Abstract
Various chemotherapeutic agents used in patients with hematopoietic malignancy cause serious side effects, including myelosuppression and immunosuppression. Immunosuppression makes patients more susceptible to infection, resulting in an increased risk of infectious complications, including the development of severe septicemia that may be life-threatening. It is necessary for dental staff to be familiar with an appropriate protocol in such cases and to share information about the chemotherapy with a hematologist. To verify the effectiveness of our dental intervention protocol, we conducted a prospective study on the incidence of complications for each myelosuppressive grade of chemotherapy in patients with hematopoietic malignancy. We compared the incidence of complications between treatment P (patients who finished all the dental treatments according to the protocol) and treatment Q (patients who did not) per grade (A, B, C, D) and incidence of systemic or oral findings. We also compared the incidence of oral complication related to the residual teeth between first chemo (patients who were undergoing chemotherapy for the first time) and prior chemo (not the first time). There were significant differences in inflammatory complications between treatment P and treatment Q. We found that both systemic and oral inflammatory complications increased with higher-grade myelosuppressive chemotherapy. Additionally, there was a significant difference between the incidence of oral complications related to the residual teeth between first chemo and prior chemo. Complete implementation of the dental intervention protocol was associated with fewer oral and systemic infectious and inflammatory complications in patients with hematopoietic malignancies undergoing chemotherapy. The incidence of oral and systemic complications also increased with grade of chemotherapy. These results support the validity of our dental intervention protocol. We should pay close attention to the oral state of de novo hematopoietic malignancy patients.
Keywords: chemotherapy, myelosuppressive grade, inflammation, dental treatment protocol, odontogenic infection, oral hygiene
Introduction
Numerous chemotherapeutic regimens for hematopoietic malignancies cause serious side effects, including myelosuppression and immunosuppression, making patients more susceptible to severe infection, such as life-threatening septicemia (Appelbaum 1996; Center for International Blood and Marrow Transplant Research et al. 2009). Important origins of infection are the apparent and inapparent endogenous foci of odontogenic lesions. In an immunosuppressive state, asymptomatic chronic foci easily convert to acute inflammatory lesions.
The maintenance of oral hygiene can reduce the risk of odontogenic infection. Also, any acute or chronic odontogenic foci should be eliminated in patients with hematopoietic malignancy so that their chemotherapeutic regimens can be completed. There are some issues, however—such as a time limitation before the initiation of chemotherapy and the onset of immunoinsufficiency because of untreated tumor volume (Elad et al. 2003; Akashi et al. 2013). To facilitate the dental intervention, an appropriate protocol is necessary for patients with hematopoietic malignancy.
We previously reported the grading of chemotherapy regimens according to their myelosuppressive intensity to enhance communication between medical and dental staff. Numerous chemotherapeutic regimens with various degrees of myelosuppression were classified into 4 categories (Akashi et al. 2013). We then formulated a novel protocol for dental intervention by modifying a protocol described by Yamagata et al. (2006) for patients who underwent hematopoietic stem cell transplantation. To verify our dental intervention protocol, we conducted the current prospective study, which evaluated the incidence of complications at each myelosuppressive grade of chemotherapy in patients with hematopoietic malignancy.
Methods
Patients
Eighty-six consecutive adult patients (52 men, 34 women) with hematopoietic malignancy were recruited for our prospective study. These subjects underwent chemotherapy, including hematopoietic stem cell transplantation at the Division of Medical Oncology/Hematology, Department of Medicine, Kobe University Hospital, between October 2012 and December 2013. Their median age was 60.5 y (range, 20 to 85 y). The numbers of patients according to the type of hematopoietic malignancy are shown in Table 1. Our institutional review board approved this study (No. 1325, 2012). Written informed consent was obtained from all subjects.
Table 1.
No. of Patients per Hematopoietic Malignancy.
| Disease | Male | Female |
|---|---|---|
| Acute myeloid leukemia | 13 | 4 |
| Acute lymphoblastic leukemia | 3 | 4 |
| Myelodysplastic syndrome | 5 | 2 |
| Chronic myeloid leukemia | 1 | 0 |
| Diffuse large B-cell lymphoma | 14 | 11 |
| Follicular lymphoma | 2 | 4 |
| Peripheral T-cell lymphoma | 4 | 1 |
| Hodgkin’s lymphoma | 1 | 1 |
| Adult T-cell leukemia/lymphoma | 2 | 0 |
| Multiple myeloma | 1 | 6 |
| Others | 6 | 1 |
Protocol for Dental Interventions
In each case, the hematologist informed the dentist of the patient’s diagnosis, the day on which chemotherapy was begun, and the intensity of the chemotherapy as classified into 1 of the 4 grades of our system (Appendix Table 1). The patient then underwent a clinical examination of the hard and soft oral tissues, supplemented by a radiographic survey by panoramic or periapical radiography. All patients were provided oral hygiene instruction by their dentist or dental hygienist with the goal of maintaining a plaque index < 20% (O’Leary et al. 1972). All dental diseases (caries, apical periodontitis, marginal periodontitis, impacted third molar) and poorly fitting dental prostheses (e.g., inlay, bridge, denture) were recorded in the patients’ charts.
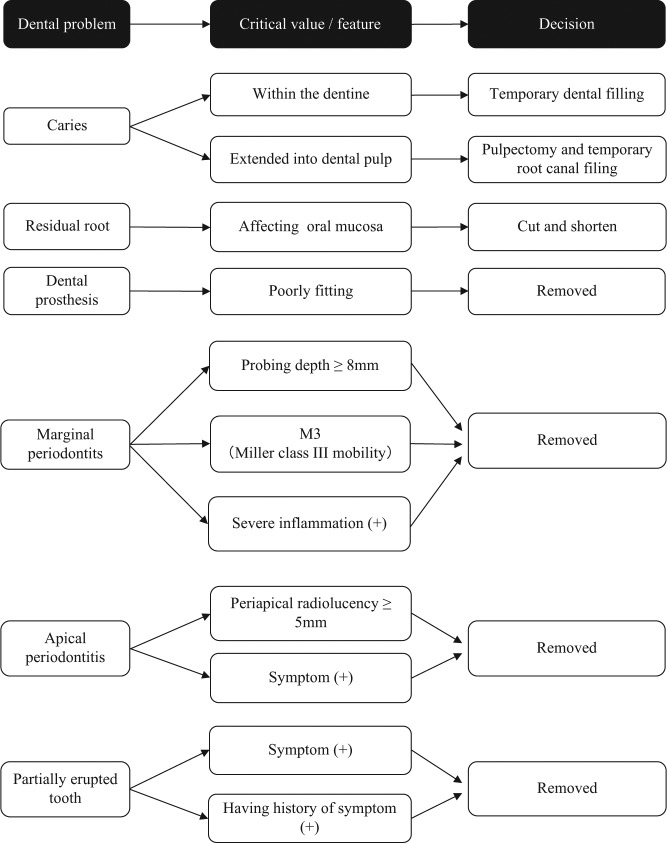
We established the dental treatment protocol shown in the Figure. For example, caries was treated as soon as possible to avoid any delay in chemotherapy initiation. If caries was confined within the dentine, it was removed and filled with a temporary dental filling. If caries extended into the dental pulp, we performed pulpectomy and temporary root canal filling. Residual root affecting the oral mucosa was cut or shortened. Poorly fitting dental prostheses (e.g., inlay, bridge, denture) were also removed when they harmed the oral mucosa or caused plaque retention. We also removed teeth with marginal periodontitis with severe gingival inflammation (pain, swelling, redness, pus discharge), a probing depth > 8 mm, or severe mobility measured as Miller class III (Miller 1985). Teeth without such signs or symptoms were not removed, and the patient was given tooth-brushing instructions, underwent scaling, and was given a periodontal antibiotic injection containing 1 mg of minocycline. In cases of apical periodontitis, teeth with apical symptoms or radiolucency with a maximum diameter of > 5 mm were removed in principle, whereas asymptomatic teeth with periapical radiolucency of < 5 mm were preserved. A partially erupted tooth (e.g., third molar) was extracted if there had been a history of pericoronitis, including pain, swelling, redness, or pus discharge. As a general rule, an antibiotic (penicillin or cephem) was administered intravenously 30 min before tooth extraction. If intravenous administration was impossible, oral administration was adopted. If a patient had an allergic reaction to penicillin or cephem, intravenous administration of clindamycin or a peroral macrolide was employed. After tooth extraction, the dentist asked the hematologist to wait a period of at least 1 wk after tooth extraction before starting the chemotherapy.
Figure.
Dental protocol for hematopoietic malignancy patients.
Subject Grouping
If chemotherapy with the same regimen was repeatedly performed in the same patient, each course was assessed as a single observation unit. The starting point of the observation was the day that chemotherapy started, and the end point was the day before the next round of chemotherapy started. If more than 4 wk elapsed from the end of the previous chemotherapy to start of the following chemotherapy round, the 28th day from the end of the previous chemotherapy was determined to be the end point of the observation unit.
In all subjects, before the beginning of the observation unit, a dentist evaluated the dental and oral status of the subject’s clinical examination and radiography. Each observation unit was then classified into 1 of 4 groups: First, the subjects were divided into 2 groups, treatment P and treatment Q. Treatment P was composed of patients who finished all the dental treatments according to the protocol before the beginning of the observation unit. Treatment Q was composed of patients who had not completed all the dental treatments. Treatment P patients finished all the dental treatments under the protocol, whereas treatment Q patients still had odontogenic foci to be treated but underwent chemotherapy before completing the dental treatments, as the chemotherapy took priority because of the state of the hematopoietic malignancy. Each of the 2 groups (treatment P and treatment Q) was partitioned into 2 further categories: first chemo and prior chemo. Category First Chemo consisted of de novo patients, who were undergoing chemotherapy for the first time. Category Prior Chemo consisted of patients who had previously undergone some chemotherapy. Subjects were thus finally divided into 4 groups: P/first chemo, P/prior chemo, Q/first chemo, Q/prior chemo. After the dental treatments were completed, treatment Q patients were transferred to treatment P. Conversely, treatment P patients who had a new lesion to be treated were transferred to treatment Q. Therefore, the same subject was seen in more than 1 group depending on the treatment.
Clinical Findings
For each observation unit, the clinical findings were recorded from the day that chemotherapy began (stage 0) to the day of the nadir of the blood cell count (stage I). Systemic clinical findings were recorded—including body temperature, respiratory symptoms (cough or sputum), neutropenia, C-reactive protein (CRP) level, and blood culture results (blood culture is examined when the patient’s body temperature is more than 38°C). If respiratory symptoms were present, chest radiography was performed and the findings recorded. Oral clinical findings also were recorded, including redness, warmth, swelling, pus discharge, and pain (spontaneous, percussion, occlusal, or tenderness) in or around teeth or periapical/periodontal tissues. Trismus was diagnosed in cases where the distance between the upper and lower incisors during mouth opening was < 20 mm. If the chemotherapy regimen was behind schedule, the reason for the delay and the number of days were recorded.
The systemic findings at stage I were body temperature > 38°C, the presence of respiratory symptoms (cough or sputum), elevated CRP level (IATRO CRP; LSI Medience, Tokyo, Japan) over 1 mg/dL compared with that at stage 0, and positive blood culture results. The acceptable error range of the CRP is within 10% of the CRP value. Therefore, an increase in the CRP level of more than 1 mg/dL is considered beyond the acceptable error range when the value is < 10 mg/dL. When there was a new appearance or worsening of oral clinical findings in stage I, it was judged to be an oral positive finding.
The incidence of systemic and oral positive findings in each group and category (according to chemotherapy grade) was calculated. The incidence of oral positive findings of teeth that needed to be extracted but were left as they were (residual teeth) was also calculated according to chemotherapy grade, first and prior chemo, and the tooth eruption site.
Statistical Analysis
The data were analyzed with the SPSS 19 (IBM Corp., New York, NY, USA). Fisher exact tests were used. Values of P < 0.05 were considered statistically significant.
Results
There were 340 observations for the 86 consecutive subjects. Table 2 shows the incidences of systemic and oral positive findings for each group (treatment P/Q) and category (first chemo/prior chemo) according to chemotherapy grade.
Table 2.
Incidence of Systemic and Oral Positive Findings for Each Group and Category According to Chemotherapy Grade.
Positive systemic clinical findings were observed in 66 units and positive oral clinical findings in 43 units. Among the systemic clinical findings were 33 units with body temperature > 38°C, 2 units with respiratory symptoms, and 66 units with an increase in the CRP level > 1 mg/dL. Blood cultures were performed in 26 units, 6 with units showing positive results.
The incidences of systemic and oral positive findings for treatment P were 15.8% (37 of 234 units) and 2.9% (7 of 234 units), respectively, and those for treatment Q were 27.4% (29 of 106 units) and 34.0% (36 of 106 units), respectively. There were significant differences between treatments P and Q regarding the incidences of systemic positive findings (P = 0.01) and oral positive findings (P < 0.0001).
In a comparative study between treatment P and treatment Q according to chemotherapy grade, there were significant differences in the incidences of oral positive findings for chemotherapy grades B (P < 0.0001) and C (P = 0.0113) and the rate of systemic positive findings for grade B (P = 0.0193). Systemic and oral positive findings were absent for chemotherapy grade A in treatment P and treatment Q. For grade D chemotherapy, there were too few unit numbers to show a significant difference.
In a comparative study of each chemotherapy grade, there was a significant difference between grades A and C (P = 0.00597), A and D (P = 0.00479), B and C (P = 0.0120), and B and D (P = 0.0001) regarding the incidence of systemic positive findings in treatment P. The rates increased in accord with increases in the chemotherapy grade. A comparative study of first and prior chemo showed no significant differences in the incidence of systemic or oral positive findings.
Seventy-one teeth were extracted in this study (Table 3). None of the sockets showed postextraction infection or incomplete healing.
Table 3.
Data for Extracted Teeth and Residual Teeth by Chemotherapy Grade.
| Residuala
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| A |
B |
C |
D |
||||||
| Parameter | Extracted | n | % | n | % | n | % | n | % |
| Probing depth ≥ 8 mm | 34 | 16 (0) | 0 | 79 (25) | 31.6 | 23 (12) | 52.2 | — | |
| Miller class III | 4 | — | 4 (2) | 50.0 | — | — | |||
| Periapical radiolucency ≥ 5 mm | 2 | 10 (0) | 0 | 5 (3) | 60.0 | 3 (1) | 33.3 | — | |
| Inflammation/symptoms | 11 | — | 13 (7) | 53.8 | 9 (8) | 88.9 | 1 (1) | 100 | |
| Historical symptoms | 12 | — | 17 (4) | 23.5 | 2 (2) | 100 | — | ||
| Residual root | 8 | — | 23 (3) | 13.0 | — | — | |||
| Total no. | 71 | 26 | 141 | 37 | 1 | ||||
Parentheses and percentages indicate number and incidence of positive findings, respectively.
The number of residual teeth was 26 in patients with grade A chemotherapy, 141 with grade B, 37 with grade C, and 1 with grade D. Table 3 shows the incidences of oral positive findings of the residual teeth with a probing depth > 8 mm, severe mobility at Miller class III, radiolucency with a maximum diameter > 5 mm, inflammation or symptoms, historical symptoms, and residual root at each myelosuppressive grade. Tooth eruption sites were classified into 3 groups: anterior, premolar, and molar. Table 4 shows the incidences of oral positive findings of residual teeth for each tooth eruption site according to chemotherapy grade. There was a significant difference between the incidences of grades B and C (P = 0.000542).
Table 4.
Incidence of Oral Positive Findings on Residual Teeth by Tooth Eruption Site—According to Chemotherapy Grade and Category.
| Anterior |
Premolar |
Molar |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | n | % | n | % | n | % | n | % | |
| Grade | |||||||||
| A | 4 (0) | 0 | 4 (0) | 0 | 18 (0) | 0 | 26 (0) | 0 | |
| B | 32 (10) | 31.3 | 26 (4) | 15.4 | 83 (30) | 36.1 | 141 (44) | 31.2 | ** |
| C | 6 (2) | 33.3 | 8 (4) | 50.0 | 23 (17) | 73.9 | 37 (23) | 62.2 | |
| D | 0 (0) | 0 | 1 (1) | 100 | 0 (0) | 0 | 1 (1) | 100 | |
| Category | |||||||||
| First chemo | 15 (9) | 60.0 | 13 (5) | 38.5 | 45 (28) | 62.2 | 73 (42) | 57.5 | *** |
| Prior chemo | 25 (3) | 12.0 | 24 (3) | 12.5 | 76 (19) | 25.0 | 125 (25) | 20.0 | |
| Total | 40 (12) | 30.0 | 37 (8) | 21.6 | 121 (47) | 38.8 | 198 (67) | 33.8 | |
Parentheses and percentages indicate number and incidence of positive findings, respectively.
P < 0.001. *** P < 0.0001.
The incidence of oral positive findings in the residual teeth at each tooth eruption site was also calculated according to the first and prior chemo categories (Table 4). There was a significant difference between the incidences in the 2 categories (P < 0.0001). The incidence of oral positive findings in the residual teeth tended to increase according to the tooth groups (anterior < premolar < molar), but there was no significant difference.
None of the units showed interruption of chemotherapy or postponement of follow-up therapy because of odontogenic infection.
Discussion
In this study, there were significant differences in inflammation between the patients who had completed the chemotherapy protocol (treatment P) and those who had not (treatment Q). This result supports the validity of our dental intervention protocol. We also found that systemic and oral infectious findings increased according to progression of chemotherapy grades (A → D). Therefore, our grading system for chemotherapeutic regimens is useful for predicting the occurrence of infectious complications. We noted that oral infections were found more frequently in de novo hematopoietic malignancy patients.
It is still uncertain whether the criteria can be applied before induction or with intensive chemotherapy. Based on our system, chemotherapy for hematopoietic stem cell transplantation was graded D, which can be distinguished from induction or intensive chemotherapy, which is graded B or C. It is important to be attentive to dental patients undergoing grade B or C chemotherapy because most of the oral positive findings in this study were detected in those with grade B or C chemotherapy. Also, in our previous study, septic shock after chronic odontogenic infection was seen in 2 patients on chemotherapy: 1 with grade B and 1 with grade C chemotherapy (Akashi et al. 2013). Despite the lack of direct bacterial evidence from the blood culture results, it was assumed that the septic shock followed a chronic odontogenic infection. In a comparative study between treatments P and Q in the current study, there was a significant difference in the incidences of oral positive findings in patients on grade B (P < 0.0001) and grade C (P = 0.0113) chemotherapy. Therefore, our protocol is suitable for patients undergoing grade B or C chemotherapy. In contrast, the findings suggested that decisions regarding tooth extraction or dental treatment before grade A chemotherapy are not essential, because there were no cases with systemic or oral positive findings in either treatment P or Q patients who were on grade A chemotherapy.
A CRP increase > 1 mg/dL in this study was considered a positive systemic finding. The CRP level in periodontitis patients was significantly reduced by appropriate periodontal therapy (Iwamoto et al. 2003; Paraskevas et al. 2008). Mean CRP levels of generalized aggressive periodontitis, chronic generalized periodontitis, and nonperiodontitis were reported to be 7.49 ± 2.31 mg/L, 4.88 ± 1.80 mg/L, and 0.68 ± 0.23 mg/L, respectively (Chopra et al. 2012). A CRP increase > 1 mg/dL (10 mg/dL) may be helpful to detect even slight progression of inflammation.
Blood culture was performed in 26 units, and only 1 unit showed a positive result for Streptococcus mitis. In this unit from P/prior chemo with grade B chemotherapy, the patient’s body temperature rose to more than 38°C. An oral indigenous bacterium was detected in the blood culture, although there was no intraoral acute inflammatory lesion during the observation period. No other unit showed a positive blood culture result. This might be explained by the broad-spectrum antibiotic that was administrated before the blood culture examination.
Yamagata et al. (2006) recommended conservative treatments such as scaling for teeth with a probing depth < 8 mm. Others reported that no cases of severe chronic periodontitis—including a probing depth > 6 mm or radiographic crestal alveolar bone loss—resulted in interruption of chemotherapy because of acute symptoms or septicemia during the period of chemotherapy-induced myelosuppression (Toljanic et al. 1999; Akintoye et al. 2002). Boku et al. (1984) demonstrated that Gram-negative rods and filaments decreased after initial treatment for adult periodontitis in patients who had a probing depth of 4 to 8 mm. We adopted a probing depth > 8 mm as one of the criteria for tooth extraction without periodontal radiography. In our study, the incidence of oral positive findings in residual teeth with a probing depth > 8 mm was 36.3% (37 of 102 teeth) in patients on grade B or C chemotherapy. On the basis of the results, we recommend that teeth with a probing depth > 8 mm be extracted before chemotherapy except in patients on grade A chemotherapy. Further studies are needed, however, to definitively decide the probing depth criteria for tooth extraction at each chemotherapy grade.
In previous dental management protocol, teeth with periapical radiolucency < 5 mm were preserved (Yamagata et al. 2011). We did not treat asymptomatic apical lesions < 5 mm before chemotherapy. In our study, some asymptomatic apical lesions > 5 mm developed acute inflammation, although these teeth did not increase infectious complications. Teeth with periapical radiolucency with a maximum diameter > 5 mm should be extracted before chemotherapy (except grade A chemotherapy).
Fernandes et al. (2009) reported the incidence of symptoms arising in previously asymptomatic impacted third molars in otherwise healthy subjects. In all, 23.1% of partially erupted teeth and 10.5% of unerupted teeth became symptomatic during the 1-y study period. However, some reports mentioned that extraction of symptomatic third molars and nonintervention for asymptomatic ones are both safe (Tai et al. 1994; Yamagata et al. 2011). With our protocol, asymptomatic impacted third molars were preserved, none of which became acute.
Systematic oral assessment, plaque control, regular encouragement of patient self-care, and consistent oral care may be the most important factors in the prevention or amelioration of oral infection during chemotherapy (Bergmann 1988; Barker 1999; Raber-Durlacher et al. 2002; Elad et al. 2003; Melkos et al. 2003; Yamagata et al. 2006; Yamagata et al. 2011; Raber-Durlacher et al. 2013). Professional oral care decreases the microbial counts of the total oropharynx and can be an important strategy to prevent aspiration pneumonia (Ishikawa et al. 2008). In our study, all patients were instructed by either the dentist or the dental hygienist to improve oral hygiene to keep the level of O’Leary plaque control < 20%. None of these patients contracted aspiration pneumonia.
There was a significant difference between the incidence of oral positive findings on residual teeth in patients who underwent chemotherapy for the first time and those who had previously undergone chemotherapy. We reported that the period around primary chemotherapy for patients with hematologic malignancy is risky with regard to the development of odontogenic infection. These infections may become severe owing to the instability of the immune system caused by the myelosuppressive chemotherapy and the high rate of hematologic tumors (Akashi et al. 2013). Why the incidence of local positive findings on residual teeth is high in patients who undergo chemotherapy with a de novo regimen remains unclear. One reason may be that these patients had a larger number of malignant hematopoietic cells causing immunosuppression. Another may be that in a number of cases, the dental disease had not developed sufficiently to trigger the criteria for treatment before the onset of chemotherapy. Based on what we know, we should pay close attention to the oral state of patients, especially those who have a hematologic malignancy and are undergoing chemotherapy for the first time. We should recognize their oral state at least a week before first chemotherapy, cooperating more with hematologist.
In conclusion, the protocol and chemotherapy grading that we introduced in this study were considered useful. Further studies with a larger number of subjects are required for confirmation.
Author Contributions
K. Tsuji, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; Y. Shibuya, contributed to conception, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; M. Akashi, contributed to conception and data interpretation, drafted and critically revised the manuscript; S. Furudoi, A. Kawamoto, A. Okamura, H. Matsuoka, T. Komori, contributed to conception and data interpretation, critically revised the manuscript; K. Yakushijin, contributed to conception, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
The authors received no financial support and declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Akashi M, Shibuya Y, Kusumoto J, Furudoi S, Inui Y, Yakushijin K, Okamura A, Matsuoka H, Komori T. 2013. Myelosuppression grading of chemotherapies for hematologic malignancies to facilitate communication between medical and dental staff: lessons from two cases experienced odontogenic septicemia. BMC Oral Health. 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akintoye SO, Brennan MT, Graber CJ, McKinney BE, Rams TE, Barrett AJ, Atkinson JC. 2002. A retrospective investigation of advanced periodontal disease as a risk factor for septicemia in hematopoietic stem cell and bone marrow transplant recipients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 94(5):581–588. [DOI] [PubMed] [Google Scholar]
- Appelbaum FR. 1996. The use of bone marrow and peripheral blood stem cell transplantation the treatment of cancer. CA Cancer J Clin. 46(3):142–164. [DOI] [PubMed] [Google Scholar]
- Barker GJ. 1999. Current practices in the oral management of the patient undergoing chemotherapy or bone marrow transplantation. Support Care Cancer. 7(1):17–20. [DOI] [PubMed] [Google Scholar]
- Bergmann OJ. 1998. Oral infections and septicemia in immunocompromised patients with hematologic malignancies. J Clin Microbiol. 26(10):2105–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boku H, Kamiya H, Okuda K, Eguchi T, Hasegawa E, Yoshie H, Hara K. 1984. The effect of initial preparation on the composition of the subgingival microbial flora in adult periodontitis. Nihon Shishubyo Gakkai Kaishi. 26(2):329–338. [DOI] [PubMed] [Google Scholar]
- Center for International Blood and Marrow Transplant Research; National Marrow Donor Program; European Blood and Marrow Transplant Group; American Society of Blood and Marrow Transplantation; Canadian Blood and Marrow Transplant Group; Infectious Disease Society of America; Society for Healthcare Epidemiology of America; Association of Medical Microbiology and Infectious Diseases Canada; Centers for Disease Control and Prevention. 2009. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 44(8):453–558. [PubMed] [Google Scholar]
- Chopra R, Patil SR, Kalburgi NB, Mathur S. 2012. Association between alveolar bone loss and serum C-reactive protein levels in aggressive and chronic periodontitis patients. J Indian Soc Periodontol. 16(1):28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad S, Garfunkel AA, Or R, Michaeli E, Shapira MY, Galili D. 2003. Time limitations and the challenge of providing infection-preventing dental care to hematopoietic stem-cell transplantation patients. Support Care Cancer. 11(10):674–677. [DOI] [PubMed] [Google Scholar]
- Fernandes MJ, Ogden GR, Pitts NB, Ogston SA, Ruta DA. 2009. Incidence of symptoms in previously symptom-free impacted lower third molars assessed in general dental practice. Br Dent J. 207(5):E10. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Yoneyama T, Hirota K, Miyake Y, Miyatake K. 2008. Professional oral health care reduces the number of oropharyngeal bacteria. J Dent Res. 87(6):594–598. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Nishimura F, Soga Y, Takeuchi K, Kurihara M, Takashiba S, Murayama Y. 2003. Antimicrobial periodontal treatment decreases serum C-reactive protein, tumor necrosis factor-alpha, but not adiponectin levels in patients with chronic periodontitis. J Periodontol. 74(8):1231–1236. [DOI] [PubMed] [Google Scholar]
- Melkos AB, Massenkeil G, Arnold R, Reichart PA. 2003. Dental treatment prior to stem cell transplantation and its influence on the posttransplantation outcome. Clin Oral Investig. 7(2):113–115. [DOI] [PubMed] [Google Scholar]
- Miller PD., Jr. 1985. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 5(2):8–13. [PubMed] [Google Scholar]
- O’Leary TJ, Drake RB, Naylor JE. 1972. The plaque control record. J Periodontol. 43(1):38. [DOI] [PubMed] [Google Scholar]
- Paraskevas S, Huizinga JD, Loos BG. 2008. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 35(4):277–290. [DOI] [PubMed] [Google Scholar]
- Raber-Durlacher JE, Epstein JB, Raber J, van Dissel JT, van Winkelhoff AJ, Guiot HF, van der Velden U. 2002. Periodontal infection in cancer patients treated with high-dose chemotherapy. Support Care Cancer. 10(6):466–473. [DOI] [PubMed] [Google Scholar]
- Raber-Durlacher JE, Laheij AM, Epstein JB, Epstein M, Geerligs GM, Wolffe GN, Blijlevens NM, Donnelly JP. 2013. Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Support Care Cancer. 21(6):1621–1627. [DOI] [PubMed] [Google Scholar]
- Tai CC, Precious DS, Wood RE. 1994. Prophylactic extraction of third molars in cancer patients. Oral Surg Oral Med Oral Pathol. 78(2):151–155. [DOI] [PubMed] [Google Scholar]
- Toljanic JA, Bedard JF, Larson RA, Fox JP. 1999. A prospective pilot study to evaluate a new dental assessment and treatment paradigm for patients scheduled to undergo intensive chemotherapy for cancer. Cancer. 85(8):1843–1848. [PubMed] [Google Scholar]
- Yamagata K, Onizawa K, Yanagawa T, Hasegawa Y, Kojima H, Nagasawa T, Yoshida H. 2006. A prospective study to evaluate a new dental management protocol before hematopoietic stem cell transplantation. Bone Marrow Transplant. 38(3):237–242. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Onizawa K, Yanagawa T, Takeuchi Y, Hasegawa Y, Chiba S, Bukawa H. 2011. Prospective study establishing a management plan for impacted third molar in patients undergoing hematopoietic stem cell transplantation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 111(2):146–152. [DOI] [PubMed] [Google Scholar]