Abstract
Neuronal loss is the most common and critical feature of a spectrum of brain traumas and neurodegenerative disorders such as Alzheimer’s disease (AD). The capacity to generate new neurons in the central nervous system diminishes early during brain development and is restricted mainly to two brain areas in the mature brain: subventricular zone and subgranular zone. Extensive research on the impact of brain injury on endogenous neurogenesis and cognition has been conducted primarily using young animals, when neurogenesis is most active. However, a critical question remains to elucidate the effect of brain injury on endogenous neurogenesis and cognition in older animals, which is far more relevant for age-related neurodegenerative disorders such as AD. Therefore, we examined the impact of neuronal loss on endogenous neurogenesis in aged animals using CaM/Tet-DTA mice, a transgenic model of hippocampal cell loss. Additionally, we investigated whether the upregulation of adult neurogenesis could mitigate cognitive deficits following substantial hippocampal neuronal loss. Our findings demonstrate that aged CaM/Tet-DTA mice that sustain severe neuronal loss exhibit an upregulation of endogenous neurogenesis. However, despite this significant upregulation, neurogenesis alone is not able to mitigate the cognitive deficits observed. Our studies suggest that the aged brain has the capacity to stimulate neurogenesis post-injury; however, multiple therapeutic approaches, including upregulation of endogenous neurogenesis, will be necessary to recover brain function after severe neurodegeneration.
Keywords: neurogenesis, neuronal loss, hippocampus, neurodegenerative disorders
INTRODUCTION
Neuronal loss is a common characteristic of a wide spectrum of brain traumas such as stroke, epilepsy, and traumatic brain injury (TBI), as well as neurodegenerative disorders such as Alzheimer’s, Parkinson’s, and Huntington’s disease (Lunn et al., 2011). The incidence and prevalence of these traumas and brain disorders are rising due to the increasing life expectancy of the human population (Salomon et al., 2012). Additionally, Alzheimer’s disease (AD) prevalence is expected to increase extensively in the USA, rising from 5.4 million to 13.8 million by 2050 (Hebert et al., 2001, 2013; Thies and Bleiler, 2013). Given the overwhelming number of people that will suffer from brain traumas and neurodegenerative disorders, we urgently need a better understanding of the endogenous mechanisms that follow neuronal loss in order to target these mechanisms for therapeutic benefit.
Neurogenesis is a critical mechanism during brain development that may also contribute to spatial and contextual memory formation in the adult brain (van Praag et al., 2002; Deng et al., 2010; Sahay et al., 2011; Pan et al., 2013). Neurogenesis in the mammalian brain is diminished early during brain development with the exception of two neurogenic regions: subventricular zone (SVZ) and subgranular zone (SGZ) (Eriksson et al., 1998; Gage, 2000; Taupin and Gage, 2002). Neurogenesis in the SVZ is generated from neural stem/progenitor cells (NSCs) that differentiate into granule and periglomerular cells (Altman, 1969). Conversely, neurogenesis in the SGZ is generated from NSCs that differentiate into neuronal and glial cells in the granular layer (Altman and Das, 1965; Cameron et al., 1993). Previous studies have shown an imperative role for neurogenesis in the SGZ for spatial and contextual memories, demonstrating an important link between adult neurogenesis and cognition (van Praag et al., 2002; Sahay et al., 2011). In addition, a recent study has shown that various forms of learning promote the survival of newborn neurons in the SGZ. (Curlik et al., 2013). Overall, these studies reveal an important role of neurogenesis in the cognitive processes.
Here we sought to assess changes in neurogenesis specifically following neuronal loss in aged animals, in order to recapitulate the cell loss found in the adult human population with neurodegenerative disorders and brain injuries. We utilized a transgenic mouse model (CaM/Tet-DTA mouse model) that allows for selectively induced neuronal loss without invasive techniques that may damage other brain areas. Thus, this transgenic model provides us with a useful tool to study the role of neurogenesis in the adult brain after selective neuronal loss. The CaM/Tet-DTA mouse model utilizes a Tet-Off inducible transgene system by crossing the TRE-DTA mice with CaMKIIα-tTA mice, producing a consistent and non-invasive lesion in the CA1 upon removal of doxycycline from the diet (Mayford et al., 1996; Lee et al., 1998). Our results indicate that 21 days of Tet-DTA induction in aged CaM/Tet-DTA mice (12 months) causes significant neuronal loss in the hippocampus (including CA1, CA3 and dentate gyrus (DG)). This massive neuronal loss in CaM/Tet-DTA lesioned mice results in substantial cognitive deficits compared to non-lesioned CaM/Tet-DTA mice. Interestingly, CaM/Tet-DTA lesioned mice display a significant upregulation in neurogenesis, both in cell proliferation and cell survival. Overall, these results indicate the aged brain is capable of producing new neurons following severe hippocampal injury; however, an upregulation of neurogenesis alone is insufficient to protect from the cognitive loss associated with severe hippocampal injury.
EXPERIMENTAL PROCEDURES
Transgenic mouse model of neuronal loss
All animal experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California, Irvine. All mice were housed with food and water ad libitum under a 12-h dark/light cycle. All the mice utilized in this study were 12-month-old CaM/Tet-DTA mice.
The CaM-Tet-DTA mouse model, characterized in the lab, utilizes a double transgene system that is capable of inducing cell ablation in the forebrain and specifically in the CA1 region of the hippocampus (Yamasaki et al., 2007). The calcium-calmodulin kinase II alpha (CamKIIα) promoter drives the expression of the first transgene, tetracycline-controlled transcriptional activator (tTA), which binds to the tetracycline-responsive element (TRE) and drives the expression of the second transgene, diphtheria toxin A chain (DTA). This model allows for an inducible Tet-off system controlled by a doxycycline diet. With doxycycline present, tTA is sequestered, thereby suppressing the DTA transgene. Upon removal of doxycycline, tTA is can freely bind to the TRE element to allow DTA expression.
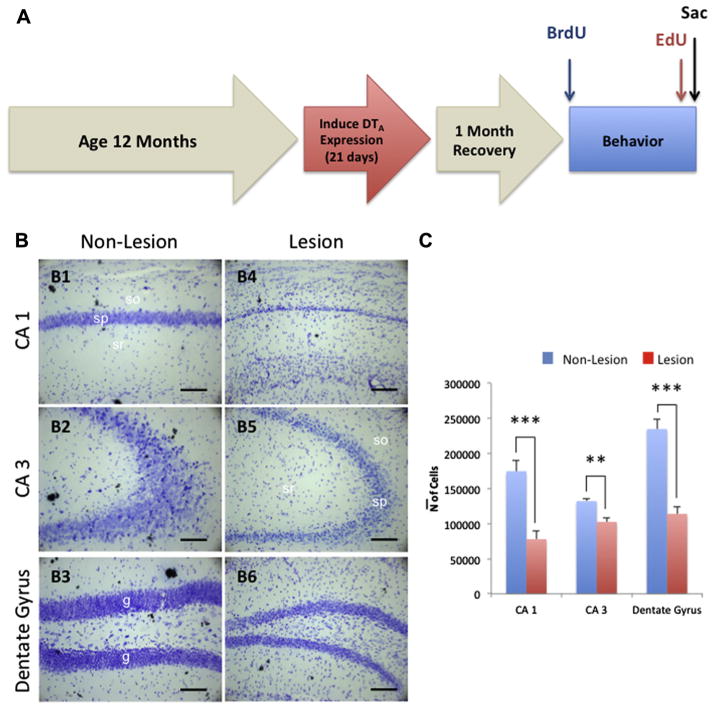
These mice were aged for 12 months from birth, after which doxycycline was removed from their diet for 21 days allowing for a 21-day lesion of the hippocampus and cortex. Post-lesion, mice were given a 1-month period to recover followed by 5 days of 5-bromo-2-deoxyuridine (BrdU) or phosphate-buffered saline (PBS) injections to allow for the visualization of newly developing neurons in the hippocampus. Behavioral assessments were then conducted, followed by 5 days of 5-ethynyl-2-deoxyuridine (EdU) injections, and immediate euthanasia was performed under sodium pentobarbital anesthesia (Fig. 1A).
Fig. 1.
Experimental timeline and hippocampal neuronal loss in aged CaM/Tet-DTA mice. (A) Mice were aged for 12 months to allow for normal development. Doxycycline was removed from the diet to induce a 21-day lesion in the CA1 of the hippocampus. The mice were given 1 month for recovery after traumatic lesion, immediately followed by a 1-week twice-daily pulse of BrdU through IP injection. After injections, mice underwent a spatial memory-dependent behavioral task, Barnes maze, and were given a 1-week pulse of EdU 1 month from BrdU injections and sacrificed immediately after. (B) Light microscopic images of Nissl staining in hippocampal CA1 (B1 and B4), CA3 (B2 and B5), and DG (B3 and B6) subfield in non-lesioned (B1 and B3) and lesioned (B4 and B6) CaM/Tet-DTA mice at 14 months of age. (C) Stereological quantifications in the pyramidal layer of CA1 and CA3 and the granular cell layer of DG hippocampal subfield show a significant decrease in the cell number in lesioned (55.45% ± 2.20%, 22.38% ± 1.15%, and 51.48% ± 1.45%, respectively) compared to non-lesioned CaM/Tet-DTA mice. The values represent the mean ± SEM (n=6). **p<0.01, ***p<0.001. so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum. Scale bars=50 μm.
Bromodeoxyuridine labeling
To label maturing endogenous neuronal stem cells, mice were given a twice-daily intraperitoneal (IP) injection of bromodeoxyuridine at 50 mg/kg (BrdU, Sigma–Aldrich, St. Louis, MO, USA), beginning 1 month post-lesion for 5 consecutive days (Fig. 1A).
Ethynyldeoxyuridine labeling
To label proliferating neuronal cells, the same cohort of mice were given a single daily IP injection of Ethynyldeoxyuridine at 50 mg/kg (EdU, Invitrogen, Grand Island, NY, USA), beginning 5 days prior to euthanasia (Fig. 1A).
Barnes maze
To evaluate spatial learning and memory after inducing neuronal loss, a 5-day Barnes maze protocol was utilized. Briefly, the Barnes maze consists of a 120-cm diameter white disk elevated 120 cm above the floor with 40 holes, 5 cm in diameter, evenly spaced around the parameter. Located beneath one of the holes, serving as the goal box, is a metallic box, 10.5-L × 6.0-W × 6.0-H cm, with torn gauze bedding on the base. The Barnes maze was stationary in a room illuminated by standard ceiling lighting with visual cues along the walls for directionality purposes. A video camera recorder was stationed approximately 30 cm from the apparatus and recorded from an angle to allow for performance monitoring.
The mice were trained for 4 days and underwent a test trial on the 5th day. Prior to the first trial on day 1, mice were placed beneath a box on the center of the apparatus for 15s. After which, the box was removed and the mouse was allowed to explore the maze for a maximum time of 120s. If the mouse found and entered the goal box, they were returned back to the cage; however if the mouse did not find and enter the box within the 120s, they were led to the goal. Mice underwent two trials a day with a 15-min inter-trial interval. After the training phase, a 24-h probe was performed, in which the goal box was removed. Mice were placed in the middle of the maze and a 120-s time limit was allotted for exploring, in which, errors, entries (target head pokes), and latency to find the target were tallied and measured.
Tissue preparation
Mice were deeply anesthetized with sodium pentobarbital and euthanized by perfusion transcardially with 0.1 mol/L PBS, pH 7.4. Half of the brain was fixed for 48 h in 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (pH7.4) and cryoprotected in 30% sucrose for immunohistochemical (IHC) analysis, where the other half of the brain was microdissected and the hippocampus was frozen in dry ice for biochemical analysis. Thick (40 μm) free-floating sections were obtained using a freezing microtome (Leica Microsystems, Bannockburn, IL, USA), and serially collected (each series contained sections that represented 1/6th of the total brain) in cold PBS and 0.02% sodium azide, and stored at 4 °C.
Protein extraction was prepared by homogenizing whole hippocampal hemisphere samples in 150 mg/ml of T-per extraction buffer (Pierce, Rockford, IL, USA) cocktail, complemented with Complete Mini Protease inhibitor Tables (Roche, Indianapolis, IN, USA) and 100 μl of 5 mM phosphatases inhibitors (Sigma–Aldrich, St. Louis, MO, USA), followed by centrifugation at 100,000g for 1 h. Protein concentration in the supernatant was determined using the Bradford Assay.
Immunohistochemistry
Immunofluorescent or light-level immunohistochemistry followed standard protocols (Blurton-Jones and Tuszynski, 2006). For fluorescent labeling, 4% paraformaldehyde fixed 40-μm-thick free-floating sections were rinsed three times in PBS for 5 min and then placed in blocking solution (PBS + 5% normal goat serum + 0.2% Triton X-100 (Tx-100)) for 1-h. Sections were then placed in primary antibodies diluted in blocking solution overnight at 4 °C. Following overnight blocking, sections were rinsed two times in PBS for 5 min, two times in PBS + 0.2% Tx-100 for 15 min, and placed in appropriate secondary antibodies conjugated to Alexa 488, 555, or 635 fluorophores for 1 h (Molecular Probes, Grand Island, NY, USA). After 1-h incubation in the respective secondary antibodies, sections were rinsed three additional times in PBS for 5 min, mounted on glass slides and cover-slipped using Fluoromount-G (Southern Biotech, Birmingham, AL, USA). Primary antibodies used consisted of NeuN (1:1000, EMD Millipore, Billerica, MA, USA), S100B (1:1000, EMD Millipore, Billerica, MA, USA), doublecortin (DCX) (1:1000, Abcam, Cambridge, UK), EdU (1:1000, Invitrogen, Grand Island, NY, USA), and BrdU (1:500, Abcam, Cambridge, UK).
Western blot
Equal amounts of protein (10–20 μg, depending on protein of interest) of hippocampal homogenates were separated on 10% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA), transferred to 0.45 μmol/L polyvinylidene difluoride membranes. Membranes were blocked for 1 h in 5% BSA in 0.2% Tween-20 Tris-buffered saline (TBS-T) (pH7.5). After blocking, the membranes were incubated overnight, at 4 °C, with a primary antibody. The membranes were washed three times in TBS-T for 5 min, incubated at room temperature with the specific secondary antibody at a dilution of 1:10,000 (Pierce Biotechnology, Rockford, IL, USA) for 60 min and rewashed three times in TBS-T for 5 min. The blots were developed using Super Signal (Pierce Biotechnology, Rockford, IL, USA). Film was digitally scanned and analyzed by Image J software (NIH, Bethesda, MD, USA) to measure signal intensity. Average signal intensity for each band was normalized to GAPDH bands, and then normalized to control. Primary antibodies used consisted of anti-Synaptophysin (1:2000, Abcam, Cambridge, UK), anti-PSD95 (1:2000, Abcam, Cambridge, UK), and anti-GAPDH (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cresyl Violet stain
Sections were mounted on slides and air-dried overnight in a dark room. Slides were re-hydrated in de-ionized H20 for 15 min followed by PBS, pH 7.4, for 15 min. Slides were then incubated in 1% Cresyl Violet Acetate solution (Merck, refK28661940, Whitehouse Station, NJ, USA) for 5 min following dehydration in graded ethanol’s (70%, 96% + acetic acid, and 100%) for 5 min, respectively. Finally, slides were placed in xylene for 5 min and cover-slipped using DPX (VWR, Visalia, CA, USA) mounting medium.
Stereological analysis
All unbiased stereological assessments were performed using StereoInvestigator software (MBF Bioscience, Williston, VT, USA) (Baglietto-Vargas et al., 2010). Briefly, an optical fractionator probe was utilized to estimate the total number of cells in the CA1 and CA3 pyramidal cell layer and in the granular cell layer of the DG. The stereological evaluation was performed on every sixth section (40 μm coronal sections) of one brain hemisphere with n=6 animals per group. A counting frame of 40 × 40 μm in a sampling grid of 150 × 150 μm was used for CA1 and a counting frame of 50 × 50 μm in a sampling grid of 250 × 250 μm was performed for the CA3 and DG. Guard zone height for both top and bottom was set at 3 μm with an optical dissector height of 10 μm. Using the 5× objective, the contour of the region of interest of the section was traced and counting of Nissl-stained cells was executed using a 100× oil objective. Quantification was focused to determine the cell loss in the CA1, CA3, and DG region of the hippocampus after 21-day induced lesioned compared to non-lesioned animals. The accuracy of the individual estimations was expressed by the total coefficient of error (CE) calculated using the CEs in each individual animal. The acceptable CE ranged between 0.02 and 0.07.
Confocal microscopy analysis
Fluorescence was detected with a Zeiss LSM 700 confocal microscope (Zeiss, Oberkochen, Germany) equipped with 488, 555, and 635 nm solid-state laser lines. ZEN 2010 (Zeiss) software suite was used for data collection and analysis. Confocal microscopy evaluation was performed on every sixth section (40-μm coronal sections) of one brain hemisphere with n=6 animals per group. When analyzing BrdU-labeled cells, slices were fluorescently stained for BrdU (488 nm), NeuN (555 nm), and S100B (635 nm). When analyzing EdU-labeled cells, slices were fluorescently stained for EdU (488 nm) and DCX (555 nm). Z stacks and X–Y position-series were taken using a confocal microscope at 40× oil magnification. Overlapping Z stacks were taken throughout the DG. BrdU+/NeuN+ cells were considered new mature neurons, BrdU+/S100B+ cells were considered new mature astrocytes, and EdU+/DCX+ cells were considered new immature neurons.
Statistical analysis
All data are expressed as the mean ± SEM. Comparisons between two groups (Lesion and Non-Lesion) were performed by unpaired t-test. The accepted level of significance for the tests was set at 95% confidence. All tests were performed using GraphPad.
RESULTS
Stereological analysis of hippocampal neuronal loss in aged CaM/Tet-DTA mice
Because neuronal loss is a primary feature of many age-related neurodegenerative disorders, we needed to determine whether CaM/Tet-DTA mice could exhibit consistent lesion-induced neuronal loss in aged animals. Previously, our laboratory has demonstrated that CaM/Tet-DTA mice show severe neuronal loss in the CA1 region at younger ages (~3 to 4 months of age) (Yamasaki et al., 2007). To examine the effects of the same lesion in older animals, 12 months CaM/Tet-DTA mice were taken off doxycycline to induce neuronal ablation for 21 days. Our stereological quantification revealed that after 21 days of lesion, the CaM/Tet-DTA mice showed significant decreases in the total number of the pyramidal cells in CA1 (55% ± 2.20%, n=6, t-test, ***p<0.001), CA3 (22.38% ± 1.15%, n=6, t-test, **p<0.01) and DG (51.48% ± 1.45%, n=6, t-test, ***p<0.001) region compared to non-lesioned CaM/Tet-DTA mice (Fig. 1B, C). These data demonstrate that this model is a useful instrument to recapitulate the massive neuronal loss observed in many neurological disorders and brain traumas experienced in the aged population.
Neuronal loss stimulates an upregulation of neurogenesis in the SGZ in lesioned CaM/Tet-DTA mice
The SGZ of the hippocampal DG is one of the two major areas of neuronal proliferation in the adult brain (Taupin and Gage, 2002). Previous work conducted by Gage and colleagues discovered that neurogenesis is decreased throughout development (Kuhn et al., 1996). However, a significant question is whether or not endogenous neurogenesis is able to be upregulated as a physiological response after brain injury or neurodegenerative disease at advanced ages. Thus, CaM/Tet-DTA mice were subjected to 5 days of BrdU pulse injections 1 month after 21 days of dietary lesioning, and were sacrificed 1 month after the last injection of BrdU to measure neuronal survival (Fig. 1A).
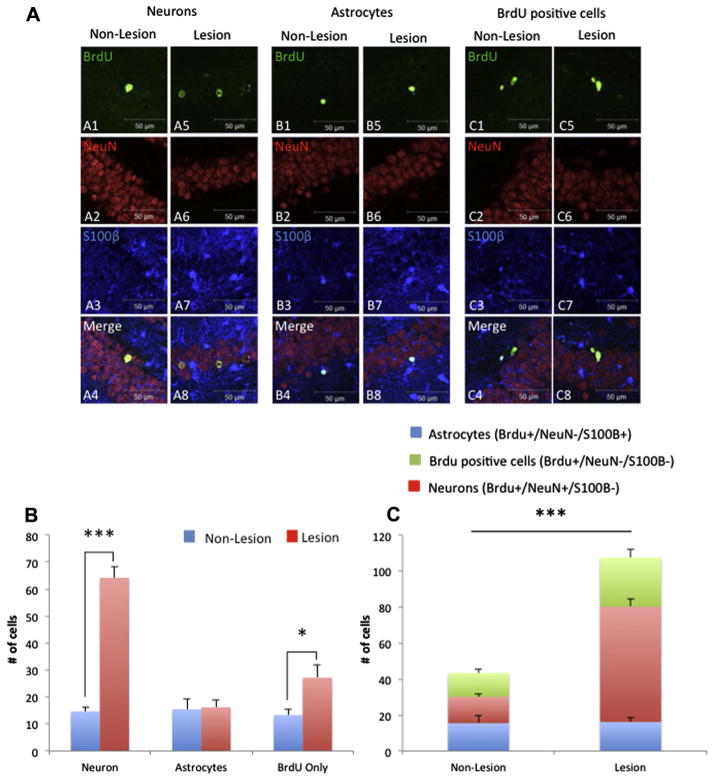
Our results show that neuronal survival, determined by double labeling BrdU+/NeuN+, is increased in CaM/Tet-DTA mice after a significant cell ablation induction compared to control mice (77.14% ± 3.76%; n=6, t-test, ***p<0.001) (Fig. 2A, B). Next, we analyzed if astrocytogenesis is altered after cell ablation. The results indicate that astrocyte cells, determined by double labeling BrdU+/S100B+, are unchanged after an induction of 21 days of cell loss (Fig. 2A, B). Lastly, our results reveal a significant upregulation of cells positive for BrdU+, and negative for S100B and NeuN, might correspond to additional gliogenesis in CaM/Tet-DTA mice after an induction of 21 days of cell ablation (50.92% ± 7.82%; n=6, t-test, *p<0.05) (Fig. 2A, B). Overall, the total number of BrdU+-positive cells is upregulated after 21 days of cell ablation in CaM/Tet-DTA mice compared to control CaM/Tet-DTA mice (59.53% ± 4.15%; n=6, t-test, ***p<0.001) (Fig. 2C). These, results reveal that neurogenesis is upregulated in aged mice as a consequence of hippocampal neuronal loss.
Fig. 2.
Neurogenesis is upregulated following hippocampal neuronal loss in aged CaM/Tet-DTA mice. (A) Double immunofluorescence confocal laser scanning images for BrdU+/NeuN+ (A1–A8), BrdU+/S100β+ (B1–B8) and BrdU+ (C1–C8) cells in non-lesioned (A1–A4, B1–B4 and C1–C4) and lesioned (A5–A8, B5–B8 and C5–C8) CaM/Tet-DTA mice. (D, E) Stereologically-based quantification reveals a significant increase in number of neurons in the lesioned mice compared to non-lesioned (lesion: 64.17 ± 3.98 cells, n=6; non-lesion: 14.67 ± 1.56 cells, n=6 ***p<0.001), no changes in astrocytogenesis between the groups (lesion: 16.17 ± 2.70 cells, n=6; non-lesion: 15.5 ± 3.96 cells, n=6), significant increase in gliogenesis in lesioned versus non-lesioned mice (lesion: 27.17 ± 4.72 cells, n=6; non-lesion: 13.33 ± 1.96 cells, n=6, *p<0.05) and a significant increase in total number of cells in lesioned mice than non-lesioned (lesion: 107.5 ± 8.19 cells, n=6; non-lesion: 43.5 ± 3.73 cells, n=6, ***p<0.001). The values represent the mean ± SEM (n=6). *p<0.05, ***p<0.001. Scale bars=50 μm (A1–C8).
Hippocampal lesion reveals increased proliferation of neurons in the SGZ
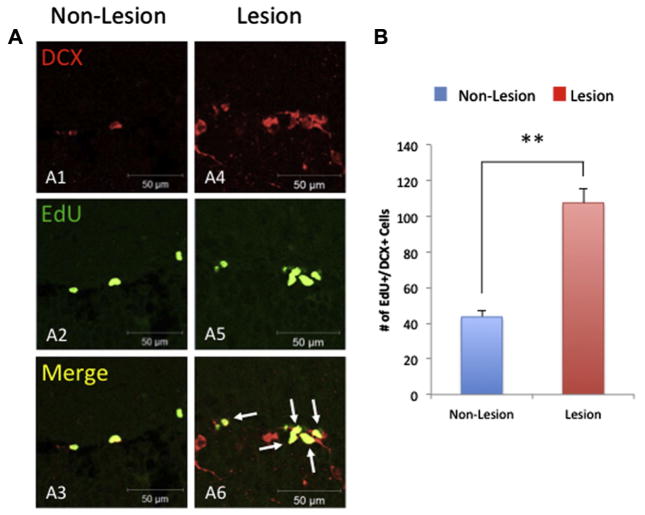
To determine whether this increase in proliferated cells in CaM/Tet-DTA is related with an increase in survival and/or proliferation, we investigated neuronal proliferation in the DG of the same cohort. Thus, CaM/Tet-DTA mice were subjected to an additional 5-day treatment of an alternative thymidine analog, EdU, prior to sacrificing to measure neuronal proliferation (Fig. 1A). Our results reveal an increase in NSC proliferation, determined by co-labeling of EdU+/DCX+, in the CaM/Tet-DTA mice lesioned compared to control mice (66.06% ± 8.78%; n=6, t-test, **p<0.01) (Fig. 3). Overall, our data indicate that cell proliferation is upregulated and contributes to the upregulation of neurogenesis observed in lesioned CaM/Tet-DTA mice.
Fig. 3.
Cell proliferation is upregulated in aged CaM/Tet-DTA mice. Hippocampal lesion and non-lesioned sections were fluorescently labeled with immature neuronal marker doublecortin (DCX) (A1, A4) and with the alternative thymidine analog, EdU, which was administered immediately before sacrifice, labeling newly dividing cells (A2, A5). Cells co-localized with EdU+/DCX+ markers confirm the labeling of immature neurons (A3, A6). (B) Quantification reveals a significant increase in the number of immature neurons in the dentate gyrus of lesioned mice versus non-lesioned (lesion: 85.83 ± 11.5 cells, n=6; non-lesion: 28.67 ± 3.96 cells, n=6, **p<0.01). The values represent the mean ± SEM (n=6). **p<0.01. Scale bars=50 μm (A1–A6).
Lesioned CaM/Tet-DTA mice exhibit cognitive deficits despite upregulation of neurogenesis
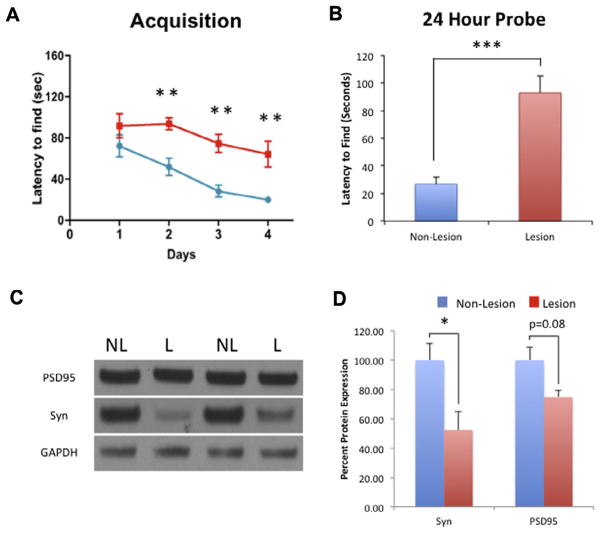
To investigate whether endogenous upregulation of neurogenesis contributes to cognitive recovery following neuronal loss, we analyzed spatial memory using the Barnes maze in CaM/Tet-DTA lesioned versus non-lesioned mice. During the training period, major data points collected were Latency to Find, measured by the time duration for the mouse to initially investigate the target. CaM/Tet-DTA lesioned mice showed significant impairment on training Latency to Find during days 2–4 compared to CaM/Tet-DTA non-lesioned mice (Fig. 4A).
Fig. 4.
Hippocampal spatial deficits in aged CaM/Tet-DTA mice. (A) 14-month-old CaM/Tet-DTA mice, lesioned and non-lesioned (cognitively normal), were trained in the Barnes maze for 4 days. Lesion CaM/Tet-DTA mice latency to find the target zone was significantly worse than non-lesioned CaM/Tet-DTA mice on days 2–4. (B) The 24-h probe trial, lesioned CaM/Tet-DTA mice required significantly more time to arrive at the target zone than the non-lesioned CaM/Tet-DTA mice (lesion: 26.845 ± 12.147, n=10; non-lesion: 92.87 ± 5.27, n=10, ***p<0.001). (C, D) Synaptophysin and PSD95 protein expression, normalized to GAPDH, was decreased in lesioned CaM/Tet-DTA mice compared to non-lesioned CaM/Tet-DTA mice (Syn: 47.48% ± 12.43%, n=6, *p<0.05; PSD 95: 25.19% ± 4.34%, n=6, p=0.08). The values represent the mean ± SEM (n=6). *p<0.05, **p<0.01, ***p<0.001.
To investigate long-term memory for the Barnes maze task, a probe trial was conducted 24-h after the last day of training. During the probe trial, we removed the target box and the mice were allowed to openly explore the maze for 120s. CaM/Tet-DTA lesioned mice exhibited significant impairment in long-term memory performance compared to CaM/Tet-DTA non-lesioned mice (71.09% ± 9.56%; t-test, ***p<0.001) (Fig. 4B).
To account for the deficit in cognition on the Barnes maze task, we analyzed the steady-state levels of pre- (synaptophysin) and post- (PSD95) synaptic protein markers in the hippocampus of non-lesioned and lesioned CaM/Tet-DTA mice. Our results showed that lesioned CaM/Tet-DTA mice exhibited a significant decrease in in synaptophysin, compared to non-lesioned CaM/Tet-DTA mice (47.48% ± 12.43%, t-test, *p<0.05) (Fig. 4C, D). Additionally, we found a decrease in the level of the post-synaptic protein PSD95, in lesioned CaM/Tet-DTA mice compared to non-lesioned CaM/Tet-DTA mice (25.19% ± 4.35%, t-test, p=0.08) (Fig. 4C, D). These data suggest that upregulation of neurogenesis in aged mice does not significantly mitigate cognitive deficits observed in aged lesioned mice nor does it alleviate synaptic loss. However, further studies will be performed using radiated mice to determine the contribution of upregulated neurogenesis to diminish cognitive deficits.
DISCUSSION
In this study, we characterized if endogenous neuronal stem/progenitor cells (NSCs) are upregulated in the SGZ in the aged brain following a significant neuronal cell loss using the innovative CaM/Tet-DTA mouse model. Notably, we showed that CaM/Tet-DTA mouse model induces significant neuronal loss in the granular cell layer of the DG (~51%) and pyramidal cell layer of the CA1 (~55%) and CA3 (~22%) regions in the hippocampus at advanced ages. Interestingly, after this significant neuronal loss, we observed an upregulation of proliferative cells in the SGZ that differentiate primarily into neurons. Additionally, we also show that despite this upregulation of neurogenesis, cognitive deficits are still observed in the CaM/Tet-DTA mouse.
Previous studies in younger animals show that different lesion approaches and/or brain injury models are able to upregulate neuronal progenitors in the DG (Gould and Tanapat, 1997; Dash et al., 2001; Richardson et al., 2007; Kernie and Parent, 2010; Kahle and Bix, 2013; Logan et al., 2013; Perederiy and Westbrook, 2013). These studies and others highlight the potential of this reservoir of precursors to produce neurons and mitigate cognitive decline induced by brain traumas and neurological disorders (Liu et al., 2007; Balu and Lucki, 2009; Christie and Turnley, 2012; Jun et al., 2012; Taylor et al., 2013). However, previous work conducted by Gage and colleagues have identified that the neurogenic proliferation phenomenon is decreased following development (Kuhn et al., 1996), suggesting that this mechanism is halted in advanced ages. Therefore, a critical question remaining in the field is whether neurogenesis might be activated at advanced ages as a mechanism to compensate for the massive neuronal loss induced by brain trauma or neurodegenerative disorders. Here, we utilized the innovative transgenic mouse model, the CaM/Tet-DTA mouse, which allows for selective neuronal loss in the hippocampus without affecting other brain areas (Yamasaki et al., 2007). The CaM/Tet-DTA mouse model enables consistent cell ablation primarily in the hippocampus by removing doxycycline from the diet. In order to model brain injury in the aged mice brain, we induced neuronal cell loss for 21 days after 12 months of normal development. Our data showed that this period of time is enough to induce extensive neuronal loss in the granular cell layer in the DG (~51%) and in the pyramidal cell layer in the CA1 (~55%) and CA3 (~22%) hippocampal region of CaM/Tet-DTA mice compared to non-lesioned mice. This significant neuronal loss observed in the hippocampus in our model is similar to post-mortem AD patients, where a previous study showed a significant loss of 25–70% of neurons (Padurariu et al., 2012). Additionally, our results reveal an upregulation of neurogenesis in the SGZ of the DG after this extensive neuronal cell loss at 14 months of age. The progenitors that are upregulated differentiate primarily to neurons, as the majority of Brdu+-positive cells co-localize with NeuN+ (~60%). Significantly, our study reveals that endogenous NSC are stimulated at advanced ages in CaM/Tet-DTA mice and the majority of the new proliferative cells differentiate to a neuronal phenotype.
As studies in younger animals have shown that upregulation of neurogenesis in the SGZ has a critical role in learning and memory process (van Praag et al., 2002), we investigated whether the upregulation of neurogenesis contributes to cognitive recovery following neuronal loss in CaM/Tet-DTA mice by performing a hippocampus-dependent task, Barnes maze. Our findings show that there was no cognitive recovery in lesioned mice, as they show a higher Latency to Find and enter the target than non-lesioned mice. Additionally, 24-h probe findings reveal significantly more entry errors prior to the correct entry in lesion CaM/Tet-DTA mice than non-lesioned CaM/Tet-DTA mice, as well as significantly less entries into the target hole and greater latency to find the target hole. Overall, our data indicate that despite seeing an upregulation in neurogenesis, cognitive deficits are not mitigated during training or probe test. Additionally, decreases in the steady-state level of pre- (synaptophysin, ~47%) and post-synaptic protein (PSD95, ~25%), is related with the cognitive deficits observed in lesioned CaM/Tet-DTA mice. The downregulation in synaptic markers indicates that neurogenic upregulation does not mitigate synaptic loss following hippocampal lesioning. A lack of cognitive recovery, despite the upregulation of endogenous neurogenesis, suggests that concomitant therapies, such as anti-depressants, anti-inflammatory and neurotrophic compounds, which can stimulate; (1) the production and signaling of plasticity-related proteins (such as neurotrophins), (2) synaptogenesis and (3) neurogenesis to facilitate functional synaptic connection and improve information processing within neuronal network to provide a more efficient functional recovery following neuronal cell loss experienced in neurodegenerative disorders and/or traumas (Castren, 2004; Bruel-Jungerman et al., 2007; Li et al., 2009; Biscaro et al., 2012; Srivastava et al., 2013). In addition, further studies blocking neurogenesis, for example by irradiation, will be necessary to determine the contribution of this upregulation of neurogenesis on cognition in aged lesioned CaM/Tet-DTA mice.
CONCLUSION
Our study demonstrates that endogenous NSCs are stimulated to proliferate, and neurogenesis is upregulated in aged CaM/Tet-DTA mice as a consequence of extensive neuronal loss. Our data also suggest that endogenous neurogenic upregulation alone is not sufficient to mitigate cognitive deficits in these aged CaM/Tet-DTA mice. Therefore, we conclude that multiple therapeutic approaches including stimulation of neurogenesis will be a better approach to recover brain function in the aged population that suffer brain traumas or neurological disorders such as AD.
Acknowledgments
We would like to thank Dr. Rahasson Ager for comments on a previous version of the manuscript.
Abbreviations
- AD
Alzheimer’s disease
- BrdU
5-bromo-2-deoxyuridine
- CamKIIα
calcium-calmodulin kinase II alpha
- CE
coefficient of error
- DCX
doublecortin
- DG
dentate gyrus
- DTA
diphtheria toxin A chain
- EdU
5-ethynyl-2-deoxyuridine
- PBS
phosphate-buffered saline
- SGZ
subgranular zone
- SVZ
subventricular zone
- TBS-T
Tween-20–Tris-buffered saline
- TRE
tetracycline-responsive element
- tTA
tetracycline-controlled transcriptional activator
- Tx-100
Triton X-100
- TBI
traumatic brain injury
- NSCs
neural stem/progenitor cells
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Baglietto-Vargas D, Moreno-Gonzalez I, Sanchez-Varo R, Jimenez S, Trujillo-Estrada L, Sanchez-Mejias E, Torres M, Romero-Acebal M, Ruano D, Vizuete M, Vitorica J, Gutierrez A. Calretinin interneurons are early targets of extracellular amyloid-beta pathology in PS1/AbetaPP Alzheimer mice hippocampus. J Alzheimers Dis. 2010;21:119–132. doi: 10.3233/JAD-2010-100066. [DOI] [PubMed] [Google Scholar]
- Balu DT, Lucki I. Adult hippocampal neurogenesis: regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener Dis. 2012;9:187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–612. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Castren E. Neurotrophic effects of antidepressant drugs. Curr Opin Pharmacol. 2004;4:58–64. doi: 10.1016/j.coph.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front Cell Neurosci. 2012;6:70. doi: 10.3389/fncel.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik DM, 2nd, Maeng LY, Agarwal PR, Shors TJ. Physical skill training increases the number of surviving new cells in the adult hippocampus. PLoS One. 2013;8:e55850. doi: 10.1371/journal.pone.0055850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15:169–173. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H, Mohammed Qasim Hussaini S, Rigby MJ, Jang MH. Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012;2012:854285. doi: 10.1155/2012/854285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle MP, Bix GJ. Neuronal restoration following ischemic stroke: influences, barriers, and therapeutic potential. Neurorehabil Neural Repair. 2013;27:469–478. doi: 10.1177/1545968312474119. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Morley G, Huang Q, Fischer A, Seiler S, Horner JW, Factor S, Vaidya D, Jalife J, Fishman GI. Conditional lineage ablation to model human diseases. Proc Natl Acad Sci U S A. 1998;95:11371–11376. doi: 10.1073/pnas.95.19.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Cai HH, Wang B, Chen L, Zhou QG, Luo CX, Liu N, Ding XS, Zhu DY. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J Neurosci Res. 2009;87:112–122. doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J. Chronic treatment with minocycline preserves adult new neurons and reduces functional impairment after focal cerebral ischemia. Stroke. 2007;38:146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- Logan TT, Villapol S, Symes AJ. TGF-beta superfamily gene expression and induction of the runx1 transcription factor in adult neurogenic regions after brain injury. PLoS One. 2013;8:e59250. doi: 10.1371/journal.pone.0059250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Baranes D, Podsypanina K, Kandel ER. The 3′-untranslated region of CaMKII alpha is a cis-acting signal for the localization and translation of mRNA in dendrites. Proc Natl Acad Sci U S A. 1996;93:13250–13255. doi: 10.1073/pnas.93.23.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Mavroudis I, Fotiou D, Baloyannis S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr Danub. 2012;24:152–158. [PubMed] [Google Scholar]
- Pan YW, Storm DR, Xia Z. Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase. Neurobiol Learn Mem. 2013;105:81–92. doi: 10.1016/j.nlm.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederiy JV, Westbrook GL. Structural plasticity in the dentate gyrus- revisiting a classic injury model. Front Neural Circuits. 2013;7:17. doi: 10.3389/fncir.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169–181. xi. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon JA, Wang H, Freeman MK, Vos T, Flaxman AD, Lopez AD, Murray CJ. Healthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010. Lancet. 2012;380:2144–2162. doi: 10.1016/S0140-6736(12)61690-0. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Evans PD. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience. 2013;239:17–33. doi: 10.1016/j.neuroscience.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Jhaveri DJ, Bartlett PF. The therapeutic potential of endogenous hippocampal stem cells for the treatment of neurological disorders. Front Cell Neurosci. 2013;7:5. doi: 10.3389/fncel.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Demen. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki TR, Blurton-Jones M, Morrissette DA, Kitazawa M, Oddo S, LaFerla FM. Neural stemcells improvememory in an inducible mouse model of neuronal loss. J Neurosci. 2007;27:11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]