Abstract
Indian Hedgehog (HH) has been shown to be involved in osteoarthritis (OA) in articular joints, where there is evidence that Indian HH blockade could ameliorate OA. It seems to play a prominent role in development of the intervertebral disc (IVD) and in postnatal maintenance. There is little work on IHH in the IVD. Hence the aim of the current study was to investigate the role of Indian Hedgehog in the pathology of facet joint (FJ) OA. 24 patients diagnosed with lumbar intervertebral disk herniation or degenerative spinal stenosis were included. Preoperative magnetic resonance imaging (MRI) and Osteoarthritis Research Society International (OARSI) histopathology grading system was correlated to the mRNA levels of GLI1, PTCH1, and HHIP in the FJs. The Weishaupt grading and OARSI scores showed high positive correlation (r = 0.894) (P < 0.01). MRI Weishaupt grades showed positive correlation with GLI1 (r = 0.491), PTCH1 (r = 0.444), and HHIP (r = 0.654) mRNA levels (P < 0.05 in each case). OARSI scores were also positively correlated with GLI1 (r = 0. 646), PTCH1 (r = 0. 518), and HHIP (r = 0.762) mRNA levels (P < 0.01 in each case). Cumulatively our findings indicate that Indian HH signaling is increased in OA and is perhaps a key component in OA pathogenesis and progression.
Facet joint (FJ) osteoarthritis (OA) is an important component of degenerative changes in the lumbar spine, and is a major contributing factor to low back pain1. However, not all individuals with FJ OA develops back pain, and patients with back pain may be radiologically negative of FJ OA2,3,4. Early detection of FJ OA before the onset of back pain-associated symptoms may help to prevent the disease progression. At present, FJ OA is diagnosed using computed tomography (CT) or magnetic resonance imaging (MRI). Due to the poorly understood mechanisms of FJ OA, there is no available marker for the early detection of this condition.
Indian Hedgehog (Hh), sonic Hh, and desert Hh constitute the Hh family, which plays critical role in growth, patterning, and morphogenesis5,6,7,8,9. Indian Hh has been found to play roles in osteoarthritis10,11,12,13,14. It has been shown that during endochondral bone growth, Indian Hh is mainly produced and secreted by pre-hypertrophic chondrocytes and regulates chondrocyte hypertrophic differentiation. Patched1 (PTCH1) and Smoothened (SMO) are two transmembrane receptors that respond to Indian Hh signaling. When Indian Hh is not present, Patched1 inhibits Smoothened, and represses the downstream gene expression by suppressing the Gli zinc finger transcription factors (GLI1, GLI2, and GLI3)15,16. When Indian Hh is present, it binds to Patched and releases Smoothened, activating the Hh signaling pathway and allowing the active Gli transcription factors to enter the nucleus and enhance the transcription level of downstream targets17. Gli1 mainly functions as a transcription factor, and increased Gli1 level indicates activation of the Hh signaling pathway18,19. The vertebrate cell surface protein Hh-interacting protein (HHIP) have also been shown to bind vertebrate Hh proteins and negatively modulate Hh signaling20.
In this study, we examined the pathological changes in the FJ OA in humans and analyzed the expression levels of Hh signaling-associated PTCH1, GLI1, and HHIP. The prospective relationship between the pathology of FJ OA and Hh signaling is discussed.
Material and Methods
Ethical Statement
All experimental protocol were approved by the Ethical Committee of The First Affiliated Hospital of Chinese PLA General Hospital. Written informed consent was obtained from all enrolled patients. All the subsequent research analyses were carried out in accordance with the approved guidelines.
Patients
This study included 24 consecutive patients (mean age of 61.7 (range 42–75 years)), including 15 males and 9 females, diagnosed with lumbar intervertebral disk herniation or degenerative spinal stenosis. These patients were treated with FJ resection en bloc at our hospital between October 2012 and April 2013.
MRI
Preoperative T2-weighted MRI images of the diseased FJs were obtained and independently evaluated by two experts in this field. Discrepancy between the two evaluators were solved by discussion. The morphological MRI grading system of Weishaupt et al. was used to evaluate the FJs21,22. This grading system utilizes joint space width, osteophytes, facet hypertrophy, bone erosions and subchondral cysts to assign grades 0 to III, where 0 and III represents normal and the most severe changes, respectively.
Pathological analysis
The diseased FJs were resected en bloc and dissected into two parts through a plane perpendicular to the joint surface. For pathological analysis, half of the FJs was fixed in 4% paraformaldehyde for 48 hours, decalcified, and embedded in paraffin. The other half of the FJs were immediately put into liquid nitrogen and used for quantitative real time polymerase chain reaction (qRT-PCR). The block was cut into 4-μm sections on the coronal plane. The sections were stained with toluidine blue and hematoxylin-eosin (HE), respectively. Osteoarthritis Research Society International (OARSI) histopathology grading system was used to evaluate the sections23,24. In this system, the grade of damage from 0 to 6 is defined as the depth of progression of OA into the cartilage and the stage of damage is defined as the horizontal extent of cartilage involvement from 0 to 4. The final score is the combined value of grade and stage (score range 0–24).
qRT-PCR
Total RNA from the facet joints (inclusive of both the cartilage and subchondral bone) was extracted using the Trizol method (Invitrogen, US). The RNA was reversely transcribed into cDNA using random hexamers. The cDNAs were subsequently used to template qRT-PCR reactions using KAPA SYBR® FAST Universal 2X qPCR Master Mix (KAPA BIOSYSTEMS, Wilmington, MA, USA) using primers mentioned in Table 1 and following manufacturer recommended reaction conditions. The data were analyzed with 2−ΔΔCt method and normalized to ACTB expression.
Table 1. Primers used for RT-PCR.
| Primer | Sequence | Product (bp) |
|---|---|---|
| GLI1-F | 5′-GGTGGTTCACATGCGCAG-3′ | 170 |
| GLI1-R | 5′-CATTGCTGAAGGCTTTACTGC-3′ | |
| PTCH1-F | 5′-CCACCAAGTGATCGTGGAAG-3′ | 244 |
| PTCH1-R | 5′-GCCAGAATGCCCTTCAGTAGA-3′ | |
| HHIP-F | 5′-TCCGGTCACATCTTGGGATT-3′ | 167 |
| HHIP-R | 5′-GTCTGTGCAGGTTGTACCGTG-3′ | |
| ACTB-F | 5′-TCCCTGGAGAAGAGCTACG-3′ | 131 |
| ACTB-R | 5′-GTAGTTTCGTGGATGCCACA-3′ |
Statistical analysis
Continuous data were expressed as mean ± SD. Correlation analysis was performed using Spearman rank coefficient with SPSS 17.0 (SPSS, US). A P-value less than 0.05 was considered statistically significant.
Results
MRI image grades
The Weishaupt grades included grade 0 in 5 cases, showing normal FJ space of 2–4 mm; grade I in 8 cases, showing narrowed FJ space of <2 mm, small osteophytes, and mild articular hypertrophy; grade II in 7 cases, showing narrowed FJ space, moderate osteophytes and articular hypertrophy, mild subchondral erosion; grade III in 4 cases, showing narrowed FJ space, large osteophytes, severe articular hypertrophy, subchondral erosion and cysts (Table 2).
Table 2. Pathological scores and mRNA levels of GLI1, PTCH1, and HHIP.
| Patient number | Weishaupt grades | OARSI scores | GLI1 (10−6) | PTCH1(10−4) | HHIIP (10−5) |
|---|---|---|---|---|---|
| 1 | 3 | 18 | 1.893 | 16.736 | 7.104 |
| 2 | 1 | 9 | 5.258 | 11.200 | 5.909 |
| 3 | 1 | 8 | 2.014 | 8.564 | 1.909 |
| 4 | 2 | 6 | 1.439 | 14.970 | 2.909 |
| 5 | 0 | 3 | 1.262 | 9.103 | 2.844 |
| 6 | 2 | 12 | 1.042 | 25.754 | 6.730 |
| 7 | 1 | 6 | 0.859 | 16.726 | 1.026 |
| 8 | 0 | 3 | 1.293 | 16.052 | 1.907 |
| 9 | 1 | 4 | 1.563 | 17.253 | 1.167 |
| 10 | 3 | 24 | 5.887 | 25.300 | 7.179 |
| 11 | 3 | 20 | 4.482 | 20.690 | 3.502 |
| 12 | 2 | 12 | 5.377 | 19.448 | 5.650 |
| 13 | 1 | 4 | 1.357 | 9.137 | 2.462 |
| 14 | 0 | 0 | 0.986 | 8.245 | 0.890 |
| 15 | 1 | 3 | 0.748 | 10.172 | 2.610 |
| 16 | 3 | 20 | 2.914 | 12.450 | 4.990 |
| 17 | 2 | 9 | 1.056 | 15.470 | 2.844 |
| 18 | 0 | 2 | 1.096 | 9.640 | 1.730 |
| 19 | 2 | 6 | 2.7212 | 14.600 | 1.026 |
| 20 | 0 | 3 | 2.7199 | 21.550 | 1.907 |
| 21 | 2 | 12 | 3.6874 | 22.680 | 5.282 |
| 22 | 1 | 8 | 2.6554 | 21.220 | 3.554 |
| 23 | 2 | 12 | 4.5783 | 23.045 | 4.113 |
| 24 | 1 | 8 | 1.9477 | 21.954 | 1.292 |
Pathological examination
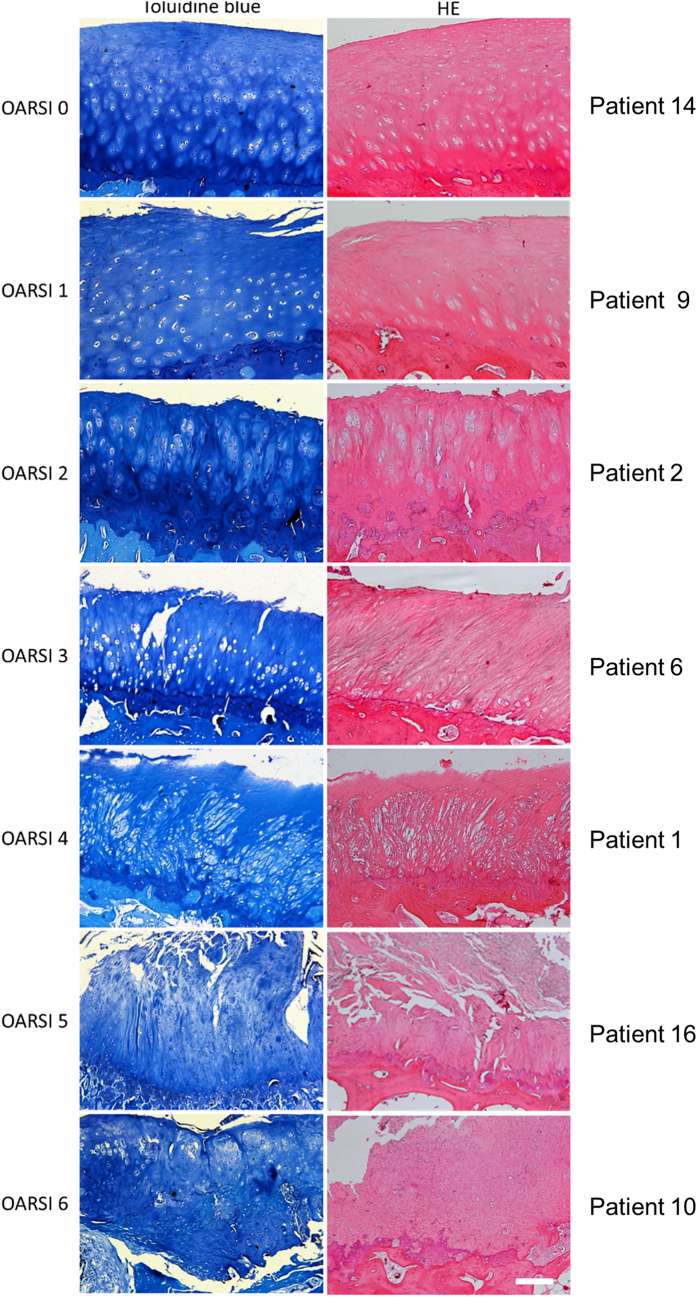
The toluidine blue and HE staining results were scored with OARSI grading system (Table 2). Typical pathological images and corresponding patient information are shown in Fig. 1. Grade 0 showed intact articular surface, clear layer structures, and normal matrix. Grade 1 showed fibrosis in the articular surface and chondrocyte hypertrophy (relative increase of chondrocyte cytoplasm compared to other chondrocytes in the histologic cartilage layer). Grade 2 showed matrix discontinuity and chondrocyte hypertrophy. Grade 3 showed cracks in the matrix and chondrocyte necrosis. Grade 4 showed matrix loss and cyst formation. Grade 5 showed exposure of the subchondral bone and fibrocartilage repair. Grade 6 showed bone reconstruction and microfracture in the fibrocartilage.
Figure 1.
Toluidine blue and HE images of the FJs showing OARSI grades 0–6.
Correlation analysis
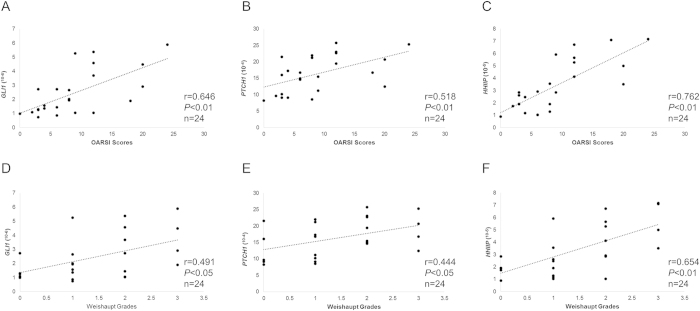
OARSI scores were positively correlated with GLI1 (r = 0. 646), PTCH1 (r = 0. 518), and HHIP (r = 0.762) mRNA expression levels (P < 0.01 in each case) (Fig. 2A–C). MRI Weishaupt grades were positively correlated with GLI1 (r = 0.491), PTCH1 (r = 0.444), and HHIP (r = 0.654) mRNA expression levels (P < 0.05) (Fig. 2D–F). Furthermore, pathological OARSI scores were positively correlated with MRI Weishaupt grades (r = 0.894, P < 0.01) (Fig. 3).
Figure 2.
Correlation analysis between OARSI scores (A–C) and Weishaupt grade (D–F) and mRNA levels of GLI1, PTCH1, and HHIP.
Figure 3.
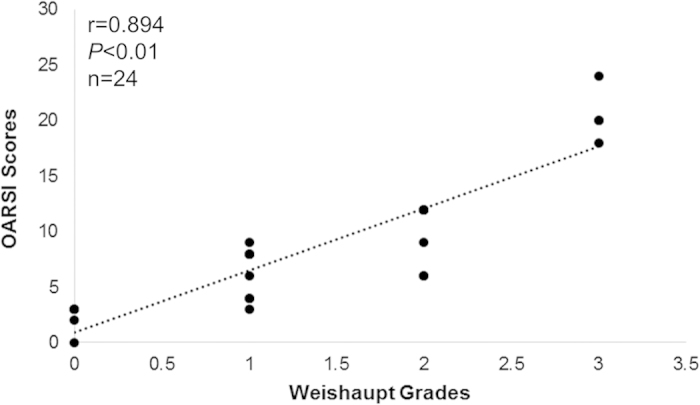
Correlation analysis between OARSI scores and Weishaupt grade.
Discussion
In this study, we collected FJs with OA from 24 patients and examined the mRNA levels of Hh signaling-associated genes. It was found that the mRNA levels of GLI1, PTCH1, and HHIP are positively associated with the MRI image and pathological scores of the FJs, suggesting a role of the Indian Hh pathway in the development of FJ OA.
It has been shown that ablation of Indian Hh from postnatal chondrocytes leads to closure of the growth plate, loss of trabecular bone, and defective skeletal growth25,26, suggesting that Indian Hh plays important roles in postnatal growth. In addition, Indian Hh is induced after femoral fracture in adults, suggesting that Indian Hh might be involved in the repair after bone injury27,28, Indian Hh plays a critical role in endochondral bone formation in embryo and limb development as well as postnatal bone formation. However, its expression starts to decrease with aging under physiological conditions29. In a human study, Indian Hh was almost undetectable in healthy adult cartilage but it increased in early cartilage damage30.
In human OA cartilage and synovial fluid, Indian Hh is upregulated and is correlated with OA progression12. Changes in gene expression and chondrocyte morphology that are consistent with chondrocyte hypertrophy and cartilage degeneration were also found in OA cartilage. These findings provide strong evidence that Indian Hh plays a critical role in OA pathology.
In addition, genetically modified mice that have elevated Hh signaling in chondrocytes showed more severe OA phenotype, and OA-caused cartilage damage can be alleviated by inhibiting Hh signaling in mice or human cartilage explants31. Lin et al. had reported that PTCH1, GLI1, and HHIP were all overexpressed in human osteoarthritic samples, as well as in the articular cartilage from a mouse model of osteoarthritis. In addition, mice with aberrant activation of the Hh pathway showed cartilage degradation and an increased tendency to develop osteoarthritis. The severity of the disease correlated with the degree of Hh pathway activation. Furthermore, genetic or pharmacologic inhibition of the Hh pathway in a mouse model of osteoarthritis significantly decreased collagen X deposition, cartilage degradation and osteoarthritis severity. Remarkably, inhibition of Hh in explant cultures of human osteoarthritic cartilage samples blocked expression of key genetic markers of osteoarthritis, including the metalloproteinases ADAMTS5 and MMP13, the transcription factor RUNX2, and COL10A131. Conditional deletion of Indian Hh in chondrocytes can attenuate OA progression in a mouse model14. Given all this information and our findings it would be rational to presume that IHH signaling is increased in OA and is perhaps a key component in OA pathogenesis and progression. However, our future endeavors would be geared towards specifically testing the same.
In our study, the mRNA expression levels of three Indian Hh-associated genes, GLI1, PTCH1, and HHIP, were found to be positively correlated with FJ OA pathological scores. This is the first study demonstrating a role of Indian Hh in the pathology of FJ OA. Future endeavors will deal with defining the precise role and underlying molecular mechanism by which Indian Hh signaling promote FJ OA.
Author Contributions
F.S. and S.X.H. wrote the main manuscript text, J.L.Z., Y.L. and Y.Z. collected data and carried out the experiments, C.L.Z. analyzed the data, J.G.T. designed the experiments. All author has approved the manuscript.
Additional Information
How to cite this article: Shuang, F. et al. Indian Hedgehog signaling pathway members are associated with magnetic resonance imaging manifestations and pathological scores in lumbar facet joint osteoarthritis. Sci. Rep. 5, 10290; doi: 10.1038/srep10290 (2015).
References
- Gellhorn A. C., Katz J. N. & Suri P. Osteoarthritis of the spine: the facet joints. Nat. Rev. Rheumatol. 9, 216–224 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman L. et al. Facet joint osteoarthritis and low back pain in the community-based population. Spine (Phila Pa 1976). 33, 2560–2565 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer P., Leboeuf-Yde C., Korsholm L., Sorensen J. S. & Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976). 30, 1173–1180 (2005). [DOI] [PubMed] [Google Scholar]
- Savage R. A., Whitehouse G. H. & Roberts N. The relationship between the magnetic resonance imaging appearance of the lumbar spine and low back pain, age and occupation in males. Eur. Spine. J. 6, 106–114 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S. et al. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 124, 53–63 (1997). [DOI] [PubMed] [Google Scholar]
- Vortkamp A. et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 273, 613–622 (1996). [DOI] [PubMed] [Google Scholar]
- Bitgood M. J. & McMahon A. P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol. 172, 126–138 (1995). [DOI] [PubMed] [Google Scholar]
- Niswander L., Jeffrey S., Martin G. R. & Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 371, 609–612 (1994). [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Riddle R. D., Laufer E. & Tabin C. Sonic hedgehog: a key mediator of anterior-posterior patterning of the limb and dorso-ventral patterning of axial embryonic structures. Biochem. Soc. Trans. 22, 569–574 (1994). [DOI] [PubMed] [Google Scholar]
- Horie M. et al. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthritis. Cartilage. 20, 1197–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y. & Im G. I. The expressions of the SOX trio, PTHrP (parathyroid hormone-related peptide)/IHH (Indian hedgehog protein) in surgically induced osteoarthritis of the rat. Cell. Biol. Int. 35, 529–535 (2011). [DOI] [PubMed] [Google Scholar]
- Wei F. et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis. Cartilage. 20, 755–763 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. Indian hedgehog in synovial fluid is a novel marker for early cartilage lesions in human knee joint. Int. J. Mol. Sci. 15, 7250–7265 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. et al. Disrupting the Indian hedgehog signaling pathway in vivo attenuates surgically induced osteoarthritis progression in Col2a1-CreERT2; Ihhfl/fl mice. Arthritis. Res. Ther. 16, R11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Tatarczuch L. & Mirams M. The skeleton: a multi-functional complex organ: the growth plate chondrocyte and endochondral ossification. J. Endocrinol. 211, 109–121 (2011). [DOI] [PubMed] [Google Scholar]
- Wuelling M. & Vortkamp A. Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatr. Nephrol. 25, 625–631 (2010). [DOI] [PubMed] [Google Scholar]
- Long F. et al. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 131, 1309–1318 (2004). [DOI] [PubMed] [Google Scholar]
- Bai C. B. & Joyner A. L. Gli1 can rescue the in vivo function of Gli2. Development. 128, 5161–5172 (2001). [DOI] [PubMed] [Google Scholar]
- Park H. L. et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 127, 1593–1605 (2000). [DOI] [PubMed] [Google Scholar]
- Chuang P. T. & McMahon A. P. Vertebrate Hedgehog signalling modulated by induction of a Hedgehog-binding protein. Nature. 397, 617–621 (1999). [DOI] [PubMed] [Google Scholar]
- Weishaupt D., Zanetti M., Boos N. & Hodler J. MR imaging and CT in osteoarthritis of the lumbar facet joints. Skeletal. Radiol. 28, 215–219 (1999). [DOI] [PubMed] [Google Scholar]
- Stelzeneder D. et al. Quantitative in vivo MRI evaluation of lumbar facet joints and intervertebral discs using axial T2 mapping. Eur. Radiol. 21, 2388–2395 (2011). [DOI] [PubMed] [Google Scholar]
- Gerwin N., Bendele A. M., Glasson S. & Carlson C. S. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis. Cartilage. 18, S24–34 (2010). [DOI] [PubMed] [Google Scholar]
- Pritzker K. P. et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis. Cartilage. 14, 13–29 (2006). [DOI] [PubMed] [Google Scholar]
- Maeda Y., Schipani E., Densmore M. J. & Lanske B. Partial rescue of postnatal growth plate abnormalities in Ihh mutants by expression of a constitutively active PTH/PTHrP receptor. Bone. 46, 472–478 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y. et al. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc. Natl. Acad. Sci. USA 104, 6382–6387 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht G. S., Silkstone D., Nadesan P., Whetstone H. & Alman B. A. Activation of hedgehog signaling during fracture repair enhances osteoblastic-dependent matrix formation. J. Orthop. Res. 32, 581–586 (2014). [DOI] [PubMed] [Google Scholar]
- Murakami S. & Noda M. Expression of Indian hedgehog during fracture healing in adult rat femora. Calcif. Tissue. Int. 66, 272–276 (2000). [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Jikko A. & Le A. X. Age-dependent effects of hedgehog protein on chondrocytes. J. Bone. Joint. Surg. Br. 81, 1076–1082 (1999). [DOI] [PubMed] [Google Scholar]
- Tchetina E. V., Squires G. & Poole A. R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 32, 876–886 (2005). [PubMed] [Google Scholar]
- Lin A. C. et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat. Med. 15, 1421–1425 (2009). [DOI] [PubMed] [Google Scholar]