Abstract
The health gains and costs resulting from using different caries detection strategies might not only depend on the accuracy of the used method but also the treatment emanating from its use in different populations. We compared combinations of visual-tactile, radiographic, or laser-fluorescence–based detection methods with 1 of 3 treatments (non-, micro-, and invasive treatment) initiated at different cutoffs (treating all or only dentinal lesions) in populations with low or high caries prevalence. A Markov model was constructed to follow an occlusal surface in a permanent molar in an initially 12-y-old male German patient over his lifetime. Prevalence data and transition probabilities were extracted from the literature, while validity parameters of different methods were synthesized or obtained from systematic reviews. Microsimulations were performed to analyze the model, assuming a German health care setting and a mixed public-private payer perspective. Radiographic and fluorescence-based methods led to more overtreatments, especially in populations with low prevalence. For the latter, combining visual-tactile or radiographic detection with microinvasive treatment retained teeth longest (mean 66 y) at lowest costs (329 and 332 Euro, respectively), while combining radiographic or fluorescence-based detections with invasive treatment was the least cost-effective (<60 y, >700 Euro). In populations with high prevalence, combining radiographic detection with microinvasive treatment was most cost-effective (63 y, 528 Euro), while sensitive detection methods combined with invasive treatments were again the least cost-effective (<59 y, >690 Euro). The suitability of detection methods differed significantly between populations, and the cost-effectiveness was greatly influenced by the treatment initiated after lesion detection. The accuracy of a detection method relative to a “gold standard” did not automatically convey into better health or reduced costs. Detection methods should be evaluated not only against their criterion validity but also the long-term effects resulting from their use in different populations.
Keywords: dental caries, fluorescence, health economics, Markov process, prevalence, radiography
Introduction
With a decreasing prevalence of cavitated caries lesions in most highly developed countries (Marthaler 2004), the focus of caries detection and treatment has shifted from cavitated to less advanced lesion stages (Ismail et al. 2007). To detect such lesions, dentists are often relying on technical aids, for example, bitewing radiography or laser fluorescence–based methods, with supposedly increased validity for detecting noncavitated lesions (Bader et al. 2001; Lussi et al. 2001), facilitating early treatment of caries. Thus, the costs associated with treating advanced lesions might be avoided or postponed, which could compensate for the possibly higher costs of the initial diagnostic process (Schwendicke et al. 2014).
However, as with all diagnostic tools, these aids nevertheless have limited sensitivity and specificity, leading to under- and overdiagnosis (Baelum et al. 2012). The relative proportion of such misdiagnoses depends on both the used cutoff for treatment initiation (e.g., treating only cavitated instead of all detected lesions) and the caries prevalence. The latter is increasingly polarized in many countries (Micheelis and Schiffner 2006; Pitts et al. 2011), thereby increasing or decreasing the predictive value of detection methods in different populations (Baelum et al. 2006; Baelum et al. 2012). Moreover, both long-term costs and health gains emanating from the detection of a lesion might depend on the subsequently performed treatment.
Occlusal surfaces are frequently afflicted by dental caries, with lesion detection being challenging for these surfaces (Bader et al. 2001; Marthaler 2004). Given the potential costs emanating from both controlling and managing these surfaces, comparative cost-effectiveness analyses for different combinations of detection and treatment strategies are relevant for clinical and nonclinical decision making. The aim of the present study was to assess the cost-effectiveness of 3 detection methods for occlusal caries in combination with different treatments initiated at different cutoffs in populations differing in their caries prevalence.
Materials and Methods
Model, Horizon, Setting, and Perspective
The present study used a Markov model (i.e., a stochastic state transition model) for comparing different combinations of detection and treatment strategies. Markov models consist of a finite number of health states, with patients, teeth, or tooth surfaces being initially placed in a certain health state, translating to other health states according to transition probabilities within given time periods (i.e., cycles) (Briggs and Sculpher 1997). The model we constructed allowed the occlusal surface of a permanent molar in a 12-y-old male patient (from age 12 y) to be followed over his remaining lifetime (TreeAge Pro 2013; TreeAge Software, Williamstown, MA). A mixed public-private perspective within German health care was chosen (see below). The sequence of events was constructed according to current evidence and the consultation of an expert panel (FS, HM-L, SP).
Comparators
We modeled only complications related to dental caries and compared 4 detection strategies within the context of German health care:
Biannual visual-tactile caries detection, including only visual, additional tactile, or combinations of visual and tactile assessment. We did not specify how visual or tactile assessment was to be performed; the resulting uncertainties were assessed using sensitivity analyses. Note that this lack of a uniform definition does not allow generalizing our results for all visual-tactile methods (Bader et al. 2001).
Biannual visual-tactile plus 2-yearly radiographic caries detection using intraoral (i.e., bitewing or periapical) radiographs. Again, we combined available validity data for different systems (e.g., analog, digital, etc.) (Bader et al. 2001).
Biannual visual-tactile plus biannual laser fluorescence–based caries detection. There are several commercial devices available for fluorescence-based caries detection (Gimenez et al. 2013). We modeled the use of only 1 system (DIAGNOdent Pen; Kavo, Biberach, Germany), since there were sufficient validity data available for this device.
Biannual visual-tactile and additional use of fluorescence-based detection only for surfaces deemed suspicious. Only if fluorescence-based detection confirmed the positive finding, a lesion was assumed to be present.
Detection methods were then combined with 1 of 2 cutoffs, determining when to initiate treatment:
treating only lesions assumed to extend into dentin or
treating all detected lesions. The assumption of a lesion reaching the dentin or not was based on the detection method used, not the standard against which the method was validated.
Eventually, 1 of 3 different treatments for a detected lesion was assigned:
Noninvasive treatment via topical fluoridation. We did not specify which kind of fluoride application was performed (e.g., gel, varnish) but simulated an “average” effect of noninvasive treatment. Note that this average effect might be achieved not only by fluoridation but also by oral hygiene counseling and so forth. We assumed noninvasive treatment to be performed by biannual fluoride application.
Microinvasive treatment via caries sealing using resin-based or glass ionomer sealants. On the basis of existing meta-analyses, we assumed 40% of sealant to require reapplication over 2 y (Kühnisch et al. 2010; Mickenautsch and Yengopal 2013).
Invasive treatment using an adhesive 1-surface composite restoration.
By combining 4 detection strategies, 2 cutoffs, and 3 treatment strategies, a total of 24 strategy combinations were compared. We did not evaluate further treatment options such as preventive resin restorations, direct restoration using glass ionomer cements or amalgams, or the immediate placement of indirect restorations.
Assumptions
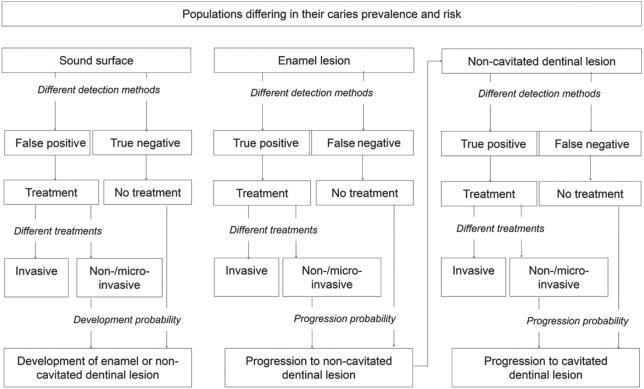
We did not simulate different jaws or teeth and did not combine different surfaces on the same tooth. However, follow-up treatments could involve more than the single surface. The model (Fig. 1) was based on several assumptions:
Figure 1.
State transition diagram. Different health states are represented by boxes. Based on prevalence, occlusal surfaces were assumed to be either sound or carious extending into enamel or dentin. Depending on the chosen detection strategy and the defined cutoff to initiate treatment (all lesions or only dentinal lesions), surfaces were detected as true or false positive or negative. For positively diagnosed surfaces, 1 of 3 treatments was allocated. For unrestored surfaces, caries development or progression was simulated based on evidence-based transition probabilities. Enamel lesions were assumed to progress to noncavitated dentinal lesions, and the latter were assumed to progress to cavitated lesions, which were assumed to be restored.
At the start of the simulation, the surface could be sound or carious, depending on the caries prevalence within the modeled population. To decide if a surface was sound or carious (i.e., to determine the prevalence), we used visual-tactile detection methods (see below).
If the surface was sound, a lesion could develop with a certain probability, depending on the simulated population and a possibly allocated treatment (see below).
If the surface was carious and not treated invasively, the lesion progressed with a certain probability, again depending on the simulated population and a possibly allocated treatment (see below).
Initially present or newly developing lesions were either confined to enamel or extended into dentin. Enamel lesions progressed to dentinal lesions with a certain probability. If the latter progressed further, cavitation was assumed, which always resulted in detection and subsequent restorative treatment (see below).
Invasive and follow-up treatments failed with certain probabilities, leading, for example, to endodontic treatment or crown placement. Repeated failure could eventually result in extraction of the tooth (Schwendicke et al. 2013).
Model validation was performed by plausibility controls, that is, internally via varying key parameters to check their impact on the results and externally by an experienced health economist (MS).
Populations and Caries Prevalence
Simulations were performed for 2 populations:
One population with low caries prevalence and low risk of sound surfaces to develop a lesion or existing lesions to progress.
One population with high prevalence and high risk of development or progression. Note that within our study, both caries activity (i.e., the predicted progression probability of lesion) and caries risk (i.e., the predicted risk of a patient to develop a new lesion) (Nyvad and Fejerskov 1997) were assumed to be a function of the simulated population.
In addition, we assumed these populations to either biannually receive baseline preventive means (i.e., noninvasive treatment via application of fluoride varnish) up to age 18 y (KZBV 2013) or not (i.e., sound surfaces only received noninvasive treatment after false-positive diagnoses).
To determine the proportion of initially carious surfaces (“surface prevalence”), we estimated both the caries experience and the proportion of children without any caries lesions reported for Germany, assuming 4 occlusal surfaces being at risk (Micheelis and Schiffner 2006; Bissar et al. 2007). The probability of a sound surface developing a lesion or a carious lesion to progress was adjusted for the high-prevalence population, assuming a 2.5-fold increase (Mejàre et al. 1999; Julihn et al. 2006) (Table 1).
Table 1.
Input Parameters.
| Enamel Lesions | Dentinal Lesions | |
|---|---|---|
| Prevalence | ||
| Low prevalencea | 0.39 (i.e., 0.0975) per surface | 0.09 (i.e., 0.0225) per surface |
| High prevalencea | 0.59 per surface | 0.14 per surface |
| Sensitivity and specificity | ||
| Sensitivity visual-tactileb | 0.199 (0.143–0.265) | 0.397 (0.355–0.440) |
| Specificity visual-tactile | 0.900 (0.865–0.928) | 0.969 (0.958–0.977) |
| Sensitivity radiographicb | 0.242 (0.111–0.423) | 0.531 (0.492–0.570) |
| Specificity radiographicb | 0.774 (0.687–0.847) | 0.899 (0.873–0.921) |
| Sensitivity fluorescence basedc | 0.749 (0.715–0.781) | 0.724 (0.672–0.772) |
| Specificity fluorescence basedc | 0.813 (0.751–0.873) | 0.718 (0.683–0.751) |
| Probability of lesion development | ||
| If untreatedd | RR = 1.26 (1.24–1.29) compared with noninvasive | |
| If noninvasively treatede | p(c) = 3.48a−1.2 | |
| If microinvasively treatedf | RR = 0.24 (0.12–0.45) compared with noninvasive | |
| Probability of lesion progression | ||
| Progression to | Dentinal lesions | Deep dentinal lesions |
| If untreatedg | p(c) = 0.045 (0.012–0.27) | p(c) = 0.450 (0.090–0.800) |
| If noninvasively treatedh | p(c) = 0.016 (0.013–0.042) | p(c) = 0.0470 |
| If microinvasively treatedi | p(c) = 0.014 (0–0.042) | p(c) = 0.0380 (0.020–0.060) |
Analyses were performed for populations with low and high caries prevalence. Sensitivity and specificity of different detection methods were synthesized or derived from existing meta-analyses. For initially sound surfaces, the per-cycle probability—p(c)—of developing a lesion was first estimated for noninvasively treated lesions and then adjusted for untreated or microinvasively treated lesions. Similarly, the progression probabilities of un-, non-, or microinvasively treated lesions were estimated. Both development and progression probabilities were calculated according to patient’s age (a) using hazard functions. If possible, we calculated mean values and 95% confidence intervals or ranges (in parentheses) to allow random sampling of estimates during microsimulation.
Prevalence was estimated from the number of patients without any caries experience at enamel or dentinal level, as assessed within the Fourth German Oral Health Study (Micheelis and Schiffner 2006) and adjusted for populations with higher prevalence (Bissar et al. 2007). For low-prevalence populations, we assumed only 1 surface at risk to be carious (Micheelis and Schiffner 2006), while for populations with high risks, all surfaces at risk were assumed to bear caries lesions. Note that this does not necessarily reflect the “true” prevalence but allows simulating possible ranges of caries prevalence.
Based on data from a systematic review regarding caries detection methods (Bader et al. 2001). For details, see Appendix Table 1.
Based on a recent meta-analysis (Gimenez et al. 2013). We used data for the pen only.
Based on reported efficacy of topical fluoride application compared with no or placebo treatment to prevent development of caries lesions (Marinho et al. 2003). Note that different fluoride applications (varnish, gel) have different preventive fractions. The used effect estimate thus represents an “average” efficacy.
Calculated as previously described (Schwendicke et al. 2014) using data from a Swedish cohort study of noninvasively treated adolescents (Mejàre et al. 2004). Since data were not reported separately for enamel and dentinal lesions, we assumed that half of the lesions would have been extending into enamel and the other half into dentin at the beginning of the simulation. The hazard function was chosen and adjusted according to the best fit.
Based on long-term effects (>5 y) of fissure sealing on caries incidence as reported within the latest Cochrane Review (Ahovuo-Saloranta et al. 2013). Note that we calculated this probability relative to the probability for noninvasively treated surfaces.
Based on untreated control groups from studies included within a systematic review regarding the efficacy of caries sealing (Griffin et al. 2008) as well as 2 further studies (Borges et al. 2012; da Silveira et al. 2012).
The probability of an enamel lesion progressing within a 6-monthly cycle was calculated based on 2 studies (Flório et al. 2001; Liu et al. 2012). Similar data for dentinal lesions were calculated from a Swedish cohort study of 14-y-olds (Ridell et al. 2008).
Progression probabilities of sealed enamel and dentinal lesions were calculated based on a systematic review (Griffin et al. 2008) or, in case of dentinal lesions, therein included studies (Frencken et al. 1998; Borges et al. 2012; da Silveira et al. 2012).
Validity Parameters and Transition Probabilities
Validity parameters were extracted from existing systematic reviews. Since there were no meta-analyses available for visual-tactile and radiographic detection, data from studies included in 1 systematic review (Bader et al. 2001) were meta-analyzed (Appendix). Transition probabilities of lesion development and progression were estimated for noninvasively treated surfaces and adjusted for untreated and microinvasively treated surfaces. If possible, we simulated age-dependent transition probabilities to account for the variability of caries rates in different ages. Eventually, allocation probabilities (i.e., allocating a tooth to a certain treatment after complications) were estimated from reviewed studies, with final consensus obtained by the panel (Table 1, Appendix).
Cost Estimation, Currency, and Discount Rate
A mixed public-private payer perspective was chosen, being characteristic in German health care. Costs were calculated in Euro based on the Public and Private Dental Fee Catalogues, Bemessungsmaßstab (BEMA), and Gebührenordnung für Zahnärzte (GOZ) (KZBV 2013). BEMA defines fee items within the public insurance, with only a few treatments not being covered or fully reimbursed. For these items, calculation was based on GOZ. Since factoring of chargeable item points is common to determine prices of private treatment in Germany, the standard multiplication factor (×2.3) was used. Items were restricted in number and character to reflect cost limitations and awareness (Appendix). Future costs were discounted at 3% per annum, as recommended for Germany (IQWIG 2009).
Health Outcome and Analyses
The health outcome (i.e., effectiveness) was measured as the time a tooth was retained. To analyze the model, we performed Monte Carlo microsimulations (i.e., stochastic tooth-level simulations), with a cohort of 1,000 independent surfaces being followed over the patient’s lifetime using 6-monthly cycles (Appendix). To introduce parameter uncertainty, we randomly sampled transition probabilities from a triangular distribution between the calculated 95% confidence interval (CI) or the range of parameters (Briggs et al. 2002). Mean point estimates for costs (c, in Euro) and effectiveness (e, in y) as well as incremental cost-effectiveness ratios (ICERs, Δc/Δe) were calculated (Drummond et al. 2005). In addition, the net benefit of each strategy combination was calculated using the following formula:
with λ denoting the ceiling threshold of willingness to pay, that is, the additional costs a decision maker is willing to sacrifice for gaining an additional unit of effectiveness (Drummond et al. 2005). If λ > Δc/Δe, an alternative intervention is considered more cost-effective than the comparator despite possibly being more costly (Briggs et al. 2002). Using this approach, we plotted the probability of being cost-effective against different λ. Finally, uni- and bivariate sensitivity analyses were performed.
Results
In populations with high prevalence, there were generally less over- and more undertreatments (Table 2). Initiating treatment for all detected lesions instead for only those lesions assumed to reach the dentin was associated with more over- and fewer undertreatments. Visual-tactile detection generally led to fewer and radiographic detection to more overtreatments, respectively. Compared with only visual-tactile detection, additional selective fluorescence-based detection for suspicious lesions was found to generate minimally fewer undertreatments and similar proportions of overtreatments.
Table 2.
Proportion of Over- and Undertreatments per Total Diagnoses.
| % Overtreatments |
% Undertreatments |
|||||||
|---|---|---|---|---|---|---|---|---|
| Detection Method | Treatment | Cutoff (Lesions) | Low Prevalence | High Prevalence | Mean | Low Prevalence | High Prevalence | Mean |
| Visual-tactile | Noninvasive | All | 32 | 22 | 27 | 1 | 8 | 4 |
| Noninvasive | Dentin | 11 | 7 | 9 | 6 | 35 | 20 | |
| Microinvasive | All | 32 | 26 | 29 | 1 | 7 | 4 | |
| Microinvasive | Dentin | 11 | 7 | 9 | 5 | 34 | 20 | |
| Invasive | All | 30 | 10 | 20 | 4 | 19 | 11 | |
| Invasive | Dentin | 10 | 4 | 7 | 17 | 55 | 36 | |
| Radiographic | Noninvasive | All | 70 | 49 | 60 | 0 | 2 | 1 |
| Noninvasive | Dentin | 33 | 22 | 27 | 7 | 35 | 21 | |
| Microinvasive | All | 71 | 58 | 65 | 0 | 2 | 1 | |
| Microinvasive | Dentin | 37 | 23 | 30 | 9 | 32 | 21 | |
| Invasive | All | 63 | 21 | 42 | 2 | 7 | 5 | |
| Invasive | Dentin | 28 | 5 | 17 | 36 | 62 | 49 | |
| Fluorescence based | Noninvasive | All | 65 | 44 | 54 | 0 | 0 | 0 |
| Noninvasive | Dentin | 53 | 35 | 44 | 6 | 34 | 20 | |
| Microinvasive | All | 62 | 45 | 54 | 0 | 0 | 0 | |
| Microinvasive | Dentin | 53 | 38 | 46 | 6 | 31 | 19 | |
| Invasive | All | 46 | 5 | 25 | 0 | 0 | 0 | |
| Invasive | Dentin | 45 | 5 | 25 | 50 | 67 | 59 | |
| Selective fluorescence based | Noninvasive | All | 32 | 24 | 28 | 1 | 8 | 4 |
| Noninvasive | Dentin | 11 | 7 | 9 | 6 | 36 | 21 | |
| Microinvasive | All | 32 | 24 | 28 | 1 | 6 | 3 | |
| Microinvasive | Dentin | 11 | 7 | 9 | 6 | 35 | 20 | |
| Invasive | All | 31 | 14 | 23 | 2 | 15 | 9 | |
| Invasive | Dentin | 10 | 4 | 7 | 14 | 50 | 32 | |
We compared different combinations of detection and treatment strategies, with treatments being initiated for all lesions or only dentinal lesions. Analyses were performed separately for populations with low and high caries prevalence. To allow comparison across subgroups, means were calculated. Note that while the validity of a detection method does not change if different treatments are performed, the chances of over- and underdiagnoses are affected, since lesions remain unrestored for different time periods, thus affecting the total number of diagnoses.
In populations with low prevalence, visual-tactile or radiographic detection combined with microinvasive treatment showed the highest cost-effectiveness, with treating only dentinal lesions being more cost-effective than treating all detected lesions (Appendix). These combinations allowed retaining teeth nearly lifelong (66 y) at lowest costs (329–333 Euro). Costs and effectiveness were generally significantly increased and decreased, respectively, for teeth in populations with high prevalence, with radiographic detection followed by microinvasive treatment (e = 63 y, c = 529 Euro) being the least costly strategy combination, and fluorescence-based and visual-tactile detections plus microinvasive treatments being similarly effective but slightly more expensive (542 and 549 Euro, respectively). Regardless of the population, combinations involving invasive treatments were found the least cost-effective, retaining teeth for a shorter time (up to −11%) at significantly increased costs (up to +133%). Generally providing preventive means regardless of any caries detection was found to minimally improve cost-effectiveness.
To control the probability of strategy combinations being cost-effective at different ceiling values, we performed net-benefit analyses (Fig. 2a, b). None of the analyzed strategy combinations had a probability >40% of being the most cost-effective option, reflecting the uncertainty of input parameters and the limited cost-effectiveness differences between those strategy combinations not involving invasive treatments.
Figure 2.
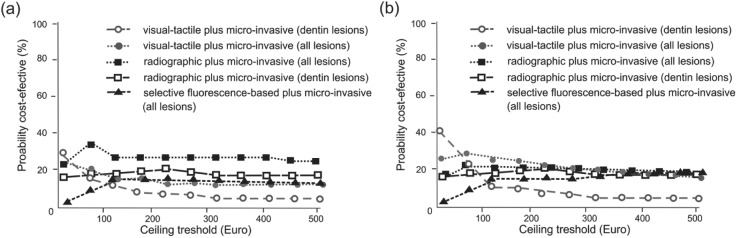
Cost-acceptability curves. For each strategy, the probability of being cost-effective is plotted against a ceiling threshold value, reflecting the maximum a decision maker is willing to invest to achieve an additional unit of effectiveness (Briggs et al. 2002). By increasing the ceiling value, the higher treatment costs of a more effective option become less important, and its probability of being cost-effective increases. We performed cost-acceptability analyses only for populations with several nondominated strategy combinations. (a) For populations with low prevalence and without baseline prevention, combining visual-tactile detection with microinvasive treatment initiated only for lesions into dentin was the least costly strategy combination, while treating all radiographically detected lesions had the highest probability of being acceptable regarding its cost-effectiveness above a ceiling value threshold of 4.41 Euro. (b) For populations with low prevalence and receiving baseline prevention during adolescence, combining visual-tactile detection with microinvasive treatment initiated only for lesions into dentin was again the least costly option, while using the same strategy but treating all detected lesions was probably more acceptable for decision makers willing to invest above 33.29 Euro. For higher ceiling thresholds, several strategies showed similar cost-effectiveness. No strategy combination had a probability >40% of being the most cost-effective choice, indicating substantial uncertainty. Strategy combinations with probabilities not exceeding 10% are not shown.
Changing the reexamination intervals from 6 to 24 mo increased the cost-effectiveness of most strategies, with decreased costs and increased effectiveness especially for those combinations involving invasive treatments (up to −70 Euro and +0.8 y). Only few strategy combinations were more cost-effective if examinations were performed more frequently. If populations with high prevalence were reexamined only every second year, combining fluorescence-based detection with microinvasive treatment was also found cost-effective (Appendix).
Further sensitivity analyses found that increasing the sensitivity and specificity of visual-tactile detection, for example, when using the International Caries Detection and Assessment System (ICDAS) (Jablonski-Momeni et al. 2012; Mitropoulos et al. 2012), did not significantly change the cost-effectiveness estimates. Similarly, decreasing the costs of noninvasive or minimal-invasive treatments to 25% of the original costs did not significantly change our estimates. Estimating costs solely based on the private fee catalogue did not significantly change the cost-acceptability, with the strategy ranking not being affected and absolute costs being only minimally changed (−2% to −5%). Last, varying the proportion of sealants requiring resealing within 2 y between 10% and 100% did also not significantly affect our results.
Discussion
Caries detection methods might no longer be recommended or rejected based only on their diagnostic (i.e., criterion) validity but also their effect on treatment allocation and the resulting health gains and long-term costs in different populations (Nyvad and Fejerskov 1997; Baelum et al. 2006; Pitts et al. 2011; Baelum et al. 2012). On one hand, additional detection methods might improve and objectify lesion detection (Lussi et al. 2001; Diniz et al. 2012), while on the other hand, such methods are prone to overdiagnose (Nyvad and Fejerskov 1997; Pereira et al. 2009; Baelum et al. 2012). However, classifying a diagnosis as being “correct” relies on a gold standard, which might be both clinically unavailable and irrelevant, given that such standard itself might not detect “the truth” and does not necessarily translate into a better treatment decision: the assumed status of a tooth surface might be less important than what is done based on that knowledge (Baelum et al. 2012), which is why the present study evaluated combinations, not single strategies, of caries detection and treatment. We showed that the harm stemming from overdiagnosis strongly depends on the allocated treatment, with methods prone to overdiagnoses still being cost-effective as long as noninvasive or microinvasive treatments were applied. Thus, overdiagnoses did not automatically start the “death spiral” of restorations (Qvist 2008; Schwendicke et al. 2013), and additional costs of, for example, inadvertently sealing occlusal surfaces were compensated by avoided or postponed follow-up treatments.
We found visual-tactile and radiographic detection of lesions cost-effective in populations with low and high caries prevalence and risk, respectively. It should be highlighted that we used average validity parameters for both detection methods, with supporting studies using different references, cutoffs, and settings. Furthermore, these studies involved different, sometimes outdated or questionable assessments systems (Bader et al. 2001). While this likely reflects the clinical reality in primary care dentistry, newer and possibly more advanced visual-tactile methods might have improved validity (Baelum et al. 2012). However, we controlled for such theoretically improved validity, with only marginally changed results when simulating the use of, for example, the ICDAS. In addition, performing fluorescence-based assessment only for suspicious surfaces did convey only minimal diagnostic benefit and was not cost-effective due to the additionally generated costs of using fluorescence-based methods.
In contrast, costs for radiographic detection were assumed to be relatively low per tooth, since not one but several teeth would be examined synchronously. Both these assumptions and the resulting cost-estimates, however, apply for German health care only, and despite our results being robust regardless of a patient’s insurance status, our cost assumptions might not hold true under different settings. This, however, applies to all such analyses, and given the magnitude of differences between the most and the least advantageous strategies, it is unlikely that potential cost-differences will significantly change or even reverse our cost-effectiveness rankings.
Based on our findings, all detection methods might yield similar effectiveness in low-risk populations as long as non- or microinvasive treatments are initiated after detecting a lesion. This was reflected by cost-effectiveness acceptability analyses as well. For populations with high prevalence, more sensitive methods seem to convey some benefit, with radiographic- but also fluorescence-based detections being effective. Given the potential harm caused by radiography, which we did not account for (by assigning costs to those harms, etc.), fluorescence-based methods could even be preferable.
Our study has several limitations. First, while our assumptions regarding the validity of different methods were usually based on broad and systematic evidence, the quality of this evidence was limited. Studies were not directly comparative but obtained from different single trials, with risk of selection and performance bias (Higgins and Green 2011). Second, we assessed the cost-effectiveness of sequential combinations of detection methods but did not simulate the complex integration of different detections into a diagnosis or treatment decision, partially since there were insufficient data for such analysis. Moreover, the chosen detection methods were performed regardless of patients’ age (i.e., the use of different methods in younger and older patients was not simulated). Third, our approach of modeling one surface per patient certainly does not reflect the clinical reality but was chosen due to limitations in data availability and technical feasibility. Considering the effects of intramouth correlation, we expect our results to underestimate rather than overestimate the true range of costs and effectiveness. Fourth, we did not simulate the effects of varying reliability of different detection methods and did not integrate lesion activity assessment into the diagnostic process. However, we integrated parameter uncertainty into the model, reflecting between-study reliability, as reported especially for fluorescence detection methods (Lussi et al. 2001; Pereira et al. 2009; Diniz et al. 2012). Such uncertainty was subsequently reflected by the certainty of our ranking. Fifth, the simulated prevalence was obtained from studies that themselves had used visual-tactile methods to detect caries lesions and should therefore not being considered “the truth,” which remains relative and therefore difficult to assess. We have, however, demonstrated the impact of varying caries prevalence and risk. Last, the chosen health outcome (years of tooth retention) can be criticized as having only limited impact on patients: however, performing cost-benefit or cost-utility analyses was not possible, since the subjective value or impact of retaining a single posterior tooth is currently unknown and might also differ between patients or countries. Multinational, long-term studies with patient-centered outcomes (quality-adjusted life years, willingness to pay) will be required to determine such values. This should also support the decision as to which perspective (patient centered, mixed patient-insurer centered, etc.) yields the most meaningful results regarding both costs and health effects and might also indicate how to integrate aspects of discomfort or pain occurring during treatment and so on.
In conclusion, using more sensitive methods to detect occlusal caries lesions increases the chance of overdiagnoses, especially in populations with low caries prevalence. Within the limitations of this study, the different detection methods generated only limited differences of cost-effectiveness, with more sensitive methods being moderately advantageous in populations with high caries prevalence and risk. More important, performing micro- or noninvasive instead of invasive treatments after detecting a lesion was found to greatly influence tooth retention and long-term costs. Caries detection methods should be evaluated not only for their criterion validity but also for the long-term health and cost effects resulting from their use in different populations.
Author Contributions
F. Schwendicke, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M. Stolpe, contributed to design, data acquisition, analysis, and interpretation, critically revised the manuscript; H. Meyer-Lueckel, contributed to data interpretation, critically revised the manuscript; S. Paris, contributed to design and data interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was funded by the authors’ institutions.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahovuo-Saloranta A, Forss H, Walsh T, Hiiri A, Nordblad A, Mäkelä M, Worthington HV. 2013. Sealants for preventing dental decay in the permanent teeth. Cochrane Database Syst Rev. 3:CD001830. [DOI] [PubMed] [Google Scholar]
- Bader J, Shugars D, Bonito A. 2001. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 65(10):960–968. [PubMed] [Google Scholar]
- Baelum V, Heidmann J, Nyvad B. 2006. Dental caries paradigms in diagnosis and diagnostic research. Eur J Oral Sci. 114(4):263–277. [DOI] [PubMed] [Google Scholar]
- Baelum V, Hintze H, Wenzel A, Danielsen B, Nyvad B. 2012. Implications of caries diagnostic strategies for clinical management decisions. Commun Dent Oral Epidemiol. 40(3):257–266. [DOI] [PubMed] [Google Scholar]
- Bissar AR, Oikonomou C, Koch MJ, Schulte AG. 2007. Dental health, received care, and treatment needs in 11- to 13-year-old children with immigrant background in Heidelberg, Germany. Int J Paediatr Dent. 17(5):364–370. [DOI] [PubMed] [Google Scholar]
- Borges BC, de Souza Borges J, Braz R, Montes MA, de Assunção Pinheiro IV. 2012. Arrest of non-cavitated dentinal occlusal caries by sealing pits and fissures: a 36-month, randomised controlled clinical trial. Int Dent J. 62(5):251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs A, Sculpher M. 1997. Commentary: Markov models of medical prognosis. BMJ. 314(7077):354–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs AH, O’Brien BJ, Blackhouse G. 2002. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Publ Health. 23(1):377–401. [DOI] [PubMed] [Google Scholar]
- da Silveira AD, Borges BC, de Almeida Varela H, de Lima KC, Pinheiro IV. 2012. Progression of non-cavitated lesions in dentin through a nonsurgical approach: a preliminary 12-month clinical observation. Eur J Dent. 6(1):34–42. [PMC free article] [PubMed] [Google Scholar]
- Diniz MB, Boldieri T, Rodrigues JA, Santos-Pinto L, Lussi A, Cordeiro RC. 2012. The performance of conventional and fluorescence-based methods for occlusal caries detection: an in vivo study with histologic validation. J Am Dent Assoc. 143(4):339–350. [DOI] [PubMed] [Google Scholar]
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. 2005. Methods for economic evaluation of health care programmes. New York (NY): Oxford University Press. [Google Scholar]
- Flório FM, Pereira AC, de Castro Meneghim M, Ramacciato JC. 2001. Evaluation of non-invasive treatment applied to occlusal surfaces. J Dent Child. 68(5–6):326–331, 301. [PubMed] [Google Scholar]
- Frencken JE, Makoni F, Sithole WD, Hackenitz E. 1998. Three-year survival of one-surface ART restorations and glass-ionomer sealants in a school oral health programme in Zimbabwe. Caries Res. 32(2):119–126. [DOI] [PubMed] [Google Scholar]
- Gimenez T, Braga MM, Raggio DP, Deery C, Ricketts DN, Mendes FM. 2013. Fluorescence-based methods for detecting caries lesions: systematic review, meta-analysis and sources of heterogeneity. PLoS One. 8(4):e60421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin SO, Oong E, Kohn W, Vidakovic B, Gooch BF. CDC Dental Sealant Systematic Review Work Group, Bader J, Clarkson J, Fontana MR, Meyer DM, Rozier RG, Weintraub JA, Zero DT. 2008. The effectiveness of sealants in managing caries lesions. J Dent Res. 87(2):169–174. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S. editors. 2011. Cochrane handbook for systematic reviews of interventions. Version 5.10 (updated March 2011). Oxford (UK): The Cochrane Collaboration. [Google Scholar]
- IQWIG. 2009. Appraisal of recommendations by the scientific board of IQWiG regarding “Methods to assess cost-effectiveness in German Public Health Insurance” [in German]. Cologne (Germany): Institute for Quality and Economy in Health Services. [Google Scholar]
- Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, Pitts NB. 2007. The International Caries Detection and Assessment System (ICDAS): an integrated system for measuring dental caries. Community Dent Oral Epidemiol. 35(3):170–178. [DOI] [PubMed] [Google Scholar]
- Jablonski-Momeni A, Stucke J, Steinberg T, Heinzel-Gutenbrunner M. 2012. Use of ICDAS-II, fluorescence-based methods, and radiography in detection and treatment decision of occlusal caries lesions: an in vitro study. Int J Dent. 2012:371595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julihn A, Barr Agholme M, Grindefjord M, Modéer T. 2006. Risk factors and risk indicators associated with high caries experience in Swedish 19-year-olds. Acta Odontol Scand. 64(5):267–273. [DOI] [PubMed] [Google Scholar]
- Kühnisch H, Reichl F, Hickel R, Heinrich-Weltzien R. 2010. Guideline fissure sealing [Leitlinie Fissurenversiegelung] [accessed on 2014 Nov 4]. http://www.dgzmk.de/uploads/tx_szdgzmkdocuments/20100300_Langfassung_Fissurenversiegelung.pdf.
- KZBV. 2013. Catalogue of Fees [Gebührenverzeichnisse]. Berlin, Germany: KZBV. [Google Scholar]
- Liu BY, Lo ECM, Chu CH, Lin HC. 2012. Randomized trial on fluorides and sealants for fissure caries prevention. J Dent Res. 91(8):753–758. [DOI] [PubMed] [Google Scholar]
- Lussi A, Megert B, Longbottom C, Reich E, Francescut P. 2001. Clinical performance of a laser fluorescence device for detection of occlusal caries lesions. Eur J Oral Sci. 109(1):14–19. [DOI] [PubMed] [Google Scholar]
- Marinho VC, Higgins JP, Logan S, Sheiham A. 2003. Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 4:CD002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler TM. 2004. Changes in dental caries 1953–2003. Caries Res. 38(3):173–181. [DOI] [PubMed] [Google Scholar]
- Mejàre I, Källest C, Stenlund H. 1999. Incidence and progression of approximal caries from 11 to 22 years of age in Sweden: a prospective radiographic study. Caries Res. 33(2):93–100. [DOI] [PubMed] [Google Scholar]
- Mejàre I, Stenlund H, Zelezny-Holmlund C. 2004. Caries incidence and lesion progression from adolescence to young adulthood: a prospective 15-year cohort study in Sweden. Caries Res. 38(2):130–141. [DOI] [PubMed] [Google Scholar]
- Micheelis W, Schiffner U. 2006. Vierte Deutsche Mundgesundheits-Studie (DMS IV). Köln: Deutscher Ärzteverlag. [Google Scholar]
- Mickenautsch S, Yengopal V. 2013. Validity of sealant retention as surrogate for caries prevention: a systematic review. PLoS One. 8(10):e77103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitropoulos P, Rahiotis C, Kakaboura A, Vougiouklakis G. 2012. The impact of magnification on occlusal caries diagnosis with implementation of the ICDAS II Criteria. Caries Res. 46(1):82–86. [DOI] [PubMed] [Google Scholar]
- Nyvad B, Fejerskov O. 1997. Assessing the stage of caries lesion activity on the basis of clinical and microbiological examination. Commun Dent Oral Epidemiol. 25(1):69–75. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Eggertsson H, Martinez-Mier EA, Mialhe FL, Eckert GJ, Zero DT. 2009. Validity of caries detection on occlusal surfaces and treatment decisions based on results from multiple caries-detection methods. Eur J Oral Sci. 117(1):51–57. [DOI] [PubMed] [Google Scholar]
- Pitts N, Amaechi B, Niederman R, Acevedo AM, Vianna R, Ganss C, Ismail A, Honkala E. 2011. Global oral health inequalities: dental caries task group—research agenda. Adv Dent Res. 23(2):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvist V. 2008. Longevity of restorations: the ‘death spiral’. In: Fejerskov O, Kidd EA, editors. Dental caries: the disease and its clinical management. Oxford (UK): Blackwell Munksgaard; p. 444–455. [Google Scholar]
- Ridell K, Olsson H, Mejàre I. 2008. Unrestored dentin caries and deep dentin restorations in Swedish adolescents. Caries Res. 42(3):167–170. [DOI] [PubMed] [Google Scholar]
- Schwendicke F, Meyer-Lueckel H, Stolpe M, Dörfer CE, Paris S. 2014. Costs and effectiveness of treatment alternatives for proximal caries lesions. PLoS One. 9(1):e86992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendicke F, Stolpe M, Meyer-Lueckel H, Paris S, Dörfer CE. 2013. Cost-effectiveness of one- and two-step incomplete and complete excavations. J Dent Res. 90(10):880–887. [DOI] [PubMed] [Google Scholar]