that moved caudad over at least fifty percent of the segment, (2) short propagations were those that moved caudad less than fifty percent of the segment, and (3) non-propagations were those that remained stationary (See notation on Figure 2).
Figure 1.
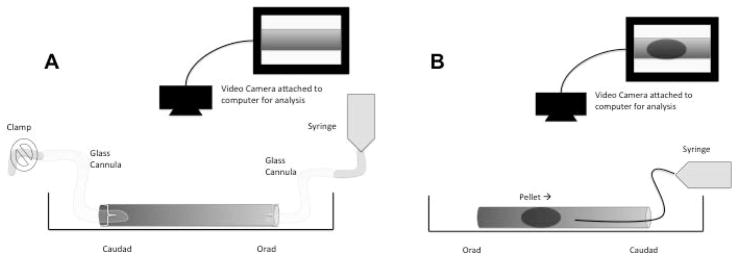
Experimental preparation of proximal colon (panel A) and distal colon (panel B). Proximal colonic segments were stimulated by intraluminal injection of Krebs buffer containing short chain fatty acids. Distal colon was intraluminally perfused at 0.1ml min−1 with Krebs buffer containing short chain fatty acids and the velocity of pellet propulsion measured.
Figure 2.
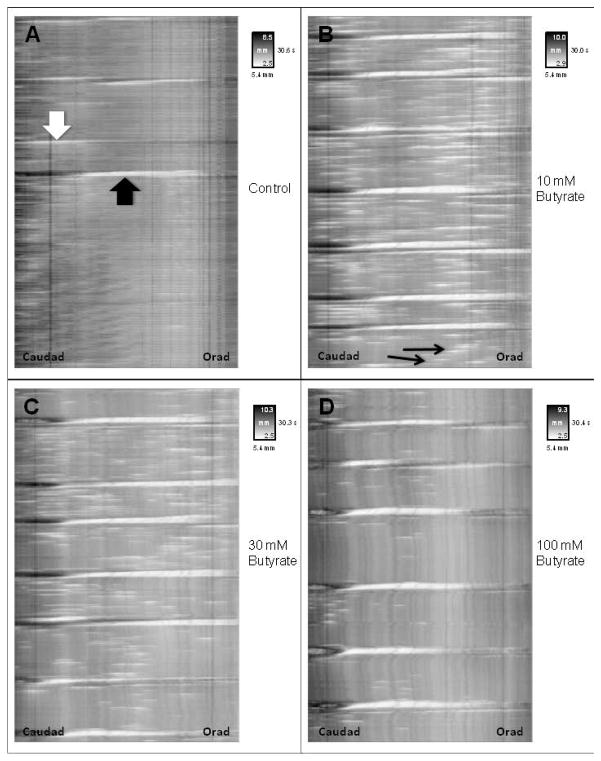
Spactiotemporal maps from 5-minute videorecording of proximal colon with or without luminal butyrate. Vertical distance along map indicates time. Horizontal distance indicates length along the segment with the anal end on the left and oral end on the right. The insert key for each map indicates time in seconds on the vertical axis and width of segment in gray scale on the vertical axis with white shading indicating narrow width (i.e. contraction) and dark shading indicating broader width (i.e. relaxation/distension). Panels illustrate typical spaciotemporal maps for intraluminal Krebs buffer (Panel A) alone and containing: (B) 10, (C) 30, and (D) 100 mM butyrate. White block arrow indicates a short propagation, black block arrow indicates a full propagation, and black thin arrows indicate non-propagations.
The distal colon was placed in warmed Krebs and allowed to expel pellets naturally. The colon was cut into 6cm segments and pinned at both ends in a GIMM organ bath and equilibrated for 15 min (Figure 1B). Clay pellets of similar size to natural fecal pellets were then inserted into the orad end and their movement through the segment recorded and velocities calculated using the pellet tracking software Catamount, St. Albans, VT). Following 2–3 control pellet trials, a solution of Krebs buffer plus 30mM butyrate, propionate, or acetate were perfused into the lumen of the distal colon using flexible PE 10 tubing advanced just caudad to the site of pellet insertion at a rate of 0.1ml min−1. The tubing was pushed out along with the pellet. In control studies, this rate of perfusion of Krebs alone and the presence of the PE 10 tubing were shown to have no effect on the velocity of pellet perfusion consistent with our previous results(25). Perfusion of Krebs buffer or Krebs buffer containing SCFA began 30 seconds before pellet insertion and continued throughout the trial. Recordings were made over at least 2 cm of the segment and velocities were expressed as mm sec−1. In all conditions, if the pellet was not propelled within the 10 min recording period, it was removed and a velocity of zero was assigned.
Preliminary studies were performed to confirm that the effects observed were due to the SCFAs themselves. As control for the additional sodium in the Krebs buffer from the added sodium salt SCFAs, Krebs buffer containing an additional 100mM NaCl was shown to have no effect versus normal Krebs buffer alone. Consistent with the pKa values for SCFAs (4.8–4.9), the pH of the solutions used in the study remained at 7.4, eliminating the possibility that observed effects were the result of pH.
Statistics were calculated using Prism GraphPad software (La Jolla, CA). One-Way ANOVA with a Dunnett’s multiple comparisons post-hoc test were used for analysis of proximal colon motility and two-tailed t-tests were used for analysis of pellet velocity in the distal colon. Graphical representations of the results represent the mean ± SEM with significance (*) representing p < 0.05. N values represent the number of animals with only one segment from each animal used with a given test agent.
Sodium salts of acetate, propionate, and butyrate; sodium chloride, potassium chloride, and D-glucose were purchased from Sigma-Aldrich, St. Louis, MO. Potassium phosphate and calcium chloride were purchased from Fischer Scientific, Pittsburgh, PA. Magnesium sulfate was purchased from JT Baker, Phillipsburg, NJ. Sodium bicarbonate was purchased from Midwest Scientific, St. Louis, MO.
Results
Response to butyrate in proximal colon
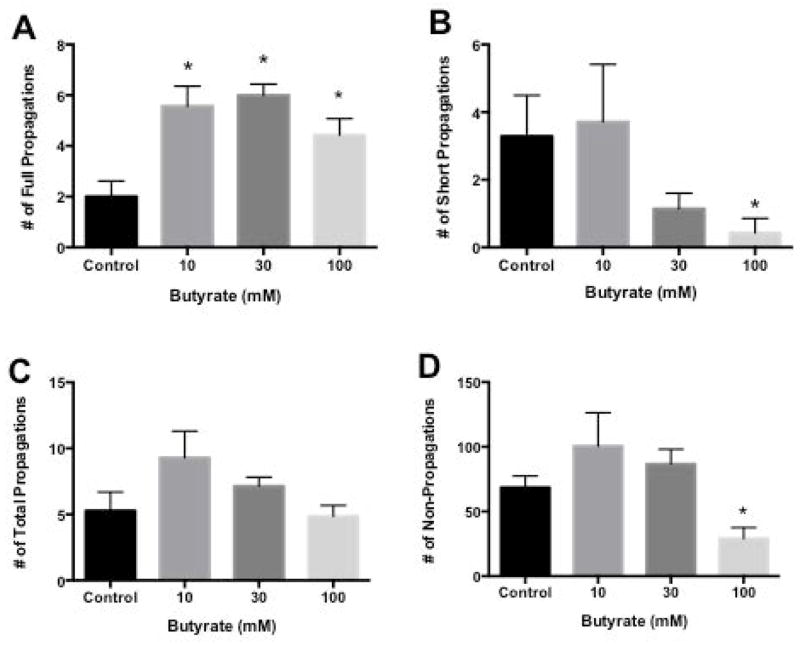
Figure 2 illustrates a typical spaciotemporal map derived from a single proximal colonic segment exposed to butyrate. The baseline frequency for full propagations in proximal colon was 2.0 ± 0.6 per 5-minute period. Following addition of 10, 30 and 100 mM butyrate the frequency of full propagations increased to 5.6 ± 0.8 (p<0.05), 6.1 ± 0.5 (p<0.05), and 4.4±0.6 (p<0.05) respectively (Figure 3A). The frequency of short propagations in the presence of 10 mM butyrate (3.7 ± 1.7 per 5-minute period) was similar to the baseline frequency (3.3 ± 1.2 per 5-minute period). Thirty mM butyrate decreased short propagation frequency to 1.1 ± 0.5 while 100 mM butyrate decreased the frequency to 0.4 ± 0.4 (p<0.05 Figure 3B). Full and short propagations were added together for a baseline frequency of 5.3 ± 1.4 total propagations per 5-minute period. Following addition of butyrate the frequency of total propagations was 9.3 ± 2.0 for 10 mM, 7.1 ± 0.7 for 30 mM and 4.9 ± 0.8 for 100 mM (Figure 3C). Baseline frequency of non-propagations was 68.6 ± 8.9 per 5-minute period. Following addition of 10 and 30 mM butyrate frequency of non-propagations increased to 100.6 ± 26.0 and 86.7 ± 11.0, respectively. 100 mM butyrate decreased non-propagations to 29.0 ± 9.0 (p<0.05) (Figure 3D).
Figure 3.
Bar graph of number of contractile responses per 5 min in proximal colon in response to butyrate. Panel A: Number of full length propagations per 5min. Full length propagations were defined as those moving more than one-half of the distance from oral to anal end of a segment. Panel B: Number of short propagations per 5min. Short propagations were defined as those moving less than one-half of the distance from oral to anal end of a segment. Panel C: Number of total propagations per 5min. Total propagations were defined as the sum of the full and short propagations. Panel D: Number of non-propagations per 5min. Non-propagations were defined as those contractions that failed to move.
Values are means ± SEM of 7 experiments. * indicate p<0.05
Response to propionate in proximal colon
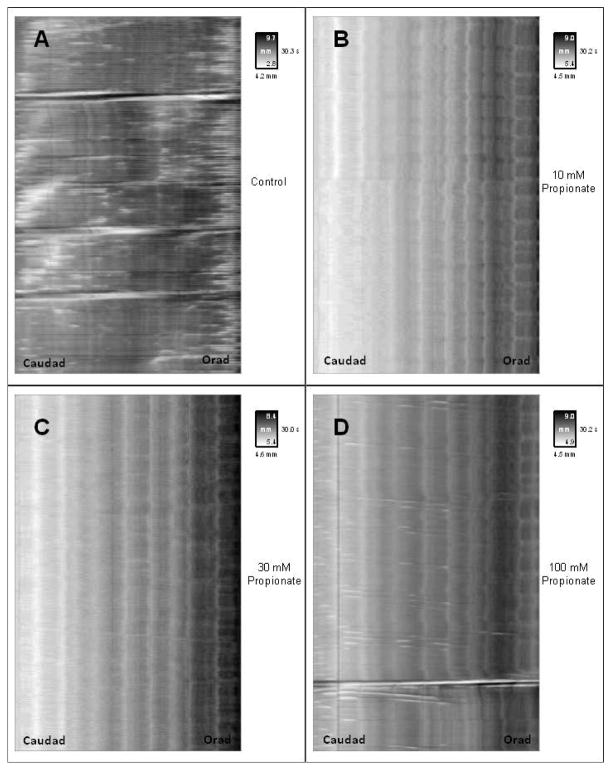
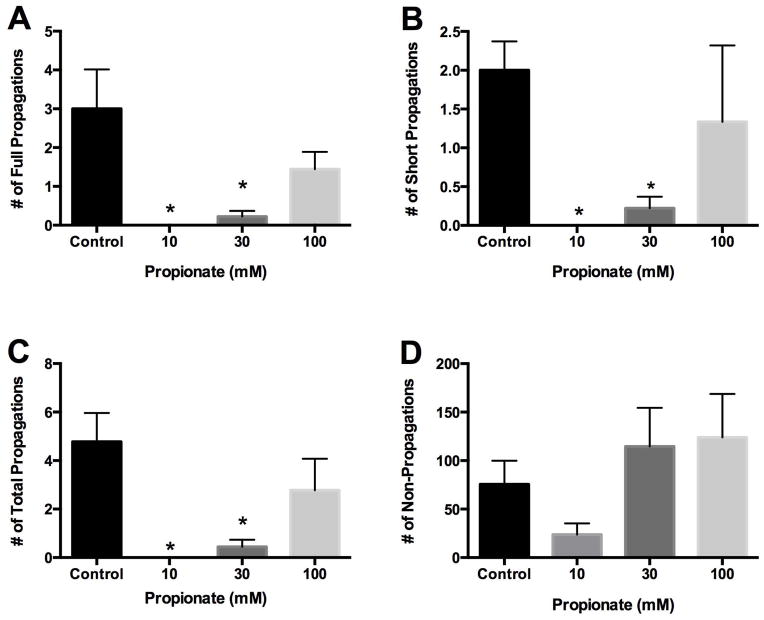
Figure 4 illustrates a typical spaciotemporal map derived from a single proximal colonic segment exposed to propionate. The baseline frequency for full propagations in proximal colon was 3.0 ± 1.0 per 5-minute period. Following addition of 10 mM propionate full propagations were abolished. Thirty mM and 100 mM propionate decreased the frequency of full propagations to 0.2 ± 0.2 (p<0.05), and 1.4±0.4 respectively (Figure 5A). The baseline frequency of short propagations was 2.0 ± 0.4 per 5-minute period and was abolished by 10 mM propionate. Thirty mM and 100 mM propionate decreased short propagations to 0.2 ± 0.2 (p<0.05) and 1.3 ± 1.0 respectively (Figure 5B). Full and short propagations were added together for a baseline frequency of 4.8 ± 1.2 total propagations per 5-minute period. Following addition of 10 mM propionate, total propagations were abolished. At 30 mM propionate the frequency of total propagations decreased to 0.4 ± 0.3 (p<0.05) and to 2.8 ± 1.3 at 100 mM propionate. (Figure 5C). The baseline frequency of non-propagations was 76 ± 24 per 5-minute period. Following addition of 10 mM propionate frequency of non-propagations tended to decrease to 24 ± 12. Thirty mM propionate increased frequency to 115 ± 40 and 100 mM propionate increased non-propagations to 124 ± 45 (Figure 5D).
Figure 4.
Spaciotemporal map from 5 minute videorecoding of proximal colon with or without luminal propionate. Vertical distance along map indicates time. Horizontal distance indicates length along the segment with the anal end on the left and oral end on the right. The insert key for each map indicates time in seconds on the vertical axis and width of segment on the horizontal axis in gray scale with white shading indicating narrow width (i.e. contraction) and dark shading indicating broader width (i.e, relaxation/distension). Panels illustrate typical spaciotemporal maps for intraluminal Krebs buffer alone and containing 10, 30 and 100 mM propionate.
Figure 5.
Bar graph of number of contractile responses per 5 min in proximal colon in response to propionate. Panel A: Number of full length propagations per 5 min. Full length propagations were defined as those moving more than one-half of the distance from oral to anal end of a segment. Panel B: Number of short propagations per 5 min. Short propagations were defined as those moving less than one-half of the distance from oral to anal end of a segment. Panel C: Number of total propagations per 5min. Total propagations were defined as the sum of the full and short propagations. Panel D: Number of non-propagations per 5min. Non-propagations were defined as those contractions that failed to move.
Values are means ± SEM of 9 experiments. * indicate p<0.05
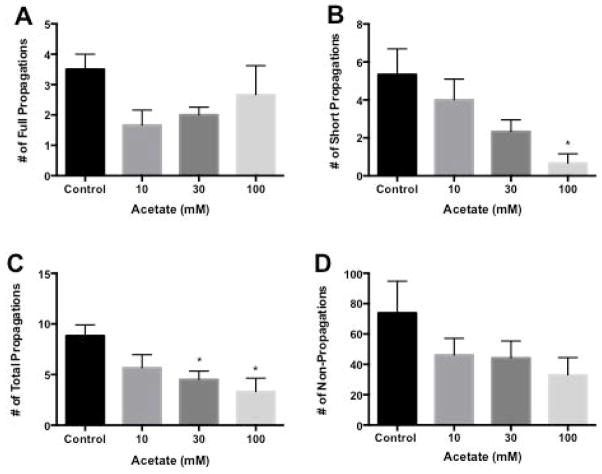
Response to Acetate in proximal colon
Figure 6 illustrates a typical spaciotemporal map derived from a single proximal colonic segment exposed to acetate. The baseline frequency for full propagations in proximal colon was 3.5 ± 0.5 per 5-minute period. Following addition of 10, 30 and 100 mM acetate the frequency of full propagations decreased to 1.7 ± 0.5, 2.0 ± 0.3, and 2.7 ± 1.0 respectively (Figure 7A). The baseline frequency of short propagations was 5.3 ± 1.4 per 5-minute period and decreased to 4.0 ± 1.1 at 10 mM, 2.3 ± 0.6 at 30 mM and 0.7 ± 0.5 (p<0.05) at 100 mM (Figure 7B). Full and short propagations were added together for a baseline frequency of 8.8 ± 1.1 total propagations per 5-minute period. Following addition of 10, 30 and 100 mM acetate total propagations were decreased to 5.7 ± 1.3, 4.5 ± 0.8 (p<0.05) and 3.3 ± 1.3 (p<0.05) respectively (Figure 7C). The baseline frequency of non-propagations was 74 ± 21 per 5-minute period. Following addition of 10, 30, and 100 mM acetate the frequency of non-propagations decreased to 46 ± 11, 44 ± 11 and 33 ± 11 respectively (Figure 7D).
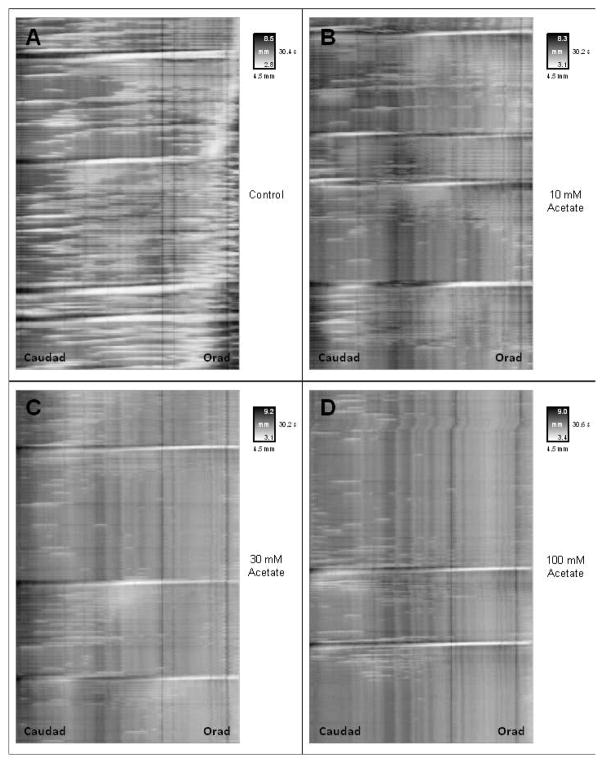
Figure 6.
Spaciotemporal map from 5 minute videorecoding of proximal colon with or without luminal acetate. Vertical distance along map indicates time. Horizontal distance indicates length along the segment with the anal end on the left and oral end on the right. The insert key for each map indicates time in seconds on the vertical axis and width of segment on the horizontal axis in gray scale with white shading indicating narrow width (i.e. contraction) and dark shading indicating broader width (i.e, relaxation/distension). Panels illustrate typical spaciotemporal maps for intraluminal Krebs buffer alone and containing 10, 30 and 100 mM acetate.
Figure 7.
Bar graph of number of contractile responses per 5 min in proximal colon in response to acetate. Panel A: Number of full length propagations per 5 min. Full length propagations were defined as those moving more than one-half of the distance from oral to anal end of a segment. Panel B: Number of short propagations per 5 min. Short propagations were defined as those moving less than one-half of the distance from oral to anal end of a segment. Panel C: Number of total propagations per 5min. Total propagations were defined as the sum of the full and short propagations. Panel D: Number of non-propagations per 5min. Non-propagations were defined as those contractions that failed to move.
Values are means ± SEM of 6 experiments. * indicate p<0.05
Response to SCFAs in distal colon
SCFAs showed similar effects in the distal colon as those observed in the proximal colon. Thirty mM butyrate perfused ahead of the pellet caused a 30% increase of pellet velocity from 1.1 ± 0.1 mm sec−1 to 1.5 ± 0.1 mm sec−1 (p<0.05) (Figure 8A); whereas, 30mM propionate perfused ahead of the pellet caused a 75% decrease of pellet velocity from 1.7 ± 0.2 mm sec−1 to 0.4 ± 0.2 mm sec−1 (p<0.05)(Figure 8B). Similar to the effects of acetate on propagation in the proximal colon, 30mM acetate decreased pellet velocity from 1.6 ± 0.1 mm sec−1 to 1.1 ± 0.3mm sec−1 (Figure 8C).
Figure 8.
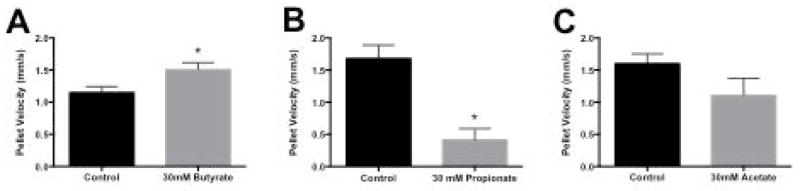
Velocity of pellet propulsion through distal colon. Segments of distal colon were prepared as described in text and illustrated in Figure 1. The lumen was perfused with Krebs buffer alone (control solid bars) or Krebs containing 30 mM of butyrate (panel A), propionate (panel B) or acetate (panel C). Artificial fecal pellets were inserted in the proximal end and allowed to be propelled to the anal end. The velocity of propulsion over a fixed distance of approximately 2 cm was determined.
Values are means ± SEM of 4 experiments for each SCFA. * indicate p<0.05
Discussion
The present study demonstrated that individual SCFAs differ from one another in their effect on colonic motility. Butyrate caused an increase in propagation; propionate and to a lesser extent acetate caused a decrease in propagation. The effects of each SCFA are consistent throughout the entire length of the colon and affect the propulsion of both fluid and solid luminal contents.
Because the SCFAs used were sodium salts, it was necessary to control for the increased sodium present in the lumen, which would be increase by 100 mM at the highest SCFA concentrations. Control solutions with 100 mM additional NaCl showed no difference in the motility from basal supporting the notion that the effects were not the result of osmotic changes. The opposing effects observed with butyrate and propionate, which contain equivalent amounts of sodium in solution, further support this notion. Additionally, since the SCFAs have the potential for altering pH of the Krebs buffer, we measured the pH of the SCFA solutions. Consistent with the use of SCFA in the form of conjugate bases with pKa in the range of 4.8–4.9, there was no effect on the pH of the buffer. This also suggests that the motility changes observed in the present study were due to the effects of the SCFA themselves rather than a secondary effect due to changes in pH.
Using video recording and analyzing spaciotemporal maps in the present study provides determination of SCFA effects on the whole colonic segment and analysis of complex motility patterns. Previous studies have focused on strips of smooth muscle with or without intact mucosa, others have measured pressure changes within the lumen(27), while still others have employed the use of strain gauges to elucidate patterns of motility in isolated whole tissue(28). The use of these different approaches as well as the use of different species has often led to different conclusions as to the effects of the SCFA in colon with both excitatory and inhibitory actions reported. The advent of spatiotemporal mapping of the gut has led to enhanced ability to analyze complex motility patterns (24).
In the present study, the overall effect of butyrate was to increase propulsive motility in the form of an increase in the number of propagations that began at the orad end and progressed throughout the proximal colonic segment to the caudad end. The effect was most notable at lower concentrations (10–30mM). The number of short propagating contraction which did not progress the full length of the colon were decreased, achieving significance at 100 mM. Similarly, the number of non-propagating contractions was increased non-significantly at the lower concentrations but decreased at the highest concentration tested. The net effect of butyrate then appears to suggest a shift from a non-propagating and partially propagating pattern to a more fully propagating type of motility. This notion of increased propagation in proximal colon is consistent with our findings in distal colon where intraluminal butyrate caused an increase in the velocity of propulsion of artificial fecal pellets. The notion that butyrate increases propulsion is consistent with our previous studies in flat-sheet preparations of rat distal colon(13). In these studies, mucosal addition of butyrate caused an increase in the ascending contraction and descending relaxation components of the peristaltic reflex. The net effect of the augmentation of these reflex components would be enhanced propulsion. Furthermore, the effect was attributed to increased release of serotonin (5-HT) and calcitonin gene-related peptide (CGRP) in response to butyrate. Interestingly, brain-derived neurotrophic factor (BDNF), which is released in conjunction with 5-HT in response to mucosal mechanical stimulation, was not released by butyrate in these studies. The stimulatory effect of butyrate has also been noted by Soret et al using muscle strips isolated from circular muscle of rat colon(12). In these studies, butyrate was shown to augment contractions induced by electrical field stimulation but not carbachol. The effect was indirectly mediated by enhancing enteric cholinergic neurons. This study also demonstrated that diets rich in starch resistant to digestion (i.e. which delivers starch to the colon for fermentation) and colonic butyrate in vivo result in increased colonic transit(12). In vivo studies in rat colon by Cherbut et al also confirm this notion. In these studies, butyrate increased the progression of colonic transit markers in spite of producing a decrease in myoelectrical activity(16). Recent studies by Suply et al (29) confirm that in rat distal colon, intraluminal butyrate enhances colonic propulsion and demonstrate that butyrate enemas result in increased numbers of cholinergic and nitrergic neurons which are key excitatory and inhibitory motor neurons mediating the peristaltic reflex and propulsion.
In contrast to butyrate, propionate and to a lesser extent acetate have a somewhat opposite effect. Propionate caused a strong inhibition of full and short propagating contractions whereas non-propagating mixing type contractions were increased with greater concentrations. Comparison of figures 2 and 4 clearly illustrate the opposite effect of butyrate and propionate on all types of contraction at each concentration tested. Acetate is somewhat intermediate in effectiveness but overall favoring a decrease in propagating and non-propagating contractions. A similar trend of increase with butyrate and decrease with propionate and acetate is also evident in distal colon (c.f. figure 8 vs. figures 3,5,7). The decrease in propagating contractions by acetate and propionate in guinea pig colon is different from our previous findings in rat colon where all three SCFA had similar effects on the peristaltic reflex(13). It is not clear what the nature of the difference is between the response to propionate and acetate in rat and guinea pig colon. It may simply be a species difference or it may reflect the fact that in the rat, the static reflex was measured whereas in the guinea pig the propagating contractile wave was measured. The differential nature of the response between species and actions of specific SCFA is well known with increases and decreases in motility and contractility being reported in the literature(11,13,16,28,30–32). The distinction between butyrate and propionate has been noted in other studies which measured actions other than gut motility. In recent studies (33) of intestinal gluconeogenesis, butyrate but not propionate was shown to activate genes (e.g. G6PC and PCK1) associated with gluconeogenesis in Caco-2 cells. This affect was attributed to the ability of butyrate but not propionate to increase cAMP. In contrast, only propionate was able to increase the expression of methylmalonyl-CoA mutase in rats fed a SCFA-rich diet. Two recent studies of Treg generation and differentiation have also identified difference in the effects of different SCFA. In a study by Arpaia et al, propionate and acetate but not butyrate promoted accumulation of Treg-cells in colon whereas butyrate promoted de novo generation of Treg-cells (15). In a similar study by Furusawa et al butyrate was shown in the induce Treg-cell differentiation whereas propionate had only moderate effects and acetate was without effect (14). Similar to the present study, both propionate and acetate were shown to have similar effects in adipocytes where they inhibited lipolysis and reduced plasma free fatty acids(34). These studies suggest that, as we have found in the present study, each SCFA generated in the colon is likely to have its own specific effect and that each SCFA needs to be examined separately for its effect on a given physiological action.
The response to SCFA is likely to be mediated by specific SCFA receptors on components of the gut wall. SCFA interact with two receptors FFAR2 (previously GPR43) and FFAR3 (previously GPR41) (34–36). SCFAs have slightly different orders of potency at these receptors with FFAR2 preferentially binding propionate and acetate and FFAR3 preferentially binds propionate and butyrate with acetate being much less potent. Both FFAR2 and FFAR3 couple to Gαi/o whereas FFAR2 but not FFAR3 also couples to Gαq(37). Recent evidence also suggests that both FFAR2 and FFAR3 are coupled to the taste receptor G protein α subunit α gustducin(38). Whether the similarity and difference of these receptors and their signaling mechanisms is responsible for the differential effect on motility observed in the present study is unknown.
The cellular site of action of SCFA could be on several cells within the gut wall, FFAR2 and/or FFAR3 have been detected on enteroendocrine cells and enteric neurons a well as mast cells and leukocytes(39,40). Interestingly, a recent study by Soret et al suggests that butyrate but not propionate or acetate increased CHAT-positive cholinergic neurons in the enteric nervous system and increased colonic circular muscle contraction(12). These effects are consistent with the increased propulsive motility induced by butyrate in the present study. In contrast, Dass et al found that SCFA were stimulatory in the rodent distal ileum and inhibitory in the colon(41). The inhibitory effect of SCFA, especially propionate, may be attributable to the release of peptide YY (PYY) from enteroendocrine cells. PYY has long been known to inhibit colonic propulsive motility(42–45). PYY and FFAR2 receptors are co-expressed in enteroendocrine cells and cell lines(40,46). Consistent with a role of PYY in mediating the inhibitory effects of propionate, a study in the enteroendocrine STC-1 cell line demonstrated that propionate but not butyrate or valerate caused an increase in PYY expression in enteroendocrine cells(46).
In summary, the present study demonstrates that short chain fatty acids have differential effects on colonic motility and that these effects are similar in proximal and distal colon. Butyrate increased propagating contractions in the proximal colon of the guinea pig but decreased non-propagating contractions. This suggests that the role of butyrate may be to enhance movement of contents through the colon. Propionate produced an opposite effect strongly inhibiting full and partial propagating contractionss while increasing non-propagating contractions. This suggests the role of propionate may be to slow movement of material through the colon and promote absorption. Thus the effect of short chain fatty acids produced by the fermentation of resistant carbohydrates by the microbiota would be the net effect of these opposing influences.
Key Messages.
Short chain fatty acids have differing effects on colonic motility depending on chain length.
The aims of the study were to identify the effects of acetate, propionate, and butyrate on proximal and distal colonic motility of the guinea pig.
Motility of isolated colonic segments was videorecorded during intraluminal installation of Krebs medium containing varying concentrations of acetate, propionate and butyrate, and spaciotemporal maps generated and analyzed.
Butyrate increased and propionate decreased propulsive contractions of the proximal colon and the velocity of pellet propulsion in the distal colon.
Acknowledgments
FUNDING: This study was supported by NIDDKD grant DK34153 (JRG). DMK was supported by the VCU Institutional Research and Academic Career Development Awards Program (IRACDA). KSM was supported by DK15564.
Footnotes
CONFLICT OF INTEREST
The authors have no competing interest.
AUTHOR CONTRIBUTIONS
Experiment concept and design: NH, DMK, KSM, JRG. Experimental procedure and data collection: NH, DMK. Data analysis: NH, DMK, JRG. Manuscript draft and review: NH, DMK, KSM, JRG
References
- 1.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Advances in immunology. 2014 Jan 3;121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9. [DOI] [PubMed] [Google Scholar]
- 2.Binder H. Role of colonic short-chain fatty acid transport in diarrhea. Annual review of physiology. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- 3.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comparative biochemistry and physiology B, Comparative biochemistry. 1987 Jan 4;86(3):439–72. doi: 10.1016/0305-0491(87)90433-0. [DOI] [PubMed] [Google Scholar]
- 4.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki S-I, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2008;59( Suppl 2):251–62. [PubMed] [Google Scholar]
- 5.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. Journal of clinical gastroenterology. 2006 Mar 3;40(3):235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Frontiers in endocrinology. 2012 Jan;3:111. doi: 10.3389/fendo.2012.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomedical Research. 2009;30(3):149156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- 8.Hudson BD, Christiansen E, Murdoch H, Jenkins L, Hojgaard Hansen A, Madsen OB, et al. Complex Pharmacology of Novel Allosteric Free Fatty Acid 3 Receptor Ligands. Molecular pharmacology. 2014 May 3; doi: 10.1124/mol.114.093294. [DOI] [PubMed] [Google Scholar]
- 9.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends in pharmacological sciences. 2013 Apr 1;34(4):226–32. doi: 10.1016/j.tips.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: the pharmacology of the free fatty acid receptors FFA2 and FFA3. British journal of pharmacology. 2009 Sep 2;158(1):146–53. doi: 10.1111/j.1476-5381.2009.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherbut C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. The Proceedings of the Nutrition Society. 2003 Feb 6;62(1):95–9. doi: 10.1079/PNS2002213. [DOI] [PubMed] [Google Scholar]
- 12.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010 May 6;138(5):1772–82. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 13.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. American journal of physiology Gastrointestinal and liver physiology. 2007 Jan 1;292(1):G429–37. doi: 10.1152/ajpgi.00376.2006. [DOI] [PubMed] [Google Scholar]
- 14.Furusawa Y, Obata Y, Fukuda S, Endo T, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 15.Arpaia N, Campbell C, Fan X, Dikiy S, Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherbut C, Ferrier L, Rozé C, Anini Y, Blottière H, Lecannu G, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. The American journal of physiology. 1998 Dec 2;275(6 Pt 1):G1415–22. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 17.Ellis M, Chambers JD, Gwynne RM, Bornstein JC. Serotonin and cholecystokinin mediate nutrient-induced segmentation in guinea pig small intestine. American journal of physiology Gastrointestinal and liver physiology. 2013 Apr 1;304(8):G749–61. doi: 10.1152/ajpgi.00358.2012. [DOI] [PubMed] [Google Scholar]
- 18.Gwynne RM, Bornstein JC. Mechanisms underlying nutrient-induced segmentation in isolated guinea pig small intestine. American journal of physiology Gastrointestinal and liver physiology. 2007 Apr;292(4):G1162–72. doi: 10.1152/ajpgi.00441.2006. [DOI] [PubMed] [Google Scholar]
- 19.Gwynne RM, Thomas EA, Goh SM, Sjövall H, Bornstein JC. Segmentation induced by intraluminal fatty acid in isolated guinea-pig duodenum and jejunum. The Journal of physiology. 2004 Apr 4;556(Pt 2):557–69. doi: 10.1113/jphysiol.2003.057182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman JM, Brooks EM, Mawe GM. Gastrointestinal Motility Monitor (GIMM) Journal of visualized experiments: JoVE. 2010 Jan 5;(46) doi: 10.3791/2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa M, Wiklendt L, Arkwright JW, Spencer NJ, Omari T, Brookes SJ, et al. An experimental method to identify neurogenic and myogenic active mechanical states of intestinal motility. Frontiers in systems neuroscience. 2013 Jan 2;7:7. doi: 10.3389/fnsys.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa M, Dodds KN, Wiklendt L, Spencer NJ, Brookes SJ, Dinning PG. Neurogenic and myogenic motor activity in the colon of the guinea pig, mouse, rabbit, and rat. American journal of physiology Gastrointestinal and liver physiology. 2013 Nov 5;305(10):G749–59. doi: 10.1152/ajpgi.00227.2013. [DOI] [PubMed] [Google Scholar]
- 23.Dinning PG, Costa M, Brookes SJ, Spencer NJ. Neurogenic and myogenic motor patterns of rabbit proximal, mid, and distal colon. American journal of physiology Gastrointestinal and liver physiology. 2012 Jul;303(1):G83–92. doi: 10.1152/ajpgi.00429.2011. [DOI] [PubMed] [Google Scholar]
- 24.Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. The Journal of physiology. 1999 Jun 2;517( Pt 2):575–90. doi: 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foxx-Orenstein AE, Jin JG, Grider JR. 5-HT4 receptor agonists and delta-opioid receptor antagonists act synergistically to stimulate colonic propulsion. The American journal of physiology. 1998 Nov;275(5 Pt 1):G979–83. doi: 10.1152/ajpgi.1998.275.5.G979. [DOI] [PubMed] [Google Scholar]
- 26.Yajima T. Contractile effect of short-chain fatty acids on the isolated colon of the rat. The Journal of physiology. 1985 Nov 5;368:667–78. doi: 10.1113/jphysiol.1985.sp015882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yajima T. Effect of sodium propionate on the contractile response of the rat ileum in situ. Japanese journal of pharmacology. 1984 Jul;35(3):265–71. doi: 10.1254/jjp.35.265. [DOI] [PubMed] [Google Scholar]
- 28.Squires PE, Rumsey RD, Edwards CA, Read NW. Effect of short-chain fatty acids on contractile activity and fluid flow in rat colon in vitro. The American journal of physiology. 1992 May 5;262(5 Pt 1):G813–7. doi: 10.1152/ajpgi.1992.262.5.G813. [DOI] [PubMed] [Google Scholar]
- 29.Suply E, de Vries P, Soret R, Cossais F, Neunlist M. Butyrate enemas enhance both cholinergic and nitrergic phenotype of myenteric neurons and neuromuscular transmission in newborn rat colon. American journal of physiology Gastrointestinal and liver physiology. 2012 Jun 5;302(12):G1373–80. doi: 10.1152/ajpgi.00338.2011. [DOI] [PubMed] [Google Scholar]
- 30.Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Effects of short-chain fatty acids on gastrointestinal motility. Scandinavian journal of gastroenterology Supplement. 1997 Jan 3;222:58–61. doi: 10.1080/00365521.1997.11720720. [DOI] [PubMed] [Google Scholar]
- 31.Kamath PS, Phillips SF. Initiation of motility in canine ileum by short chain fatty acids and inhibition by pharmacological agents. Gut. 1988 Jul 5;29(7):941–8. doi: 10.1136/gut.29.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamath PS, Phillips SF, Zinsmeister AR. Short-chain fatty acids stimulate ileal motility in humans. Gastroenterology. 1988 Dec 4;95(6):1496–502. doi: 10.1016/s0016-5085(88)80068-4. [DOI] [PubMed] [Google Scholar]
- 33.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014 Jan 4;156(1–2):84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Hara T, Ichimura A, Hirasawa A. Therapeutic role and ligands of medium- to long-chain Fatty Acid receptors. Frontiers in endocrinology. 2014 Jan 3;5:83. doi: 10.3389/fendo.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soldavini J, Kaunitz JD. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Digestive diseases and sciences. 2013 Oct 2;58(10):2756–66. doi: 10.1007/s10620-013-2744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL. Short chain fatty acids and their receptors: new metabolic targets. Translational research: the journal of laboratory and clinical medicine. 2013 Mar 5;161(3):131–40. doi: 10.1016/j.trsl.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. The Journal of biological chemistry. 2003 Mar 5;278(13):11312–9. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Kokrashvili Z, Mosinger B, Margolskee RF. Gustducin couples fatty acid receptors to GLP-1 release in colon. American journal of physiology Endocrinology and metabolism. 2013 Mar 5;304(6):E651–60. doi: 10.1152/ajpendo.00471.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013 Oct 2;154(10):3552–64. doi: 10.1210/en.2013-1142. [DOI] [PubMed] [Google Scholar]
- 40.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell and tissue research. 2006 Jun 4;324(3):353–60. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- 41.Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2007 Jan 1;19(1):66–74. doi: 10.1111/j.1365-2982.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Gourcerol G, Yuan P-QQ, Wu SV, Million M, Larauche M, et al. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. American journal of physiology Gastrointestinal and liver physiology. 2010 Jan 5;298(1):G45–56. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993 Sep 3;105(3):733–9. doi: 10.1016/0016-5085(93)90890-o. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JM, Tatemoto K, Terenius L, Hellström PM, Mutt V, Hökfelt T, et al. Localization of peptide YY (PYY) in gastrointestinal endocrine cells and effects on intestinal blood flow and motility. Proceedings of the National Academy of Sciences of the United States of America. 1982 Jul 4;79(14):4471–5. doi: 10.1073/pnas.79.14.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cherbut C, Ferrier L, Rozé C, Anini Y, Blottière H, Lecannu G, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. The American journal of physiology. 1998 Dec 2;275(6 Pt 1):G1415–22. doi: 10.1152/ajpgi.1998.275.6.G1415. [DOI] [PubMed] [Google Scholar]
- 46.Hand KV, Bruen CM, O’Halloran F, Panwar H, Calderwood D, Giblin L, et al. Examining acute and chronic effects of short- and long-chain fatty acids on peptide YY (PYY) gene expression, cellular storage and secretion in STC-1 cells. European journal of nutrition. 2013 Jun 6;52(4):1303–13. doi: 10.1007/s00394-012-0439-9. [DOI] [PubMed] [Google Scholar]