Abstract
Background
Alopecia in captive primates continues to receive attention from animal care personnel and regulatory agencies. However, a method that enables personnel to reliably score alopecia over time and under various conditions has proven difficult to achieve.
Methods
The scoring system developed by the behavioral and veterinary staffs at the Washington National Primate Research Center (WaNPRC) uses the rule of 9s to estimate the percentage of the body affected with alopecia (severity) and how the alopecia presents itself (pattern). Training and scoring can conveniently be managed using photographic images, cage-side observations, and/or physical examinations.
Results
Personnel with varying degrees of experience were quickly trained with reliability scores ranging from 0.82 to 0.96 for severity and 0.82 to 0.89 for pattern using Cohen’s κ.
Conclusions
This system allows for reliable and consistent scoring across species, sex, age, housing condition, seasons, clinical or behavioral treatments, and level of personnel experience.
Keywords: alopecia, alopecia scoring, colony management, hair loss, hair pulling, macaque, molt, rule of 9s, self-injurious behavior, trichotillomania
Introduction
Historically, there has been an assumption that alopecia in captive non-human primates (NHP) is indicative of poor health and/or poor psychological well-being and is due to physical or psychological stressors. However, alopecia may be a result of a variety of physiological or psychological processes, stress being only one of them [20], and it is a misconception to assume that all alopecia is abnormal or pathological in origin.
Natural biological processes such as aging [20], sex [14], seasonality [2, 24, 26], and pregnancy or hormonal changes [2, 20, 26] have all been shown to affect the coat condition of rhesus macaques (Macaca mulatta). In fact, alopecia was described as a normal seasonal phenomena (‘molt’) related to reproductive hormone variations in free-ranging rhesus on Cayo Santiago and at the La Parguera Primate Facility in Puerto Rico almost 45 years ago [26]. In 2009, Novak [20] published a series of photographs of a pregnant rhesus, illustrating the remarkable amount of hair loss present during the month parturition occurred. Genetics may also play a role. Male stump-tailed macaques have a common pattern baldness that is genetically inherited [4]. Additionally, various physiological and biological dysfunctions such as nutritional imbalance [25], endocrine disorders, immunologic disease, bacterial or parasitic infections, and atopic dermatitis can also cause poor coat condition and alopecia [20].
Other factors that can affect hair coat and alopecia include housing conditions [1, 2] and social stress [24]. Something as simple as the amount of time a body part is in physical contact with a cage may be correlated with alopecic severity [27]. Hair pulling and over-grooming by social partners [20], as well as self-directed hair pulling [7, 18], have also been related to alopecia, although the causal mechanisms for such behaviors are still not well understood in non-human primates. Self-directed hair pulling is an atypical/undesirable behavior that has been commonly suspected of causing alopecia and has been proposed as an NHP model of human trichotillomania [21]. However, it should be noted that the mere presence of alopecia does not imply that animals are hair pullers. In a recent survey of four National Primate Research Centers, approximately 50% of rhesus macaques were found to have alopecia, but only 8% were determined to be hair pullers [18]. Research within the Washington National Primate Research Center (WaNPRC) corroborated this disparity. Hair pullers comprised 19% of our sample, but 57–69% of animals with alopecia were not identified as hair pullers [16]. In addition, Kramer [15] obtained skin biopsies from 17 alopecic rhesus macaques but found that they rarely demonstrated trichomalacia or intrafollicular hemorrhage consistent with trichotillomania, suggesting an etiology other than hair pulling.
Because coat condition can imply such a wide range of physical and psychological conditions, the implementation of a quantitative and consistent alopecia scoring system would be an effective method for health and welfare management. A scoring system should be nonintrusive, easy to train and use, and allow for high inter-and intra-rater reliability between both experienced and inexperienced personnel. It should also take a minimum amount of time to score, therefore making it appropriate for scoring large numbers of animals. Such a scoring system should ideally allow for the identification of animals in need of further diagnosis to ascertain the underlying etiology of their alopecia – whether it is one of the many physiological conditions known to cause alopecia or whether it is self-induced due to the abnormal behavior of hair pulling. This same scoring system should then allow for a continuous assessment of alopecia over a range of species, sexes, ages, seasons, and housing conditions and could be used as a tool for measuring the efficacy of long-term clinical or behavioral therapies used to treat such alopecia.
Although a number of alopecia scoring systems have been developed, few are geared toward practical implementation in a colony management setting. Only a small number of scoring systems have assessed inter-rater or intra-rater reliability, most often within a small number of raters [2, 10]. Other studies have used a single rater to avoid the complications involved in training and attaining reliability with multiple personnel [13, 22, 28]. If a system is to be used for colony management purposes, it should be reliable across (and within) multiple raters due to the sheer numbers of animals requiring assessment. The need to cross-train personnel from different departments or facilities who may have different levels of access to, or familiarity with, primates and the need to maintain a core group of trained personnel in an environment where staff may change frequently are also significant considerations.
Another limitation of many scoring systems is that they only rate a portion of the body or score alopecia on an ordinal scale [2, 3, 10]. Some authors have recently noted that there may be species differences in how alopecia presents itself [16] and that hair loss in different parts of the body may be indicative of different etiologies [15]. If coat condition is rated only in selected body areas, the score could potentially be unrepresentative of the animal’s true condition. Animals can also exhibit a wide range of variation in coat condition from one part of the body to another, making a gestalt rating of the coat condition potentially unwieldy, especially when large numbers of animals must be assessed. Thus, an ideal scoring system for colony management would include an assessment of the entire body as well as retain the ability to examine the involvement of specific body parts when a pattern of hair loss is of clinical or scientific interest.
With these issues in mind, members of the veterinary and behavioral staffs at the Washington National Primate Research Center (WaNPRC) developed and implemented an alopecia scoring system in 2008. This scoring system quantifies the extent of alopecia over the entire body using the rule of 9s, which is commonly used in medical practice to assess the extent of burn injuries in human patients [12]. Our goals were to (i) easily train multiple raters including both experienced and inexperienced personnel; (ii) attain high inter- and intra-rater reliability scores; (iii) provide maximum flexibility using several different methods to train and score animals, including non-invasive methods (scoring awake animals or photographs), as well as scoring sedated animals during physical examinations; (iv) allow for pattern and severity to be scored separately; (v) assess a large number of animals (regardless of species, sex, or age) in an efficient amount of time; (vi) rate the entire body while at the same time providing the option to rate individual body parts separately if necessary; (vii) identify animals with moderate to severe alopecia for further diagnosis and treatment; (viii) provide quantitative measures to guide clinical decisions regarding treatment of the more serious cases; and (ix) allow for accurate assessments of the long-term effects of ameliorative therapies.
The ability to use several methods of training adds flexibility to the system, so that it can be tailored to the needs of specific personnel. For example, it may be more convenient for clinical staff that routinely sees sedated animals during physical examinations, to be initially trained using photographs with reliability later assessed on sedated animals. Personnel normally scoring awake animals can also be trained using photographs, but reliability can then be assessed after further cage-side training. Scoring pattern and severity separately allow this system to be tailored to various clinical or research needs. For some needs, such as tracking the demographics of alopecia at our center, the overall severity score may suffice. In other circumstances, such as assessing a particular type of ameliorative therapy, the pattern score may also need to be utilized.
Materials and methods
Humane care guidelines
The WaNPRC is accredited by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International, and all research was conducted under protocols approved by the University of Washington’s Institutional Animal Care and Use Committee (IACUC). The research adhered to the American Society of Primatologists’ Principles for the Ethical Treatment of Nonhuman Primates and complied with national standards including the United States Public Health Service Policy on the Humane Care and Use of Laboratory Animals as well as the Animal Welfare Act. Animals were maintained in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals [19]. All animals participated in the WaNPRC Environmental Enhancement plan and were fed a nutritionally balanced diet, supplemented at least three times per week with additional fruit and vegetables.
Scoring system
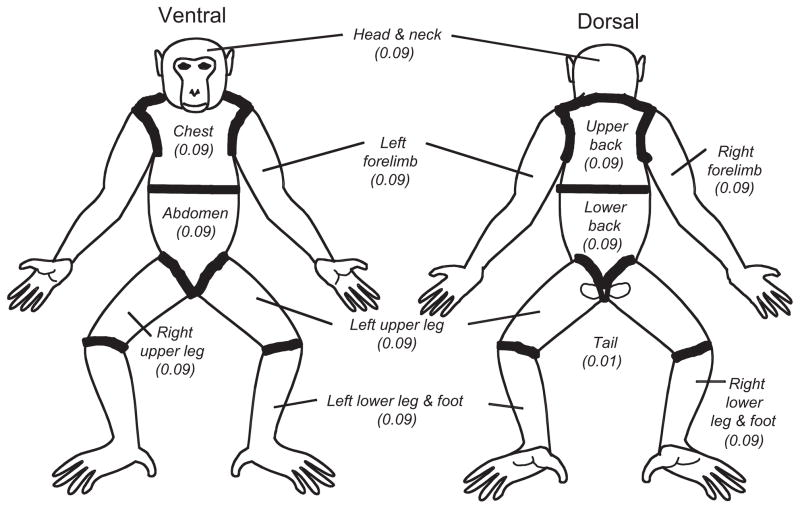
With a diagram as reference (Fig. 1), a generic primate body is visually separated into 12 body parts or sections: head and neck, left arm, right arm, chest, abdomen, upper back, lower back, left upper leg, left lower leg, right upper leg, right lower leg, and tail. Each body part comprises 9% of the body surface except for the tail which makes up the final 1%. If alopecia larger than half an inch is noted in one of these sections, that body part is considered ‘affected’. Areas that have been shaved by research or veterinary personnel or areas that appear thin due to infancy, scars, calluses, cowlicks, or sexual swelling are not defined as alopecia. The total number of ‘affected’ body parts is then added to determine the overall alopecia severity score (Table 1, Figs 2 and 3). This allows the recording and monitoring of alopecia over the entire body surface of the animal.
Fig. 1.
Generic primate body illustrating the ‘rule of 9s’. Note that the tail is not shown but counts for 1% of the total score.
Table 1.
Using the rule of 9s to determine the alopecia severity score
| Number of body parts (bp) affected | Percentage of body affected, % | Alopecia severity score |
|---|---|---|
| 0 bp | 0 | 0 |
| Tail | 1 | 1 |
| 1 bp | 9 | 1 |
| 1 bp + tail | 10 | 1 |
| 2 bp | 18 | 1 |
| 2 bp + tail | 19 | 1 |
| 3 bp | 27 | 2 |
| 3 bp + tail | 28 | 2 |
| 4 bp | 36 | 2 |
| 4 bp + tail | 37 | 2 |
| 5 bp | 45 | 2 |
| 5 bp + tail | 46 | 2 |
| 6 bp | 54 | 3 |
| 6 bp + tail | 55 | 3 |
| 7 bp | 63 | 3 |
| 7 bp + tail | 64 | 3 |
| 8 bp | 72 | 3 |
| 8 bp + tail | 73 | 3 |
| 9 bp | 81 | 4 |
| 9 bp + tail | 82 | 4 |
| 10 bp | 90 | 4 |
| 10 bp + tail | 91 | 4 |
| 11 bp | 99 | 4 |
| 11 bp + tail (aka 12 bp) | 100 | 4 |
Fig. 2.
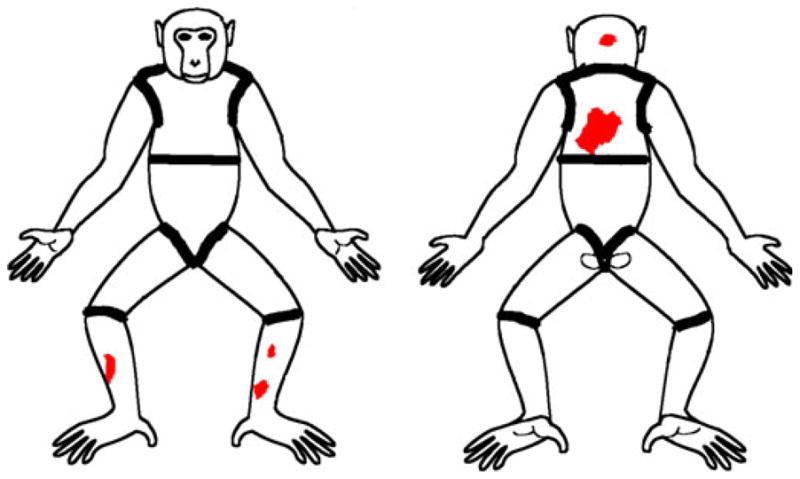
Alopecia severity score 2 (four body parts affected – head, upper back, L lower leg, R lower leg).
Fig. 3.
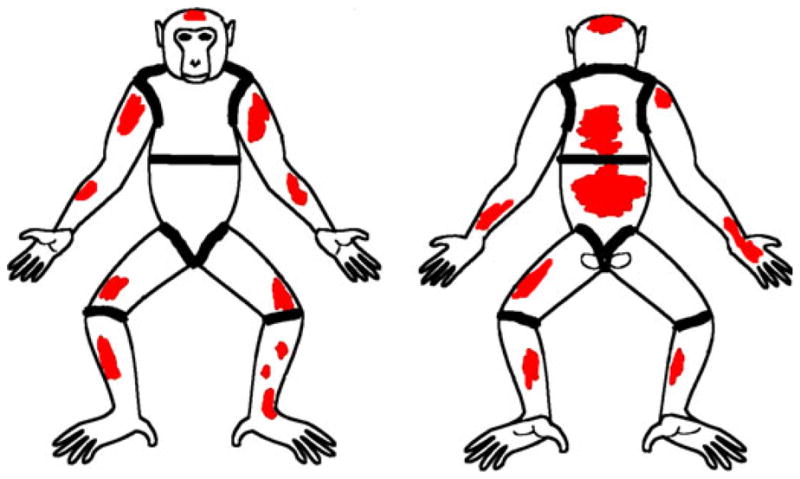
Alopecia severity score 4 (nine body parts affected – head, L arm, R arm, L upper leg, R upper leg, L lower leg, R lower leg, upper back, lower back).
The alopecia pattern score is descriptive in nature. It applies to the most prevalent pattern in any alopecic areas of a particular animal (Table 2). An animal with mostly thinning hair receives a pattern score of 1 ‘Diffuse Thinning’ (Fig. 4); an animal with patches of intact hair mixed with patches of alopecia is scored 2 ‘Patchy’ (Fig. 5); and an animal with mostly bare areas is scored 3 ‘Bare’ (Fig. 6). If two alopecia patterns are present in equal amounts on the body (for example, one bare limb and one patchy limb), the pattern with the higher numerical score is recorded (in this case bare). Because alopecia severity and alopecia pattern scores are separated in this scoring system, either one or both can be used to score an individual animal based upon colony management or research needs. Reliability for alopecia severity and alopecia pattern can also be analyzed separately, providing additional flexibility in training and implementation of the scoring system.
Table 2.
Alopecia pattern scores
| Numerical score | Pattern description | Pattern definition |
|---|---|---|
| 1 | Diffuse thinning | Thinning hair; dispersed; fewer hairs per given surface area |
| 2 | Patchy | Patches of bare skin surrounded or infiltrated by intact hair; localized |
| 3 | Bare | Bald, no hair; more skin visible than hair in affected areas; identifiable discrete borders of hair vs. skin |
Fig. 4.
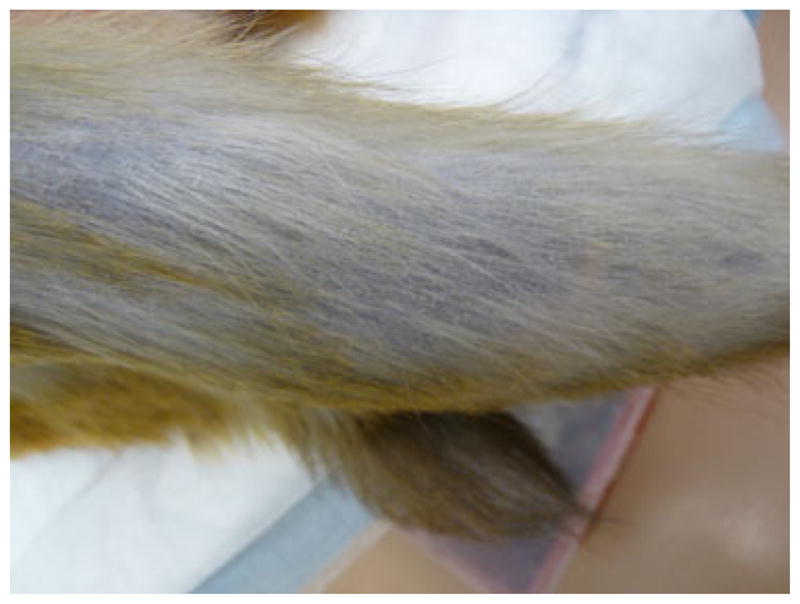
Example: Diffuse alopecia pattern.
Fig. 5.
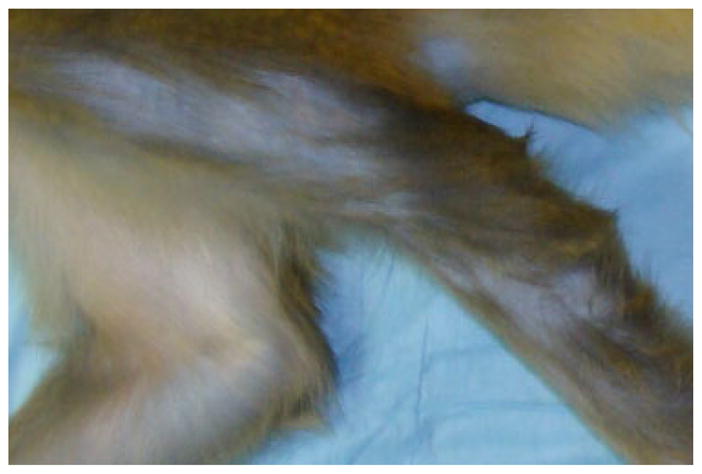
Example: Patchy alopecia pattern.
Fig. 6.
Example: Bare alopecia pattern.
Training
Personnel are trained to recognize varying degrees of alopecia using photographs of monkeys with and without alopecia, live observations of monkeys in their home cages, and/or physical examinations of monkeys that are sedated by veterinary or research support staff for routine physical examinations or procedures. After being trained, personnel then score an additional set of photographs and Cohen’s κ [5] is used to determine reliability. After reliability is reached using photographs, additional scoring can then be conducted cage side, and Cohen’s κ can again be used to determine reliability.
Results
Reliability for alopecia severity score
All personnel had at least some familiarity (~1 month or more) with our WaNPRC primate population (which currently includes Macaca nemestrina, Macaca fascicularis, M. mulatta, Papio c. anubis, and Saimiri sciureus) prior to training on the alopecia scoring system. Three individuals with previous alopecia scoring experience (‘expert’) and three individuals with no alopecia scoring experience (‘novice’) were chosen as raters. One 30-minute photographic training session was provided to each rater by the first author (RB), during which 24 photographs and the applicable score for each photograph were discussed in detail. After this initial training session, raters independently scored an additional set of 24 photographs using the alopecia severity score only. All six raters were reliable with the first author as determined by Cohen’s κ scores ranging from 0.83 to 0.96. A subsequent round of cage-side scoring between the first author and one of the other expert raters resulted in a Cohen’s κ reliability score of 0.95. An experienced rater could score 24 photographs for alopecia severity in 5 minutes or less or one individual animal cage side in 1 minute or less.
To check intra-rater reliability, the first author and another expert rater scored the same 24 photographs nearly twelve months after their first scoring round. Cohen’s κ scores were 0.91 for rater one and 0.87 for rater two. Inter-rater reliability between these two raters a year after their initial scoring round was 0.82. Thus, inter- and intra-reliability on the alopecia severity score are both relatively fast and easy to achieve and maintain using this system.
Reliability for alopecia pattern score
Two expert raters conducted cage-side scoring to assess reliability for alopecia patterns. After an initial trial run, it became apparent that additional guidelines were needed to produce accurate scores when more than one pattern was present. This resulted in the scoring rule cited above (i.e., if two patterns are present in equal amounts, the one with the higher numerical score is recorded). A second round of cage-side scoring was conducted on nineteen animals within 24 hours of the first trial run. The resulting Cohen’s κ was 0.89. Scoring alopecia pattern from photographs resulted initially in a κ score of 0.72. Both raters reviewed the photographs and determined that some additional descriptive terms (1 = Diffuse Thinning – dispersed; fewer hairs per given surface area; 2 = Patchy – localized; small areas of loss surrounded or infiltrated by areas of hair; 3 = Bare –bald; more skin visible than hair in affected areas; identifiable discrete borders of hair vs. skin) should be added to the pattern definitions to account for the difficulty in assessing a 3-dimensional pattern using a two-dimensional photograph. A subsequent reliability test on 24 additional photographs resulted in a κ score of 0.82. An experienced rater could score 24 photographs for alopecia pattern in 5 minutes or less or one individual animal cage side in 1 minute or less.
Reliability was not conducted on sedated animals because it occurred infrequently at the time of this analysis.
Discussion
A number of detailed alopecia scoring systems have been published. Runeson et al. [22] developed a scoring system that rated the entire body via individual body section scores. The body was divided into 12 sections (head, shoulders, dorsal torso, ventral torso, ventral and dorsal left and right forelimbs, and ventral and dorsal left, and right hind limbs), and body sections were assigned weights roughly corresponding to their relative surface areas. The section scores were then multiplied by their corresponding weights and summed to produce a total body alopecia rating ranging from 0 to 1.0. Garner et al. [9] used a generic mouse body map upon which alopecic areas were hand-drawn. This information was then imported into custom-made software, which calculated the overall proportion of skin denuded for the entire body as well as for separate body areas. Ryan et al. [23] developed a similar system of mapping alopecia for non-human primates. This system involves circumscribing alopecic areas on digital photographs and using ‘Image J’ software (National Institutes of Health, Bethesda, MD, USA) to calculate total proportion of the body that is affected by alopecia. Horenstein et al. [11] used a method that involved actually counting the number of hairs in specific body areas of squirrel monkeys. Detailed systems such as these are useful in assessing response to ameliorative therapies or for conducting research on a small number of animals. However, because these particular systems are relatively complex, the amount of time necessary to train raters and to produce accurate and reliable alopecia scores would likely preclude their use for large numbers of animals. In addition, some of these systems would likely necessitate sedation to properly score the animals, something that is potentially invasive as well as time- and resource-consuming.
There are other systems that would be more practical for large numbers of animals. Most of these score alopecia on an ordinal scale. Honess et al. [10] developed a scale with ratings from 1 (very good coat condition; complete back cover) to 5 (back completely bald, i.e., more skin visible than hair). Beisner and Isbell [1, 2] adapted this system to include nine score levels with whole and half integers that ranged from 1 (perfect coat); 1.5 (one to two small patches of fur missing, etc.,) to 5 (bald or nearly bald). Berg et al. [3] developed a similar six-point scale for scoring alopecia on the body and tail of ring-tailed lemurs (Lemur catta). These ordinal scales potentially lose specificity because they incorporate aspects of both alopecia severity and alopecia pattern into one total score. Ellison et al. [7] indicated that different alopecia patterns (patchy, bald, etc.) may derive from different etiologies. Therefore, separating pattern information from overall severity information may be beneficial when trying to assess both etiology and the effects of therapeutic interventions.
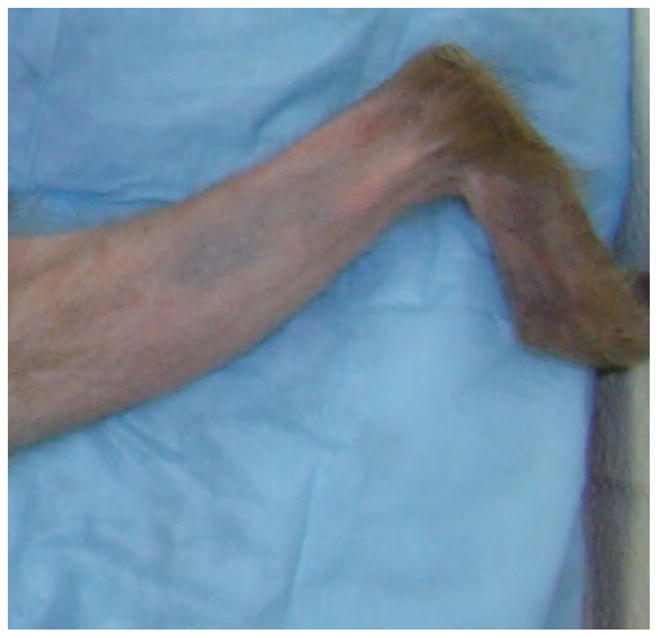
Some scoring systems propose to score alopecia on selected body parts instead of the whole animal. Honess [10] rates only the backs of rhesus macaques, and Zhang [28] scores heads and backs of Japanese macaques (Macaca fuscata). While this strategy may greatly simplify scoring and is appropriate to address some specific research questions, it may also present a drawback because they do not rate the animal in its entirety. Presentation of alopecia on certain body parts may be significant and important. For example, Kramer et al. [15] suggested that alopecia affecting the distal limbs is psychogenic in origin. Although we do not routinely track which specific body parts are affected in an individual animal, our scoring system does retain that ability. Each body part could be scored individually as to the presence or the absence of alopecia severity and pattern.
Of the previously published systems for scoring alopecia in primates, very few sufficiently address inter-rater and intra-rater reliability. Some research studies have restricted observations to one individual [13, 22, 28], thus circumventing the inter-rater reliability issue. Other studies have assessed and reported inter-rater reliability with varying degrees of success. Beisner and Isbell [2] provide reliability information for two observers on a subsample of animals. Honess [10] reports a correlation of 0.92 between the scores of two observers under laboratory conditions. Berg’s system [3] was developed in a field research setting with ring-tailed lemurs (Lemurcatta). Inter-rater reliability was assessed only as differences between raters in group mean scores. As evidenced by both Berg [3] and Zhang [28], there are additional complexities when trying to score alopecia in a field research environment. As such, it is difficult to determine whether these particular scoring systems allow sufficient reliability to be of use in a colony management setting.
WaNPRC is not the only facility to implement an alopecia scoring system based upon the rule of 9s. Staff at the Oregon National Primate Research Center (ONPRC) implemented a rule of 9s scoring system in 2006 [8] that includes a three-point scale for quantifying alopecia and a five-point scale for distinguishing patterns. Specific reliability scores were not published, but the time it takes to score an animal’s alopecic condition was estimated to be 5 minutes. The Behavioral Management Consortium of the National Primate Research Centers is currently in the process of testing a more complex scoring system that is also based upon the rule of 9s [6, 17]. Their system uses a six-point ordinal scale for estimating the percentage of body surface affected and a six-point pattern scale for estimating how the alopecia presents itself in a particular animal. Initial reliability scores appear promising for raters scoring a series of photographs, but information on the amount of time it takes to train personnel or whether raters can reach reliability while scoring animals cage side has not yet been published.
As previously described, our system incorporates enough simplicity and flexibility to foster high inter-and intra-reliability scores for personnel of varying experience. The ability to score animals under a variety of conditions (cage side, via photographs, or while sedated) as well as the ability to score the severity and pattern separately reduces the amount of time it might take to train such personnel. One 30-minute training session was enough to attain reliability for six raters while scoring photographs, which is identical to the training time reported by Honess [10], wherein only the back was scored. Using our system, scoring an awake animal cage side usually requires <1 minute, which is an improvement over that reported by Ellison [8]. Because it is such a streamlined system, we are able to use it to score all primates housed at the WaNPRC (n = ~600–900) at four time points over the course of each year. From these data, we have demonstrated effects of various demographic and environmental variables, as well as hair pulling behavior, on alopecia [16]. This provides confirmation that our scoring system is sensitive enough to identify important differences in alopecia between and within animals. This system can also be used to track an individual’s alopecia over time, and animals of specific interest can easily be scored more frequently (e.g., weekly) without creating an undue burden for personnel. Veterinary and behavioral staff can use the severity score to guide decisions regarding interventions and to gauge long-term responses to treatment. We have successfully used this system to identify animals who may engage in hair pulling or self-injurious behavior in the form of skin picking for further evaluation. We currently implement therapies for animals with severity scores of 3 or 4.
While this scoring system is useful for identifying alopecia cases, it cannot determine the underlying cause or etiology of a specific animal’s alopecia. As we have illustrated in a previous publication [16], animals identified as hair pullers were more likely to have severe alopecia, but the majority of animals with alopecia were not hair pullers. Because alopecia may be caused by several different factors, identifying a particular etiology in a colony setting will require more in-depth assessment and diagnostics than this scoring system will provide.
Our scoring system may not be sensitive enough to assess short-term ameliorative therapies either. An animal exhibiting some hair regrowth on six body parts, for instance, would still receive a relatively high severity score using our system because the body parts are still affected with some degree of alopecia. Visual mapping systems that could potentially provide a more detailed assessment of hair regrowth, such as Garner’s [9] or Ryan’s [23], or systems that incorporate more detail about each specific body part (such as that used by Runeson [22]) would likely be more appropriate for use in these circumstances.
Conclusion
The WaNPRC alopecia scoring system is appropriate for assessment of large numbers of animals by multiple personnel. Scoring individual animals takes a minimal amount of time, and training raters to reliability is quick and easy. We believe this is in part because severity and pattern are scored as separate elements in this system. Our method also allows for scoring the entire body of an animal, while still retaining the ability to score specific body parts separately if so desired.
Our scoring system excels in the ability to easily train multiple raters, including those with no prior experience, to quickly and easily reach high inter- and intra-rater reliability scores and to score awake animals, sedated animals, as well as photographs for greater flexibility in scoring and training. Our system allows us to assess a large number of animals over time to track demographic information while still providing us with the ability to monitor an individual’s alopecia scores over the long term.
We recognize that diverse research questions and situations may drive the development of diverse scoring systems. However, we hope that this system may provide a flexible framework that allows for multiple aspects of information gathering while still providing some consistency for alopecia research. Although it may not be sensitive enough to evaluate therapeutic interventions over the short term, it does provide the ability to assess the effects long term and it provides enough information to identify and guide treatment for the more serious cases.
Future research into alopecia scoring could focus on capturing enough detail (such as hair regrowth) to allow short-term therapeutic assessments while still maintaining the ease of training and the high reliability scores of our current alopecia scoring system.
Acknowledgments
This research was supported by NIH Grant P51 OD010425 (Thomas Baillie, PI) and by NIH Grant R24OD01180-15 to Melinda Novak at the University of Massachusetts through Harvard Medical School, with a subcontract to the University of Washington, Seattle (Julie Worlein, PI).
Footnotes
No authors on this study have conflict of interests related to this research. Thank you to Diana Christie and Katherine Forshee for their alopecia scoring efforts.
References
- 1.Beisner BA, Isbell LA. Ground substrate affects activity budgets and hair loss in outdoor captive groups of rhesus macaques (Macaca mulatta) Am J Primatol. 2008;70:1–9. doi: 10.1002/ajp.20615. [DOI] [PubMed] [Google Scholar]
- 2.Beisner BA, Isbell LA. Factors influencing hair loss among female captive rhesus macaques (Macaca mulatta) Appl Anim Behav Sci. 2009;119:91–100. [Google Scholar]
- 3.Berg W, Jolly A, Rambeloarivony H, Andrianome V, Rasamimanana H. A scoring system for coat and tail condition in ringtailed lemursLemur catta. Am J Primatol. 2009;71:183–90. doi: 10.1002/ajp.20652. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein JA, Didier PJ. Nonhuman primate dermatology: a literature review. Vet Dermatol. 2009;20:145–56. doi: 10.1111/j.1365-3164.2009.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 6.Crockett CM, Baker KC, Lutz CK, Coleman K, Fahey MA, Bloomsmith MA, McCowan B, Sullivan J, Weed JL. Developing a reliable laboratory primate alopecia scoring system for interfacility collaboration and on-line training. Am J Primatol. 2009;71:73. [Google Scholar]
- 7.Ellison R, Hobbs T, Maier A, Coleman K. An assessment of temperament and behavior in Rhesus macaques with alopecia. Am J Primatol. 2006;68(Suppl 1):107. [Google Scholar]
- 8.Ellison R. Standardizing alopecia assessment: eliminating bare areas in hair loss evaluation. Tech Talk. 2006;11:2–3. [Google Scholar]
- 9.Garner JP, Dufour B, Gregg LE, Weisker SM, Mench JA. Social and husbandry factors affecting the prevalence and severity of barbering (“whisker trimming”) by laboratory mice. Appl Anim Behav Sci. 2004;89:263–82. [Google Scholar]
- 10.Honess PE, Gimpel JL, Wolfensohn SE, Mason GJ. Alopecia scoring: the quantitative assessment of hair loss in captive macaques. Altern Lab Anim. 2005;33:193–206. doi: 10.1177/026119290503300308. [DOI] [PubMed] [Google Scholar]
- 11.Horenstein VD, Williams LE, Brady AR, Abee CR, Horenstein MG. Age-related diffuse chronic telogen effluvium-type alopecia in female squirrel monkeys (Saimiri boliviensis boliviensis) Comp Med. 2005;55:169–74. [PubMed] [Google Scholar]
- 12.Hussain S, Ferguson C. Assessing the size of burns: which method works best? Emerg Med J. 2009;26:664–6. doi: 10.1136/emj.2009.081380. [DOI] [PubMed] [Google Scholar]
- 13.Jolly A. Coat condition of ringtailed lemurs, Lemur catta, at Berenty reserve, Madagascar: I. Differences by age, sex, density and tourism, 1996–2006. Am J Primatol. 2009;71:191–8. doi: 10.1002/ajp.20647. [DOI] [PubMed] [Google Scholar]
- 14.Kramer J, Fahey M, Santos R, Carville A, Wachtman L, Mansfield K. Alopecia in Rhesus macaques correlates with immunophenotypic alterations in dermal inflammatory infiltrates consistent with hypersensitivity etiology. J Med Primatol. 2010;39:112–22. doi: 10.1111/j.1600-0684.2010.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer JA, Mansfield KG, Simmons JH, Bernstein JA. Psychogenic alopecia in rhesus macaques presenting as focally extensive alopecia of the distal limb. Comp Med. 2011;61:263–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Kroeker R, Bellanca RU, Lee GH, Thom JP, Worlein JM. Alopecia in three macaque species housed in a laboratory environment. Am J Primatol. 2013 doi: 10.1002/ajp.22236. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luchins KR, Baker KC, Gilbert MH, Blanchard JL, Liu DX, Myers L, Bohm RP. Application of the diagnostic evaluation for alopecia in traditional veterinary species to laboratory rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2011;50:926– 38. [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz CK, Coleman K, Worlein J, Novak MA. Hair loss and hair pulling in rhesus monkeys (Macaca mulatta) J Am Assoc Lab Anim Sci. 2013;52:454–7. [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 20.Novak MA, Meyer JS. Alopecia: possible causes and treatments, particularly in captive nonhuman primates. Comp Med. 2009;59:18–26. [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhardt V. Hair pulling: a review. Lab Anim. 2005;39:361–9. doi: 10.1258/002367705774286448. [DOI] [PubMed] [Google Scholar]
- 22.Runeson EP, Lee GH, Crockett CM, Bellanca RU. Evaluating paint rollers as an intervention for alopecia in monkeys in the laboratory (Macaca nemestrina) J Appl Anim Welf Sci. 2011;14:138–49. doi: 10.1080/10888705.2011.551626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan AM, Menard MT, Coleman K, Lutz CK, Worlein JM, Novak MA. Visual assessment of alopecia and hair loss patterns in laboratory- housed rhesus macaques using open source Image J software. Am J Primatol. 2013;75(Suppl 1):60. [Google Scholar]
- 24.Steinmetz HW, Kaumanns W, Dix I, Heistermann M, Fox M, Kaup FJ. Coat condition, housing condition and measurement of faecal cortisol metabolites–a non-invasive study about alopecia in captive rhesus macaques (Macaca mulatta) J Med Primatol. 2006;35:3–11. doi: 10.1111/j.1600-0684.2005.00141.x. [DOI] [PubMed] [Google Scholar]
- 25.Swenerton H, Hurley LS. Zinc-deficiency in rhesus and bonnet monkeys, including effects on reproduction. J Nutr. 1980;110:575–83. doi: 10.1093/jn/110.3.575. [DOI] [PubMed] [Google Scholar]
- 26.Vessey SH, Morrison JA. Molt in free-ranging Rhesus monkeysMacaca mulatta. J Mammal. 1970;51:89–93. [Google Scholar]
- 27.West AM, Leland SP, Lorence MA, Welty TM, Wagner WL, Erwin JM. Behavioral correlates of alopecia severity in laboratory rhesus macaques (Macaca mulatta) Am J Primatol. 2008;70(Suppl 1):51. [Google Scholar]
- 28.Zhang P. A non-invasive study of alopecia in Japanese macaques Macaca fuscata. Curr Zool. 2011;57:26–35. [Google Scholar]