Abstract
Synovial fluid of the joint decreases friction between the cartilage surfaces and reduces cartilage wear during articulation. Characteristic changes of synovial fluid have been shown in patients with osteoarthritis (OA) in the temporomandibular joint (TMJ). OA is generally considered to be induced by excessive mechanical stress. However, whether the changes in synovial fluid precede the mechanical overloading or vice versa remains unclear. In the present study, our purpose was to examine if the breakdown of joint lubrication affects the frictional properties of mandibular condylar cartilage and leads to subsequent degenerative changes in TMJ. We measured the frictional coefficient in porcine TMJ by a pendulum device after digestion with hyaluronidase (HAase) or trypsin. Gene expressions of interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2), matrix metalloproteinases (MMPs), type II collagen, and histology were examined after prolonged cyclic loading by an active pendulum system. The results showed that the frictional coefficient increased significantly after HAase (35%) or trypsin (74%) treatment. Gene expression of IL-1β, COX-2, and MMPs-1, -3, and -9 increased significantly in enzyme-treated TMJs after cyclic loading. The increase in the trypsin-treated group was greater than that in the HAase-treated group. Type II collagen expression was reduced in both enzyme-treated groups. Histology revealed surface fibrillation and increased MMP-1 in the trypsin-treated group, as well as increased IL-1β in both enzyme-treated groups after cyclic loading. The findings demonstrated that the compromised lubrication in TMJ is associated with altered frictional properties and surface wear of condylar cartilage, accompanied by release of pro-inflammatory and matrix degradation mediators under mechanical loading.
Keywords: lubrication, cyclic loading, hyaluronan, superficial zone protein, osteoarthritis, matrix metalloproteases
Introduction
Human synovial fluid contains various molecules such as mucinous glycoproteins, serum albumin, phospholipids, hyaluronan (HA), and superficial zone protein (SZP). SZP is homologous to the molecules referred to as lubricin or proteoglycan 4 (PRG4). Among them, HA imparts viscosity to the synovial fluid and is a major component in fluid film lubrication. The large size and negative charge of HA contribute to its structural fluid dynamics (Benz et al. 2004). Conversely, SZP has also been implicated as a crucial factor in the lubrication of synovial joints (Schumacher et al. 1994). SZP provides boundary lubrication of articular surfaces under high contact pressure and low sliding speed (Jay, Tantravahi, et al. 2001). The lubrication mechanisms of synovial joints generally accepted are fluid film and boundary lubrication. Fluid film lubrication is the dominant mechanism, and joints can withstand the loading as long as it is not excessive. However, after prolonged overloading, only solid contact may exist between the articular surfaces, and boundary lubrication takes over (Tanaka et al. 2008).
Osteoarthritis (OA) in the temporomandibular joint (TMJ) is a degenerative disease characterized by the loss of the extracellular matrix (ECM) network of mandibular condylar cartilage, which affects the underlying bone and surrounding musculoskeletal components. Based on recent findings, disease models predict that mechanical overloading triggers a cascade of molecular events leading to OA in TMJ (Haskin et al. 1995). Several mediators play a central role in the progression of the disease. Interleukin-1β (IL-1β) is one of the most prevalent pro-inflammatory cytokines in patients with temporomandibular disorder (TMD) (Kim et al. 2012). Prostaglandin E2 (PGE2), which is catalyzed by cyclooxygenase-2 (COX-2), has been characterized as a catabolic mediator found in patients with TMD (Alstergren and Kopp 2000). The proteolytic cleavage of collagen fibers is mediated by several matrix metalloproteases (MMPs) in pathologic TMJ (Yoshida et al. 2006). Under the effects of these factors, destruction of cartilage ECM is an irreversible consequence. In addition to cartilage tissue, characteristic changes in synovial fluid have also been shown to be associated with OA. The molecular-weight distribution of HA is altered to lower molecular-weight forms in synovial fluid from patients with TMD (Takahashi et al. 2004). Patients with TMJ and OA present a much lower lubricin concentration in their synovial fluid (Wei et al. 2010). It has been indicated that these contents could be modulated by oxidative stress (Grootveld et al. 1991) or direct mechanical stress (Yanagida-Suekawa et al. 2013). However, whether the change in synovial fluid may precipitate the TMJ into mechanical overloading or not remains unclear. It has been proposed that the impairment of joint lubrication may cause disc displacement in TMJ (Nitzan 2001). When synovial fluid degrades, a decrease in viscosity and loss of lubricating ability between the articular surfaces may occur (Forster and Fisher 1996). We speculate that this deficient lubrication will alter the frictional characteristics of the articular surfaces, which may reduce the capacity of the joint’s load-absorbing system. As a result, the joint may be overloaded or subjected to tissue damage. In the present study, our purpose was to examine if the breakdown of joint lubrication affects the frictional properties of mandibular condylar cartilage and leads to subsequent degenerative changes. We measured the frictional coefficient in porcine TMJs after proteolytic treatment with hyaluronidase (HAase) or trypsin. Furthermore, an active pendulum system was used to apply cyclic loading to mimic TMJ load. Gene expressions of inflammatory mediators, MMPs, type II collagen, and histology were examined after 24 h of loading and compared with those of groups without loading.
Materials and Methods
Measurement of Frictional Coefficient
Thirty porcine cranial heads (aged 6–9 mo) were obtained at a local slaughterhouse. The protocol for the experiment was approved by the Animal Care and Use Committee at Hiroshima University. Crania were cut in half and all soft tissues were carefully removed, except the TMJ capsule. Next, 1 mL of phosphate-buffered solution (pH 7.4, PBS; Mitsubishi Kagaku, Tokyo, Japan), HAase (3,000 units/mL in pH 5.5 acetate buffer solution, H3506; Sigma-Aldrich, St. Louis, MO, USA), or trypsin (0.5% in pH 7.4 PBS; Nacalai Tesque, Kyoto, Japan) was injected into both TMJ cavities with syringes, followed by digestion for 1 h at 37°C (n = 10). A pendulum-type tester was then used to measure the frictional coefficient (Fig. 1A). The measuring procedure was based on a previous study (Tanaka et al. 2004). Briefly, the swing motion was measured by a three-dimensional dynamic angle sensor (GU-3024; Data Tek, Tokyo, Japan) placed on the upper plate. Readings (sampling rate, 60 times per s; resolution, 0.5°) were transmitted to a computer and recorded. The initial swing was approximately 5°. The frictional coefficient μ was calculated by the following equation: μ = L Δθ/4r, where L is the pendulum arm length, Δθ is the average amplitude difference in pitch angle between the third swing and the twelfth swing on a decreasing curve, and r is the radius of the condylar head (Fig. 1B).
Figure 1.
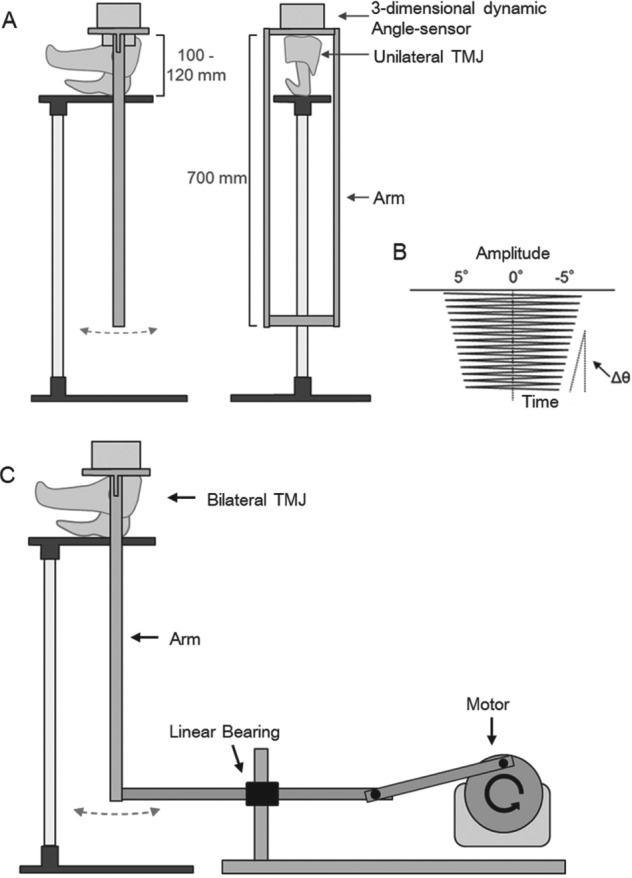
Schematic illustration of device used in the experiment. Pendulum-type tester for measuring frictional coefficient (A) and a sample of damping curve recorded by the angle sensor (B). Active cyclic loading device modified from the friction tester is used to apply mechanical load (C).
Application of Cyclic Load to TMJ
Six porcine cranial heads with bilateral intact TMJs were used in this experiment. All soft tissues except the TMJ capsule were carefully removed. One side of the TMJ was injected with 1 mL of PBS, and the contralateral side was injected with either HAase (3,000 units/mL in pH 5.5 acetate buffer solution, H3506; Sigma-Aldrich) or trypsin (0.5% in pH 7.4 PBS; Nacalai Tesque) with syringes (n = 3). This time, the arm of the pendulum unit was connected to a rotary motor through a series of linkages and a linear bearing to generate active cyclic loading (Fig. 1C). The cyclic loading device was driven automatically for 24 h at a rate of 1 Hz. A warming device was used to keep the temperature around the TMJ at 37°C. Unloaded groups underwent the same injection procedure except for loading by the pendulum unit.
Real-time RT-PCR Analysis
After being loaded, the joint capsules were opened, and the stressed area was identified by an indentation on the disc (15 mm in diameter). Half of the cartilage from the load-bearing area was harvested. Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To quantify target gene expression, we used real-time reverse transcription polymerase chain-reaction (RT-PCR) with a LightCycler system (Roche Diagnostics, Penzberg, Germany) and SYBR Green (Toyobo, Osaka, Japan). The sequences of primers used are shown in the Table. Porcine glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control gene. Normalized cycle threshold (Ct) values were compared relative to those of controls. Data are shown as relative expression calculated by the comparative Ct method. Each sample was analyzed in triplicate to ensure accuracy.
Table.
Primer Sequences for Real-time RT-PCR.
| Gene | Sequence 5′-3′ | |
|---|---|---|
| IL-1β | Forward: | ACCTCAGCCCTCTGGGAGA |
| Reverse: | GGGCTTTTGTTCTGCTTGAG | |
| COX-2 | Forward: | CTTACTGGAACATGGCATCAC |
| Reverse: | CTCTGCTCTGGTCGATTGA | |
| MMP-1 | Forward: | CTACACTTCGGGGAGAACTA |
| Reverse: | CGGACTTCATCTCTATCGG | |
| MMP-3 | Forward: | ACTGGATTTGCCAAGAAGTG |
| Reverse: | GCATAGGCATGAGCCAAAAC | |
| MMP-9 | Forward: | ACACACACGACATCTTCCAG |
| Reverse: | AGGTCACGTAGCCCACATAG | |
| Type II collagen | Forward: | CCATCTGGCTTCCAGGGAC |
| Reverse: | CCACGAGGCCAGGAGCT | |
| GAPDH | Forward: | TCATCCCTGCTTCTACCG |
| Reverse: | CAGATCCACAACCGACAC | |
Histological Examination
The condylar head with the remaining half of the cartilage was separated and fixed with 10% neutral buffered formalin (Sigma-Aldrich). Specimens were decalcified in EDTA (Sigma-Aldrich) and dehydrated by gradient ethanol. After being embedded in paraffin wax, all slides were subjected to immunohistochemical (IHC) stain. Briefly, slides were blocked with serum and primary antibody: 1:400 IL-1β (MAB681; R&D Systems, Minneapolis, MN, USA), 1:800 MMP-1 (F-67; Daiichi Fine Chemical, Toyama, Japan), or 1:500 PRG4 (AB2200; Millipore, Darmstadt, Germany) diluted in PBS and applied for overnight incubation. After being washed, the slides were covered with 1:200 anti-mouse secondary biotinylated antibody followed by Elite ABC reagent (both Vectalab, Burlingame, CA, USA), and DAB (Nichirei, Tokyo, Japan) was applied for colorimetric detection. For the detection of SZP, fluorescent anti-rabbit secondary antibody (Alexa Fluor 488; Life Technologies, Carlsbad, CA, USA) was used, and DAPI (SouthernBiotech, Birmingham, AL, USA) was applied for counterstain. All images were captured under a digital microscope (Keyence, Osaka, Japan).
Statistical Analysis
Reproducibility of these results was confirmed by repeated experiments conducted in the same manner. All data are expressed as means ± standard deviation. One-way analysis of variance (ANOVA) with the Tukey-Kramer post-hoc test was used to evaluate the frictional coefficient of TMJ cartilage and gene expression. Resulting P values of less than 0.05 were regarded as statistically significant, and those of less than 0.01 were considered to be highly significant.
Results
Effects of Enzymatic Degradation on Frictional Coefficient of TMJ
The frictional coefficient of TMJs treated with PBS was 0.0113 (SD 0.0005) (Fig. 2). Frictional coefficients of those treated with HAase and trypsin increased significantly to 0.0165 (SD 0.0020) and 0.0241 (SD 0.001), respectively. Collectively, proteolytic treatment for 1 h raised the frictional coefficient by 35% with HAase and by 74% with trypsin.
Figure 2.
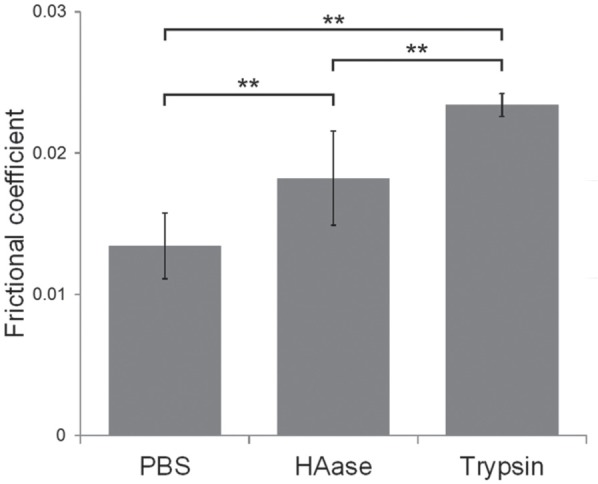
Effects of enzyme treatment on frictional coefficient of temporomandibular joint (TMJ). Frictional coefficient of TMJ after 1 h of phosphate-buffered solution (PBS) or hyaluronidase (HAase) (3,000 U/mL) or trypsin (0.5%) treatment. Data are expressed as means ± SD, n = 10, ** P < 0.01.
Effects of Enzymatic Degradation on Gene Expression in Mandibular Condylar Cartilage
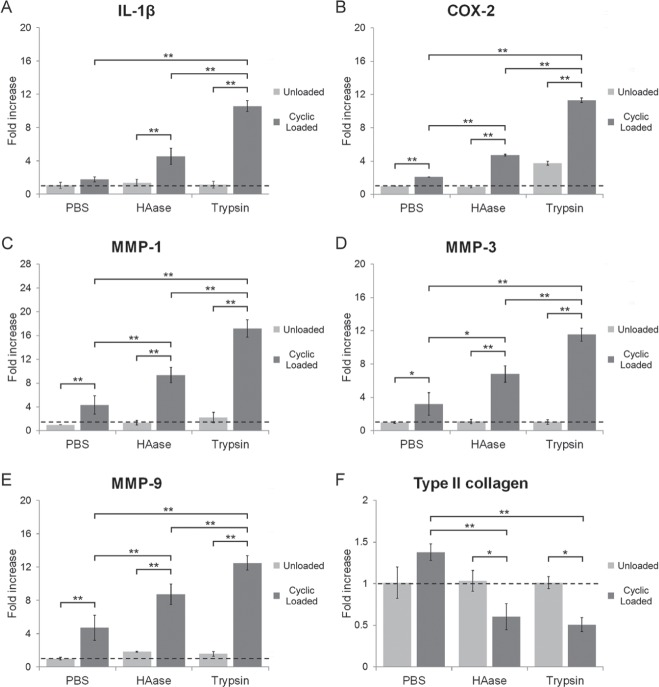
Without cyclic loading, gene expression of IL-1β, MMPs-1, -3, and -9, and type II collagen after 24-hour treatment with HAase or trypsin remained the same as with PBS treatment. In contrast, the expression of IL-1β, COX-2, and MMPs increased significantly in TMJs treated with HAase or trypsin after 24-hour cyclic loading (Fig. 3A–E). The increase in gene expression in the trypsin-treated group was greater than that in the HAase-treated group. Conversely, gene expression of type II collagen decreased after treatment with HAase and trypsin to 0.6- and 0.5-fold, respectively, compared with that in the unloaded group (Fig. 3F).
Figure 3.
Effects of enzyme treatment on gene expression in TMJ cartilage. Gene expression of IL-1β (A), COX-2 (B), MMP-1 (C), MMP-3 (D), MMP-9 (E), and type II collagen (F) in TMJ cartilage after 24 h of PBS, HAase (3,000 U/mL), or trypsin (0.5%) treatment with or without cyclic loading. Data are expressed as means ± SD, n = 3, * P < 0.05, ** P < 0.01.
Effects of Enzymatic Degradation on Histologic Changes in Mandibular Condylar Cartilage
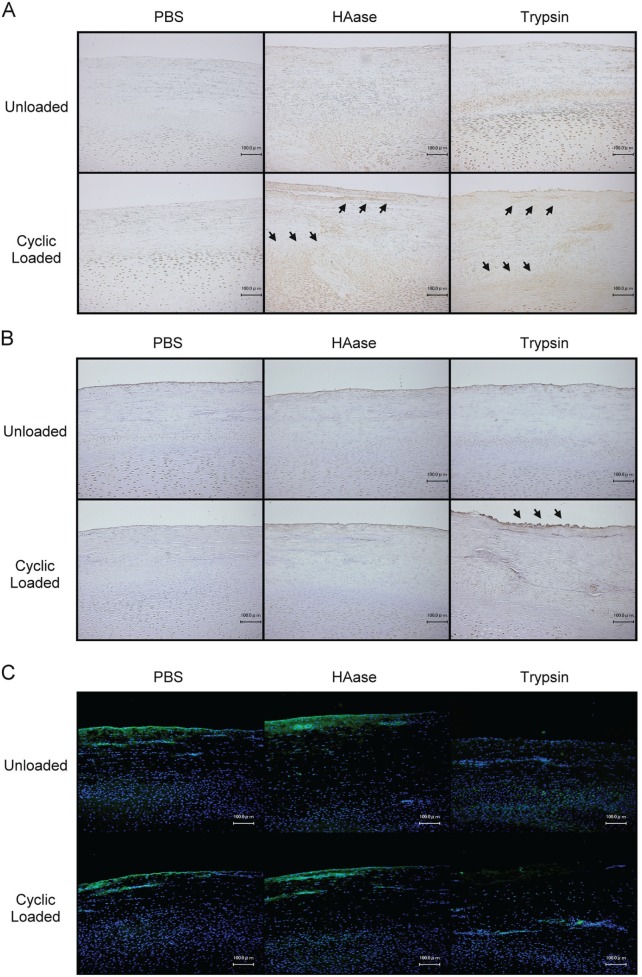
IHC revealed that both the HAase- and trypsin-treated groups with cyclic loading showed more intense staining of IL-1β at the superficial and proliferative cartilage zones (Fig. 4A). Extensive staining of MMP-1 was found at the superficial zone in the trypsin-treated group after cyclic loading (Fig. 4B). Meanwhile, surface fibrillation was evident in the trypsin-treated group with cyclic loading (Fig. 4); however, Safranin-O staining revealed no change in the proteoglycan contents in all groups (data not shown). The removal of SZP in trypsin-treated groups was confirmed by fluorescent IHC (Fig. 4C).
Figure 4.
Effects of enzymatic degradation on histologic changes in TMJ cartilage. Representative IHC staining of IL-1β (A), MMP-1 (B), and SZP (C) of TMJ cartilage after 24 h of PBS, HAase (3,000 U/mL), or trypsin (0.5%) treatment with or without cyclic loading. Extensive staining areas are indicated by arrowheads.
Discussion
Previous studies have measured the frictional coefficient under various conditions of TMJs (Kawai et al. 2004; Tanaka et al. 2006). This is the first attempt to report the effect of proteolytic disruption on frictional property in TMJ cartilage. HAase specifically hydrolyzes the N-acetyl-D glucosamine of HA, which does not affect the lubricity of SZP (Jay, Harris, et al. 2001). Trypsin, in contrast, is a broad-spectrum protease that degrades proteins at the carboxyl-terminal domains in lysine and arginine residues. SZP contains an abundance of both residues, particularly in the mucin region (Flannery et al. 1999). The sensitivity of lubricin to trypsin has been well-established (Chan et al. 2010). Investigation is now focused on identifying the enzymes responsible for the loss of lubricating ability in synovial fluid. Large numbers of serine proteases have been found in knee synovial fluid from patients with OA and rheumatoid arthritis (RA), including thrombin, trypsin-like protease, elastase, and urokinase (Jay et al. 2004). From our results, we confirmed that enzyme degradation affects the frictional properties in TMJ, as seen in other articular joints (Teeple et al. 2007; Chan et al. 2011). The frictional coefficient increased more in the trypsin-treated group than the HAase-treated group. Although degradation of HA could affect the frictional coefficient, a complex formed between SZP and HA may circumvent this diminution in function (Kwiecinski et al. 2011). The result suggested that SZP plays a crucial role in protecting the lubricating ability. Numerous medications such as NSAIDs, cyclooxygenase 2 inhibitors, and steroids for the treatment of OA act by inhibiting joint degradation. However, the effectiveness of OA prevention in clinical practice is limited. A recent study demonstrated that the recombinant lubricin has significant chondroprotective effects during the progression of OA in an animal model, which suggested the potential use of recombinant lubricin molecules in novel approaches for the treatment of OA (Flannery et al. 2009).
Repetitive dynamic loading is more representative of TMJ motion than is static loading. It has been shown that repetitive forced-mouth opening induced cartilage degradation and osteoarthritic lesions of TMJ in a rabbit model (Fujisawa et al. 2003). In a previous study of the knee joint, SZP-knockout mice showed increased baseline frictional coefficient and did not resist further friction caused by cyclic loading (Drewniak et al. 2012). In this study, induction of pro-inflammatory mediators and MMPs was observed in trypsin-treated TMJ after cyclic loading. The results are consistent with the characteristics of stress-induced osteoarthritic chondrocytes in vitro (Wang et al. 2013). This was further evidenced by precocious joint failure in individuals with camptodactyly arthropathy coxa vara pericarditis (CACP) syndrome, a disease linked to deficiency in the gene encoding lubricin, and by progressive TMJ degeneration observed in SZP-null mice (Hill et al. 2014). At the histological level, collagen fibers are damaged at the superficial layer, as seen in early signs of OA (Poole et al. 2002). In a computational biomechanical analysis, it is predicted that friction primarily causes increased shear stress in the articular cartilage layers, but hardly in the articular disc (Koolstra 2012). From our results, we provide evidence that deterioration of lubricating ability in a joint can result in the generation of stress on the articular surfaces (Rahamim et al. 2001). The increased frictional stress may further contribute to the degradation of ECM contents. However, it should be realized that the amount and nature of loading used in the present study do not represent the actual TMJ dynamics in vivo. Furthermore, during functional movements of the jaw, part of the loading can be absorbed by involuntary muscle contractions responding to proprioceptive sense. These limiting factors were eliminated in this simplified joint-loading model.
In conclusion, the findings demonstrated the crucial role of lubrication in TMJ. We provide evidence that the compromised lubrication in TMJ is associated with altered frictional properties and surface wear of condylar cartilage, accompanied by release of pro-inflammatory and matrix degradation mediators under mechanical loading.
Author Contributions
Y. Asakawa-Tanne, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; S. Su, contributed to data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; R. Kunimatsu, N. Hirose, contributed to data acquisition and analysis, drafted the manuscript; T. Mitsuyoshi, Y. Okamoto, contributed to data acquisition, drafted the manuscript; E. Tanaka, contributed to conception and data analysis, critically revised the manuscript; K. Tanne, contributed to conception and design, drafted and critically revised the manuscript; K. Tanimoto, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This research was supported by a Grant-in-Aid (No. 23792429) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alstergren P, Kopp S. 2000. Prostaglandin E2 in temporomandibular joint synovial fluid and its relation to pain and inflammatory disorders. J Oral Maxillofac Surg. 58(2):180–186. [DOI] [PubMed] [Google Scholar]
- Benz M, Chen N, Israelachvili J. 2004. Lubrication and wear properties of grafted polyelectrolytes, hyaluronan and hylan, measured in the surface forces apparatus. J Biomed Mater Res A. 71(1):6–15. [DOI] [PubMed] [Google Scholar]
- Chan SM, Neu CP, DuRaine G, Komvopoulos K, Reddi AH. 2010. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthritis Cartilage. 18(7):956–963. [DOI] [PubMed] [Google Scholar]
- Chan SM, Neu CP, Komvopoulos K, Reddi AH. 2011. Dependence of nanoscale friction and adhesion properties of articular cartilage on contact load. J Biomech. 44(7):1340–1345. [DOI] [PubMed] [Google Scholar]
- Drewniak EI, Jay GD, Fleming BC, Zhang L, Warman ML, Crisco JJ. 2012. Cyclic loading increases friction and changes cartilage surface integrity in lubricin-mutant mouse knees. Arthritis Rheum. 64(2):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, Caterson B. 1999. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Commun. 254(3):535–541. [DOI] [PubMed] [Google Scholar]
- Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermúdez MA, Blanchet T, Gleghorn JP, Bonassar LJ, Bendele AM, et al. 2009. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 60(3):840–847. [DOI] [PubMed] [Google Scholar]
- Forster H, Fisher J. 1996. The influence of loading time and lubricant on the friction of articular cartilage. Proc Inst Mech Eng H. 210(2):109–119. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Kuboki T, Kasai T, Sonoyama W, Kojima S, Uehara J, Komori C, Yatani H, Hattori T, Takigawa M. 2003. A repetitive, steady mouth opening induced an osteoarthritis-like lesion in the rabbit temporomandibular joint. J Dent Res. 82(9):731–735. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Henderson EB, Farrell A, Blake DR, Parkes HG, Haycock P. 1991. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem J. 273(Pt 2):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskin CL, Milam SB, Cameron IL. 1995. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Crit Rev Oral Biol Med. 6(3):248–277. [DOI] [PubMed] [Google Scholar]
- Hill A, Duran J, Purcell P. 2014. Lubricin protects the temporomandibular joint surfaces from degeneration. PLoS One. 9(9):e106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, Chichester CO. 2004. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 31(3):557–564. [PubMed] [Google Scholar]
- Jay GD, Harris DA, Cha CJ. 2001. Boundary lubrication by lubricin is mediated by O-linked beta(1-3)Gal-GalNAc oligosaccharides. Glycoconj J. 18(10):807–815. [DOI] [PubMed] [Google Scholar]
- Jay GD, Tantravahi U, Britt DE, Barrach HJ, Cha CJ. 2001. Homology of lubricin and superficial zone protein (SZP): products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 19(4):677–687. [DOI] [PubMed] [Google Scholar]
- Kawai N, Tanaka E, Takata T, Miyauchi M, Tanaka M, Todoh M, van Eijden T, Tanne K. 2004. Influence of additive hyaluronic acid on the lubricating ability in the temporomandibular joint. J Biomed Mater Res A. 70(1):149–153. [DOI] [PubMed] [Google Scholar]
- Kim YK, Kim SG, Kim BS, Lee JY, Yun PY, Bae JH, Oh JS, Ahn JM, Kim JS, Lee SY. 2012. Analysis of the cytokine profiles of the synovial fluid in a normal temporomandibular joint: preliminary study. J Craniomaxillofac Surg. 40(8):e337–e341. [DOI] [PubMed] [Google Scholar]
- Koolstra JH. 2012. Biomechanical analysis of the influence of friction in jaw joint disorders. Osteoarthritis Cartilage. 20(1):43–48. [DOI] [PubMed] [Google Scholar]
- Kwiecinski JJ, Dorosz SG, Ludwig TE, Abubacker S, Cowman MK, Schmidt TA. 2011. The effect of molecular weight on hyaluronan’s cartilage boundary lubricating ability—alone and in combination with proteoglycan 4. Osteoarthritis Cartilage. 19(11):1356–1362. [DOI] [PubMed] [Google Scholar]
- Nitzan DW. 2001. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. J Oral Maxillofac Surg. 59(1):36–45. [DOI] [PubMed] [Google Scholar]
- Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, et al. 2002. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum. 61(Suppl 2):ii78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamim E, Better H, Dagan A, Nitzan DW. 2001. Electron microscope and biochemical observations of the surface active phospholipids on the articular surfaces and in the synovial fluid of the temporomandibular joint: a preliminary investigation. J Oral Maxillofac Surg. 59(11):1326–1332. [DOI] [PubMed] [Google Scholar]
- Schumacher BL, Block JA, Schmid TM, Aydelotte MB, Kuettner KE. 1994. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 311(1):144–152. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tominaga K, Takano H, Ariyoshi W, Habu M, Fukuda J, Maeda H. 2004. A decrease in the molecular weight of hyaluronic acid in synovial fluid from patients with temporomandibular disorders. J Oral Pathol Med. 33(4):224–229. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Dalla-Bona DA, Iwabe T, Kawai N, Yamano E, van Eijden T, Tanaka M, Miyauchi M, Takata T, Tanne K. 2006. The effect of removal of the disc on the friction in the temporomandibular joint. J Oral Maxillofac Surg. 64(8):1221–1224. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Detamore MS, Tanimoto K, Kawai N. (2008). Lubrication of the temporomandibular joint. Ann Biomed Eng. 36(1):14–29. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Kawai N, Tanaka M, Todoh M, van Eijden T, Hanaoka K, Dalla-Bona DA, Takata T, Tanne K. 2004. The frictional coefficient of the temporomandibular joint and its dependency on the magnitude and duration of joint loading. J Dent Res. 83(5):404–407. [DOI] [PubMed] [Google Scholar]
- Teeple E, Fleming BC, Mechrefe AP, Crisco JJ, Brady MF, Jay GD. 2007. Frictional properties of Hartley guinea pig knees with and without proteolytic disruption of the articular surfaces. Osteoarthritis Cartilage. 15(3):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Guan PP, Guo C, Zhu F, Konstantopoulos K, Wang ZY. 2013. Fluid shear stress-induced osteoarthritis: roles of cyclooxygenase-2 and its metabolic products in inducing the expression of proinflammatory cytokines and matrix metalloproteinases. FASEB J. 27(12):4664–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Xiong H, Li B, Cheng Y, Long X. 2010. Boundary-lubricating ability and lubricin in synovial fluid of patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 68(10):2478–2483. [DOI] [PubMed] [Google Scholar]
- Yanagida-Suekawa T, Tanimoto K, Tanne Y, Mitsuyoshi T, Hirose N, Su S, Tanne K, Tanaka E. 2013. Synthesis of hyaluronan and superficial zone protein in synovial membrane cells modulated by fluid flow. Eur J Oral Sci. 121(6):566–572. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Takatsuka S, Hatada E, Nakamura H, Tanaka A, Ueki K, Nakagawa K, Okada Y, Yamamoto E, Fukuda R. 2006. Expression of matrix metalloproteinases and aggrecanase in the synovial fluids of patients with symptomatic temporomandibular disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 102(1):22–27. [DOI] [PubMed] [Google Scholar]