Abstract
Background
We aimed to examine whether pre-existing impaired estimated glomerular filtration rate (eGFR) and proteinuria were associated with mortality following community-acquired pneumonia or sepsis among people aged ≥65 years with diabetes mellitus, without end-stage renal disease.
Methods
Patients were followed up from onset of first community-acquired pneumonia or sepsis episode in a cohort study using large, linked electronic health databases. Follow-up was for up to 90 days, unlimited by hospital discharge. We used generalized linear models with log link, normal distribution and robust standard errors to calculate risk ratios (RRs) for all-cause 28- and 90-day mortality according to two markers of chronic kidney disease: eGFR and proteinuria.
Results
All-cause mortality among the 4743 patients with pneumonia was 29.6% after 28 days and 37.4% after 90 days. Among the 1058 patients with sepsis, all-cause 28- and 90-day mortality were 35.6 and 44.2%, respectively. eGFR <30 mL/min/1.73 m2 was a risk marker of higher 28-day mortality for pneumonia (RR 1.27: 95% CI 1.12–1.43) and sepsis (RR 1.32: 95% CI 1.07–1.64), adjusted for age, sex, socio-economic status, smoking status and co-morbidities. Neither moderately impaired eGFR nor proteinuria were associated with short-term mortality following either infection.
Conclusions
People with pre-existing low eGFR but not on dialysis are at higher risk of death following pneumonia and sepsis. This association was not explained by existing co-morbidities. These patients need to be carefully monitored to prevent modifiable causes of death.
Keywords: chronic kidney disease, community-acquired infections, electronic health records, infection/mortality, proteinuria
INTRODUCTION
Chronic kidney disease (CKD) affects an estimated 1.8 million people in England, 98% of whom do not require renal replacement therapy [1]. CKD is defined by reduced estimated glomerular filtration rate (eGFR) or evidence of kidney damage such as proteinuria and is commonest among older people [2, 3]. Most patients with CKD are managed in primary care [4].
Infection is an important cause of mortality among older people [5, 6]. Both reduced eGFR and proteinuria are associated with an increased rate of infection-related mortality, which could be partly explained by increased incidence of infection [7–9]. It is less clear whether CKD is also associated with poorer prognosis following infection. When clinicians assess patients with community-acquired infection, developing complications such as acute kidney injury (AKI) may not yet be apparent, but they will know which patients have pre-existing CKD. If pre-existing CKD is a risk marker for short-term mortality, this would be useful for risk stratification and clinical management of patients with infections, especially in primary care where clinicians may not have access to immediate laboratory tests. While the implications of acute changes in eGFR during infection are a focus of current research, few studies have investigated the role of pre-existing CKD [10]. Low baseline eGFR has been found to be associated with mortality following sepsis and community-acquired pneumonia, but rarely examined according to clinically meaningful categories of eGFR [11–15]. To the best of our knowledge, proteinuria has not been examined as a potential risk marker for mortality following infection [12, 15, 16].
Among older people, CKD frequently co-exists with other co-morbidities [3]. An association of CKD with poor prognosis of infections could thus be due to confounding from these co-morbidities. For example, CKD is strongly associated with cardiovascular disease, which may be complicated by infection, resulting in post-infection mortality driven by the underlying cardiovascular disease [17]. Such deaths would largely follow hospitalization for cardiovascular events. CKD is associated with healthcare-associated pneumonia, which carries a worse prognosis than community-acquired pneumonia [18, 19]. Focusing on community-acquired infections should exclude infections arising as short-term sequelae of cardiovascular events and improve understanding of the relationship between pre-existing CKD markers and infection prognosis.
Older people with diabetes mellitus form a large and growing population in primary care who suffer a high incidence of community-acquired pneumonia and sepsis [20]. Forty per cent of adults with diabetes have CKD, of whom three-quarters have proteinuria, and CKD among these patients is associated with a greater all-cause excess mortality than among patients without diabetes [21]. If proteinuria is a risk marker for mortality among older patients with diabetes who develop community-acquired pneumonia or sepsis, this could inform clinical management of a large primary care patient population with appreciable mortality following infection [20].
This study aimed to examine whether baseline eGFR and proteinuria were independent risk markers for short-term mortality following community-acquired pneumonia or sepsis among older people with diabetes mellitus, using large, linked electronic health record databases.
MATERIALS AND METHODS
Data sources
The Clinical Practice Research Datalink (CPRD) is an anonymized UK dataset, comprising primary care records (including diagnoses, prescriptions and test results) for 12.8 million patients in May 2011 when data were extracted. The CPRD population is representative of the general UK population and validity of recorded diagnoses is generally high [22, 23]. Monitoring of eGFR and proteinuria in primary care is standard practice for people with diabetes and has been financially incentivized by the Quality Outcomes Framework since April 2004 [24].
Data linkage is available within England subject to practice-level consent. Records of all patients in CPRD with available linkage to Office for National Statistics (ONS) mortality data formed the study dataset [25]. We additionally used linked Hospital Episodes Statistics admissions data, which were available for all patients [26].
Study population
The study population was a subset of a population described in more detail previously [20]. It comprised people aged ≥65 years with diabetes mellitus who experienced a first community-acquired pneumonia or sepsis, with a valid serum creatinine result and no history of renal replacement therapy.
A valid serum creatinine result was one recorded in primary care after the latest time-point of diabetes diagnosis, 65th birthday, 1 year after patients' practice registration, date the practice reached CPRD quality control standards or 1 January 1998. Study exit occurred at the first time-point of death, patient leaving the practice, last data collection from the practice, last ONS data linkage date, renal replacement therapy (kidney transplant or dialysis) or 31 March 2011. Patients with a history of renal replacement therapy were excluded.
Definition of infections
Infection was identified by a diagnostic Read code in primary care records, or a diagnostic International Classification of Disease 10 (ICD-10) code as the primary cause of hospital admission in secondary care records. The first consultation for infection was treated as the date of infection onset. Any community-acquired infection with onset at least 28 days after the first valid serum creatinine result, and before study exit, was included in the study.
Hospital-acquired infections were identified and excluded as described previously [20]. Briefly, infections were designated as hospital-acquired if onset was during or within 14 days of discharge from a hospitalization. Hospital-acquired infections continued until 28 days had passed without a diagnostic code for the infection or 28 days after hospital discharge, whichever was the later. After this, patients re-entered follow-up for community-acquired infection.
Study outcomes
The outcomes were death from any cause recorded in ONS mortality data within 28 days (primary outcome) or within 90 days (secondary outcome) of infection onset.
Definition of CKD
CKD was described in terms of eGFR and proteinuria, using primary care records. We estimated eGFR from serum creatinine test results using the CKD Epidemiology Collaboration (CKD-EPI) equation including adjustment for ethnicity [27]. We excluded serum creatinine results <28 days prior to infection onset to avoid misclassification of CKD status, as a developing infection could disrupt serum creatinine levels.
Clinically, CKD diagnosis is based on two GFR estimates at least 3 months apart [2]. Using a single GFR estimate can result in over-ascertainment of CKD due to creatinine fluctuation [28]. If more than one serum creatinine result was recorded between the start of patient follow-up and 28 days prior to infection onset, we used the higher eGFR from the latest two results that were at least 3 months apart, to obtain conservative estimates of eGFR [28].
We aimed to categorize eGFR according to thresholds corresponding to those used in diagnosing CKD stage. Due to the small number of outcomes in the category eGFR < 15 mL/min/1.73 m2, we collapsed Stages 4 and 5 to categorize eGFR as < 30, 30–44, 45–59 and ≥60 mL/min/1.73 m2 [2].
A history of proteinuria was defined by either a positive urine protein test result (excluding results on the same day as a urinary tract infection diagnosis) or a diagnosis of proteinuric renal disease. We did not count trace results as positive.
Other variables
Age was defined in 5-year age-bands up to a final category of ≥85 years. Socio-economic status was assigned by quintile at an individual level, using 2007 ONS estimates of the Index of Multiple Deprivation, a composite area-level marker of deprivation [25]. If this was not available, it was supplemented by the socio-economic status for the patient's primary care practice. Smoking status was defined by the most recent record before infection onset when available, otherwise by the first subsequent record. Non-cardiovascular co-morbidities (chronic lung disease, dementia, cancer, connective tissue disorders, hypertension and cerebrovascular disease) and cardiovascular co-morbidities (congestive heart failure and ischaemic heart disease) were defined by diagnostic CPRD Read codes, and diabetic medication history by CPRD prescription records prior to infection onset. HbA1C test results within 28 days before infection were excluded as these could reflect disturbed glycaemic control during the early stages of infection.
Data analysis
Pneumonia and sepsis analyses were conducted separately. A patient could be included in both the pneumonia and sepsis analyses if they experienced both infections, but not for multiple episodes of either pneumonia or sepsis. We excluded patients with no smoking status or HbA1C result available.
We described mortality using Kaplan–Meier survival curves stratified by eGFR status. We calculated risk ratios (RRs) using a generalized linear model with log link, normal distribution and robust standard errors, according to a pre-specified analysis plan [29]. Our first model adjusted for age, sex, socio-economic status and infection onset prior to 1 April 2004 (when Quality Outcomes Framework guidelines financially incentivizing recording of CKD status among people with diabetes in primary care were introduced) [24]. Our second model adjusted for confounding by smoking status, characteristics of diabetes (HbA1C and diabetic medication history) and non-cardiovascular co-morbidities. Our final model additionally adjusted for congestive heart failure and ischaemic heart disease, which could confound or mediate an association between CKD and post-infection mortality. We repeated the final model with additional adjustment for peripheral vascular disease as a sensitivity analysis. We looked for effect modification between eGFR and proteinuria in the final model.
We focused on whether pre-existing CKD was a risk marker for short-term mortality following infection. Data on acute electrolyte changes during infection were not routinely available, and so potential causal mechanisms such as AKI could not be explored [30].
Causes of death in ONS mortality data are recorded using ICD-10 codes from 1 January 2001, and ICD-9 codes prior to this, which are not easily comparable [31]. We therefore described cause of death among patients who died after 1 January 2001.
Stata version 13.1 was used for data analysis. All code lists are available on request.
Ethics
The study was approved by the Independent Scientific Advisory Group of the CPRD (ISAC reference 11_033A) and the London School of Hygiene & Tropical Medicine Ethics Committee (LSHTM reference 6116).
RESULTS
We identified 4957 individuals with community-acquired pneumonia and 1114 individuals with community-acquired sepsis. Data were missing (for smoking status and/or HbA1C results) for 212/4957 individuals with pneumonia (4.3%) and 56/1114 individuals with sepsis (5.0%). These patients were excluded. Among patients with pneumonia, patients with missing data were older (median age 83 years, IQR: 78–88) compared with those included (median age 80 years, IQR: 74–85), with a higher 28-day mortality (excluded 88/214, 41.1%: included 1406/4743, 29.6%) but a similar distribution of baseline eGFR. A similar pattern was seen for sepsis.
Estimated GFR was based on the higher of two results for 4029 patients with pneumonia (84.9%) and 919 patients with sepsis (86.9%); for the remaining patients, only a single valid serum creatinine result was available. CKD prevalence was high: almost half of the patients had eGFR <60 mL/min/1.73 m2 and a third had proteinuria. Patients with eGFR <60 mL/min/1.73 m2 were older, with a higher prevalence of ischaemic heart disease and congestive heart failure than patients with eGFR ≥60 mL/min/1.73 m2 (Table 1).
Table 1.
Baseline characteristics of the study population
| Pneumonia |
Sepsis |
|||
|---|---|---|---|---|
| eGFR < 60 mL/min/1.73 m2 | eGFR ≥ 60 mL/min/1.73 m2 | eGFR < 60 mL/min/1.73 m2 | eGFR ≥ 60 mL/min/1.73 m2 | |
| Age (years) | ||||
| Median (IQR) | 82 (77–87) | 78 (72–83) | 81 (75–86) | 76 (71–82) |
| n (col %) | n (col %) | n (col %) | n (col %) | |
| Gender | ||||
| Female | 1231 (53.0) | 1106 (42.0) | 294 (55.7) | 268 (45.7) |
| Onset prior to 1 April 2004 | 584 (25.1) | 527 (20.0) | 124 (23.5) | 120 (20.5) |
| Socio-economic status (IMD quintile)a | ||||
| 1 (least deprived) | 383 (16.5) | 438 (16.6) | 109 (20.6) | 106 (18.1) |
| 2 | 554 (23.8) | 595 (22.6) | 116 (22.0) | 127 (21.7) |
| 3 | 494 (21.3) | 582 (22.1) | 120 (22.7) | 137 (23.4) |
| 4 | 502 (21.6) | 574 (21.8) | 110 (20.8) | 108 (18.4) |
| 5 (most deprived) | 391 (16.8) | 444 (16.9) | 73 (13.8) | 108 (18.4) |
| Smoking status | ||||
| Current | 321 (13.8) | 549 (20.9) | 70 (13.3) | 103 (17.6) |
| Ex-smoker | 1185 (51.0) | 1351 (51.3) | 235 (44.5) | 284 (48.5) |
| Non-smoker | 776 (33.4) | 698 (26.5) | 207 (39.2) | 193 (32.9) |
| Missing | 42 (1.8) | 35 (1.3) | 16 (3.0) | 6 (1.0) |
| Comorbidities | ||||
| Chronic lung disease | 503 (21.6) | 686 (26.1) | 59 (11.2) | 95 (16.2) |
| Hypertension | 1623 (69.8) | 1662 (63.1) | 372 (70.5) | 381 (65.0) |
| Congestive heart failure | 734 (31.6) | 430 (16.3) | 147 (27.8) | 95 (16.2) |
| Ischaemic heart disease | 966 (41.6) | 887 (33.7) | 223 (42.2) | 207 (35.3) |
| Cerebrovascular disease | 692 (29.8) | 638 (24.2) | 152 (28.8) | 145 (24.7) |
| Other dementia | 174 (7.5) | 190 (7.2) | 42 (8.0) | 23 (3.9) |
| Cancer | 382 (16.4) | 474 (18.0) | 99 (18.8) | 120 (20.5) |
| Connective tissue disorders | 228 (9.8) | 215 (8.2) | 36 (6.8) | 52 (8.9) |
| HbA1C | ||||
| Good <7% | 1176 (50.6) | 1401 (53.2) | 251 (47.5) | 308 (52.6) |
| Borderline 7–10% | 966 (41.6) | 1033 (39.2) | 213 (40.3) | 230 (39.3) |
| Poor >10% | 108 (4.7) | 131 (5.0) | 41 (7.8) | 34 (5.8) |
| None recorded | 74 (3.2) | 68 (2.6) | 23 (4.4) | 14 (2.4) |
| Prior antidiabetes medication | ||||
| Insulin | 122 (5.3) | 126 (4.8) | 34 (6.4) | 23 (3.9) |
| Oral medications | 1139 (49.0) | 1426 (54.2) | 247 (46.8) | 312 (53.2) |
| Both | 462 (19.9) | 384 (14.6) | 130 (24.6) | 114 (19.5) |
| None | 601 (25.9) | 697 (26.5) | 117 (22.2) | 137 (23.4) |
| Total | 2324 | 2633 | 528 | 586 |
eGFR, estimated glomerular filtration rate.
aIndex of multiple deprivation (IMD) score for patient's postcode where available, otherwise, practice-level IMD score.
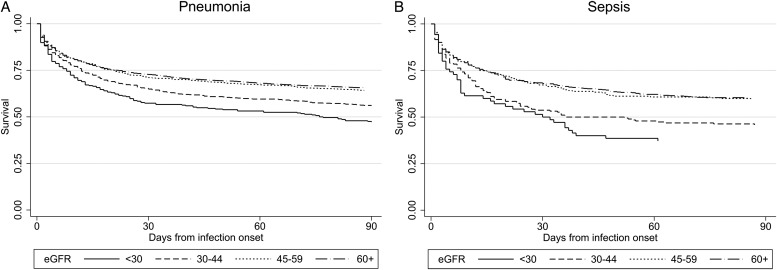
Patients with pneumonia experienced 29.6% 28-day all-cause mortality (1406 deaths). Patients with sepsis experienced 35.6% 28-day all-cause mortality (377 deaths) (Table 2). Survival curves showed high mortality at infection onset, declining over ∼30 days to a more stable rate for the next 60 days following both pneumonia and sepsis (Figure 1). RRs for 28-day mortality were higher among people with eGFR <30 mL/min/1.73 m2 compared with people with eGFR ≥60 mL/min/1.73 m2 for pneumonia (RR = 1.27; 95% CI 1.10–1.47) and sepsis (RR = 1.42; 1.10–1.84), adjusted for age, sex, socio-economic status and onset prior to April 2004. Adjustment for smoking status, co-morbidities and characteristics of diabetes had minimal effect on these RRs for pneumonia (fully adjusted RR = 1.27; 1.12–1.43) or sepsis (fully adjusted RR = 1.32; 1.07–1.64). There was no evidence of associations between intermediate levels of eGFR and 28-day mortality for either infection, nor for an association between proteinuria and 28-day mortality. The pattern of associations of eGFR and proteinuria with 90-day mortality was similar to those for 28-day mortality (Table 2). Results were unchanged by additional adjustment for peripheral vascular disease. There was no good evidence of effect modification between eGFR and proteinuria, and in particular no evidence of any association of proteinuria with 28-day mortality within any category of eGFR status (data not shown).
Table 2.
Short-term mortality by CKD status (n = 4743 for pneumonia, n = 1058 for sepsis)a
| Number (column %) | 28-Day mortality (row %) | 90-Day mortality (row %) | RRs for 28-day mortality (95% CI) |
RRs for 90-day mortality (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for demographicsb | adjusted for non-CVD co-morbiditiesc | Fully adjustedd | Adjusted for demographicsb | Adjusted for non-CVD co-morbiditiesc | Fully adjustedd | |||||
| Pneumonia | ||||||||||
| Proteinuria (adjusted for eGFR) | ||||||||||
| Yes | 1611 (34.0) | 499 (31.0) | 625 (38.8) | 1.07 (0.97–1.17) | 1.07 (0.98–1.18) | 1.07 (0.98–1.18) | 1.05 (0.97–1.13) | 1.06 (0.98–1.14) | 1.05 (0.98–1.14) | |
| No | 3132 (66.0) | 907 (29.0) | 1150 (36.7) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| eGFR (mL/min/1.73 m2) (adjusted for proteinuria) | ||||||||||
| <30 | <15 | 23 (0.5) | 12 (52.2) | 12 (52.2) | 1.27 (1.10–1.47) | 1.31 (1.13–1.52) | 1.30 (1.12–1.52) | 1.26 (1.12–1.42) | 1.29 (1.14–1.45) | 1.27 (1.12–1.43) |
| 15–29 | 263 (5.6) | 110 (41.8) | 139 (52.9) | |||||||
| 30–44 | 764 (16.1) | 265 (34.7) | 336 (44.0) | 0.98 (0.86–1.10) | 0.99 (0.88–1.13) | 0.99 (0.88–1.12) | 1.02 (0.93–1.13) | 1.04 (0.94–1.15) | 1.03 (0.94–1.14) | |
| 45–60 | 1162 (24.5) | 332 (28.6) | 418 (36.0) | 0.91 (0.82–1.02) | 0.94 (0.84–1.04) | 0.93 (0.84–1.04) | 0.91 (0.83–1.00) | 0.93 (0.85–1.02) | 0.92 (0.84–1.01) | |
| ≥60 | 2531 (53.4) | 687 (27.1) | 870 (34.4) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Total | 4743 | 1406 (29.6) | 1775 (37.4) | |||||||
| Sepsis | ||||||||||
| Proteinuria (adjusted for eGFR) | ||||||||||
| Yes | 358 (33.8) | 128 (35.8) | 159 (44.4) | 0.98 (0.82–1.17) | 1.01 (0.85–1.21) | 1.01 (0.85–1.21) | 0.98 (0.86–1.13) | 0.96 (0.84–1.10) | 0.96 (0.84–1.10) | |
| No | 700 (66.2) | 249 (35.6) | 309 (44.1) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| eGFR (mL/min/1.73 m2) (adjusted for proteinuria) | ||||||||||
| <30 | <15 | 8 (0.8) | 2 (25.0) | 5 (62.5) | 1.42 (1.10–1.84) | 1.41 (1.08–1.84) | 1.37 (1.05–1.79) | 1.39 (1.14–1.70) | 1.32 (1.07–1.63) | 1.32 (1.07–1.64) |
| 15–29 | 62 (5.9) | 32 (51.6) | 39 (62.9) | |||||||
| 30–44 | 190 (18.0) | 88 (46.3) | 103 (54.2) | 1.25 (1.01–1.55) | 1.24 (0.99–1.55) | 1.24 (0.99–1.54) | 1.14 (0.96–1.36) | 1.12 (0.94–1.33) | 1.11 (0.94–1.32) | |
| 45–60 | 232 (21.9) | 76 (32.8) | 95 (41.0) | 0.95 (0.75–1.19) | 0.91 (0.72–1.15) | 0.91 (0.72–1.14) | 0.92 (0.77–1.11) | 0.89 (0.74–1.07) | 0.89 (0.75–1.07) | |
| ≥60 | 566 (53.5) | 179 (31.6) | 226 (39.9) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | |
| Total | 1058 | 377 (35.6) | 468 (44.2) | |||||||
aExcluding patients with missing smoking or HbA1C data.
bAge, gender, socio-economic status, onset prior to 1 April 2004.
cAge, gender, socio-economic status, onset prior to 1 April 2004, smoking status, chronic lung disease, dementia, cancer, connective tissue disorders, hypertension, cerebrovascular disease, diabetes medications, latest HbA1C.
dAge, gender, socio-economic status, onset prior to 1 April 2004, smoking status, chronic lung disease, dementia, cancer, connective tissue disorders, hypertension, cerebrovascular disease, diabetes medications, latest HbA1C, congestive heart failure, ischaemic heart disease.
FIGURE 1:
Survival curves of short-term mortality following infection onset by eGFR status for (A) pneumonia and (B) sepsis.
The underlying causes of death following pneumonia and sepsis were similar for patients with eGFR above and below 60 mL/min/1.73 m2. Causes of 28-day mortality following sepsis were predominantly sources of infection (Table 3). Following pneumonia onset, pneumonia was recorded as an underlying or contributory cause of death for 83.9% (1191/1419) of deaths within 28 days and 76.3% (1366/1790) of deaths within 90 days. Among patients with eGFR <60 mL/min/1.73 m2, renal disease was recorded as an underlying or contributory cause for 10.6% (77/724) of those who died within 28 days of pneumonia onset and 16.6% (152/913) of those who died within 90 days. Recording of CKD as a cause of death increased with lower eGFR (Table 4).
Table 3.
Top five underlying causes of death by ICD-10 code for short-term mortality following pneumonia and sepsis (deaths after 2001)a
| eGFR |
||
|---|---|---|
| eGFR <60 mL/min/1.73 m2 | eGFR ≥60 mL/min/1.73 m2 | |
| 28-Day mortality following pneumonia, n = 1419 | J18 Pneumonia, organism unspecified, n = 256, 35.4% J44 Other chronic obstructive pulmonary disease, n = 63, 8.7% I25 Chronic ischaemic heart disease, n = 47, 6.5% I50 Heart failure, n = 43, 5.9% I64 Stroke, not specified as haemorrhage or infarction, n = 37, 5.1% |
J18 Pneumonia, organism unspecified, n = 216, 31.1% J44 Other chronic obstructive pulmonary disease, n = 63, 9.1% I64 Stroke, not specified as haemorrhage or infarction, n = 35, 5.0% C34 Malignant neoplasm of bronchus and lung, n = 34, 4.9% I25 Chronic ischaemic heart disease, n = 29, 4.2% |
| Total = 724 | Total = 695 | |
| 29–90 Day mortality following pneumonia, n = 371 | J18 Pneumonia, organism unspecified, n = 38, 20.1% I25 Chronic ischaemic heart disease, n = 24, 12.7% J44 Other chronic obstructive pulmonary disease, n = 13, 6.9% C34 Malignant neoplasm of bronchus and lung, n = 12, 6.4% I21 Acute myocardial infarction, n = 12, 6.4% |
J18 Pneumonia, organism unspecified, n = 20, 11.0% C34 Malignant neoplasm of bronchus and lung, n = 20, 11.0% J44 Other chronic obstructive pulmonary disease, n = 19, 10.4% I25 Chronic ischaemic heart disease, n = 9, 5.0% I64 Stroke, not specified as haemorrhage or infarction, n = 9, 5.0% |
| Total = 189 | Total = 182 | |
| 28-Day mortality following sepsis, n = 387 | N39 Other disorders of urinary systemb, n = 33, 15.9% J18 Pneumonia, organism unspecified, n = 30, 14.4% E14 Unspecified diabetes mellitus, n = 12, 5.8% A41 Other sepsis, n = 11, 5.3% L03 Cellulitis, n = 11, 5.3% |
N39 Other disorders of urinary systemb, n = 20, 11.2% A41 Other sepsis, n = 18, 10.1% J18 Pneumonia, organism unspecified, n = 18, 10.1% E14 Unspecified diabetes mellitus, n = 10, 5.6% J44 Other chronic obstructive pulmonary disease, n = 6, 3.4% =K55 Vascular disorders of intestine, n = 6, 3.4% =L03 Cellulitis, n = 6, 3.4% |
| Total = 208 | Total = 179 | |
aDeaths prior to 2001 were recorded using ICD-9 codes and have not been included.
bAll incidences of code N39 were N39.0 Urinary tract infection, site not specified.
Table 4.
Recording of renal disease as a cause of deatha following pneumonia among patients with reduced eGFR
| eGFR (mL/min/1.73 m2) | Deaths within 28 days of pneumonia onset |
Deaths within 90 days of pneumonia onset |
||||
|---|---|---|---|---|---|---|
| Total | N18 CKD, n (%) | Renal disease,b n (%) | Total | N18 CKD, n (%) | Renal disease,b n (%) | |
| <15 | 8 | 6 (75.0) | 7 (87.5) | 8 | 6 (75.0) | 7 (87.5) |
| 15–29 | 109 | 19 (17.4) | 41 (37.6) | 139 | 26 (18.7) | 53 (38.1) |
| 30–44 | 275 | 14 (5.1) | 2 (15.3) | 347 | 20 (5.8) | 56 (16.1) |
| 35–59 | 332 | 8 (2.4) | 27 (8.1) | 419 | 10 (2.4) | 36 (8.6) |
| Total | 724 | 47 (6.5) | 77 (10.6) | 913 | 62 (6.8) | 152 (16.6) |
aDeaths prior to 2001 were recorded using ICD-9 codes and have not been included.
bAny ICD-10 code from Chapter XIV ‘Diseases of the genitourinary system’ except N10 ‘Acute tubule-interstitial nephritis’, which is used for pyelonephritis, N30 ‘Cystitis’, N34 ‘Urethritis’ or N39.0 ‘Urinary tract infection, site not specified’.
DISCUSSION
Among this population of older people with diabetes mellitus, eGFR <30 mL/min/1.73 m2 was a risk marker of higher 28- and 90-day mortality following community-acquired pneumonia and sepsis, compared with patients with eGFR ≥60 mL/min/1.73 m2. The relationship between eGFR and mortality did not change with adjustment for co-morbidities. Neither moderately impaired eGFR nor proteinuria was associated with higher short-term mortality following either infection.
The strengths of this study follow from the analysis of a focused question using large, linked datasets for a highly monitored primary care population with a cohort study design. Our study identifies that the association between eGFR and post-infection mortality persists when patients with end-stage renal disease (ESRD) are excluded (and is not explained by renal replacement therapy), when considering fixed-term rather than in-hospital mortality (thus is not due to differences in hospital stay) and when exclusively community-acquired infections are considered (so does not result from increased risk of healthcare-associated infections). The linked datasets allowed us to identify infections both among patients presenting directly to hospital and those managed in the community, maximized ascertainment of mortality and enabled description of the causes of death. The highly monitored population allowed good ascertainment of CKD status. The cohort study design has less potential for selection bias than an equivalent case–control study.
A limitation is our assumption that the absence of a record implies a negative status for proteinuria and co-morbidities. Under-ascertainment of co-morbidities could result in residual confounding, with unpredictable effects, but the high prevalence of co-morbidities observed suggests that ascertainment was not markedly incomplete. We observed a high prevalence of proteinuria, and this is a highly monitored population (with financial incentives for standardized recording of proteinuria since 2004), but under-ascertainment of proteinuria could result in underestimation of any association between proteinuria and mortality [24]. Residual confounding from undiagnosed cardiovascular disease should have been minimized by adjustment for cardiovascular disease risk factors including smoking, hypertension and characteristics of diabetes.
Our findings for eGFR provide further detail to build on previous findings that baseline eGFR <60 mL/min/1.73 m2 or renal disease are risk factors for short-term mortality following (hospital- or community-acquired) sepsis and for in-hospital mortality following community-acquired pneumonia (including patients receiving dialysis) [11–15]. A more comparable Canadian study examined the associations between eGFR and 30-day mortality following community-acquired pneumonia among the general population aged ≥65 years, excluding patients with ESRD [16]. Fully adjusted hazard ratios for 30-day mortality were 1.22 (95% CI 1.01–1.49) for eGFR 45–59, 2.03 (1.64–2.50) for eGFR 30–44 and 4.94 (3.94–6.19) for eGFR < 30, compared with eGFR 60–104 mL/min/1.73 m2. These are somewhat greater than the associations we observed. The difference may be explained by the different study populations. Both studies required a baseline serum creatinine result for inclusion. Our study population of older people with diabetes were routinely monitored for CKD (with financial incentivization in primary care) [32]. Creatinine testing of the Canadian study population may have been encouraged by co-morbidities or health-behaviours associated with CKD (such as smoking), which increase post-infection mortality, resulting in over-estimation of the association of eGFR and post-infection mortality. Our study population is less vulnerable to differential ascertainment of CKD. Alternatively, the association between eGFR and post-infection mortality may be smaller among patients with diabetes.
To the best of our knowledge, our examination of any association between proteinuria and mortality following community-acquired pneumonia and sepsis is novel. A history of proteinuria, although a marker for mortality in general, does not appear to be a risk marker for short-term mortality following community-acquired infection. This is unlikely to be due to chance, as the study was large, with findings consistent across both infections. We designed our study to produce conservative estimates and may have under-estimated the association between proteinuria and post-infection mortality due to under-ascertainment of proteinuria. Alternatively, any potential relationship between proteinuria and mortality may have been mitigated by clinical care of patients with infection who had pre-existing proteinuria, for example, through swift recognition of AKI.
The survival curves demonstrate a steep initial mortality following infection onset, and a high proportion of deaths had the underlying cause assigned to infection. Since 2001 in England, co-morbidities are assigned as the underlying cause of death when pneumonia has occurred in the context of, for example, malignancy or respiratory disease [25]. This suggests that the associations we observed are driven by an association between eGFR and infection prognosis, not merely high underlying baseline mortality among patients with impaired eGFR. This is supported by previous research which found 7.7-fold elevated mortality in the 30 days following community-acquired pneumonia [19]. Estimates for associations between eGFR and mortality were not substantially altered by adjustment for co-morbidities, suggesting that any causal relationship between eGFR and mortality is not mediated through co-morbidities.
Our findings apply to the large population of older patients with community-acquired pneumonia or sepsis with diabetes mellitus who do not have ESRD. Inclusion criteria are unlikely to have limited generalizability appreciably. Practices which consent to data linkage could be more research oriented, providing good primary care management of risk factors for infection-related mortality (such as smoking cessation), but this is unlikely to affect the relationship between CKD and short-term mortality post-infection. Lack of pre-existing creatinine test results is likely to reflect limited time potentially eligible for the study rather than CKD status among this highly monitored population. Missing data on smoking status and HbA1C may be a marker of low patient engagement: caution should be used in generalizing our results to patients who are not actively managed in primary care.
We found that CKD is a useful clinical risk marker for post-infectious mortality. Whether this relationship is causal is less clear; but the association does not appear to be explained by age, co-morbidities or hospital attendance. Potential mechanisms include immune system dysfunction, but also more preventable complications such as AKI. Combinations of risk factors may be important: for example, patients with post-operative AKI have higher mortality if they also have pre-existing CKD [30].
Our results have implications for patient management and future research. Patients with baseline eGFR <30 mL/min/1.73 m2 and community-acquired infection need careful monitoring, particularly in the 28 days following infection. Future research should investigate preventable mechanisms by which low baseline eGFR could be related to post-infection mortality, for example, fluid management, AKI and drug dosage in patients with low renal clearance.
ACKNOWLEDGEMENTS
This work was supported by National Institute for Health Research (CDF 2010-03-32 to S.L.T.) and Kidney Research UK (ST2/2011 to H.I.M.). The study funders had no role in the design nor conduct of the study, nor the collection, management, analysis, nor interpretation of data, nor the preparation, review, nor approval of the manuscript, nor the decision to submit it for publication. The views expressed in this publication are those of the authors and not necessarily those of the UK National Health Service, the National Institute for Health Research, the Department of Health or Kidney Research UK. The results presented in this paper have not been published previously in whole or part, except in abstract format.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Kerr M, Bray B, Medcalf J, et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012; 27(Suppl. 3): iii73–iii80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266 [PubMed] [Google Scholar]
- 3.Roderick PJ, Atkins RJ, Smeeth L, et al. Detecting chronic kidney disease in older people; what are the implications? Age Ageing 2008; 37: 179–186 [DOI] [PubMed] [Google Scholar]
- 4.Burden R, Tomson C. Identification, management and referral of adults with chronic kidney disease: concise guidelines. Clin Med 2005; 5: 635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies S. Annual Report of the Chief Medical Officer, Volume 1, 2011, On the State of the Public's Health. Department of Health, London, 2012 [Google Scholar]
- 6.Millett ER, Quint JK, Smeeth L, et al. Incidence of community-acquired lower respiratory tract infections and pneumonia among older adults in the United Kingdom: a population-based study. PLoS One 2013; 8: e75131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HE, Gamboa C, Warnock DG, et al. Chronic kidney disease and risk of death from infection. Am J Nephrol 2011; 34: 330–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 2005; 16: 3728–3735 [DOI] [PubMed] [Google Scholar]
- 9.McDonald HI, Thomas SL, Nitsch D. Chronic kidney disease as a risk factor for acute community-acquired infections in high-income countries: a systematic review. BMJ Open 2014; 4: e004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10: 193–207 [DOI] [PubMed] [Google Scholar]
- 11.Maizel J, Deransy R, Dehedin B, et al. Impact of non-dialysis chronic kidney disease on survival in patients with septic shock. BMC Nephrol 2013; 14: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James MT, Laupland KB, Tonelli M, et al. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med 2008; 168: 2333–2339 [DOI] [PubMed] [Google Scholar]
- 13.Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med 2002; 165: 766–772 [DOI] [PubMed] [Google Scholar]
- 14.Marrie TJ, Carriere KC, Jin Y, et al. Factors associated with death among adults <55 years of age hospitalized for community-acquired pneumonia. Clin Infect Dis 2003; 36: 413–421 [DOI] [PubMed] [Google Scholar]
- 15.Viasus D, Garcia-Vidal C, Cruzado JM, et al. Epidemiology, clinical features and outcomes of pneumonia in patients with chronic kidney disease. Nephrol Dial Transplant 2011; 26: 2899–2906 [DOI] [PubMed] [Google Scholar]
- 16.James MT, Quan H, Tonelli M, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 2009; 54: 24–32 [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 18.Chalmers JD, Taylor JK, Singanayagam A, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis 2011; 53: 107–113 [DOI] [PubMed] [Google Scholar]
- 19.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the pneumonia patient outcomes research team cohort study. Arch Intern Med 2002; 162: 1059–1064 [DOI] [PubMed] [Google Scholar]
- 20.McDonald HI, Nitsch D, Millett ER, et al. New estimates of the burden of acute community-acquired infections among older people with diabetes mellitus: a retrospective cohort study using linked electronic health records. Diabet Med 2013; 31: 606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69: 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walley T, Mantgani A. The UK General Practice Research Database. Lancet 1997; 350: 1097–1099 [DOI] [PubMed] [Google Scholar]
- 24.Hobbs H, Stevens P, Klebe B, et al. Referral patterns to renal services: what has changed in the past 4 years? Nephrol Dial Transplant 2009; 24: 3411–3419 [DOI] [PubMed] [Google Scholar]
- 25.Office for National Statistics. Office for National Statistics, 2012 www.ons.gov.uk (14 September 2012, date last accessed)
- 26.The Health and Social Care Information Centre. Hospital Episode Statistics, 2014; http://www.hscic.gov.uk/hes (11 October 2014, date last accessed)
- 27.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lusignan S, Tomson C, Harris K, et al. Creatinine fluctuation has a greater effect than the formula to estimate glomerular filtration rate on the prevalence of chronic kidney disease. Nephron Clin Pract 2011; 117: c213–c224 [DOI] [PubMed] [Google Scholar]
- 29.Cummings P. Methods for estimating adjusted risk ratios. Stata J 2009; 9: 175–196 [Google Scholar]
- 30.Wu VC, Huang TM, Lai CF, et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 2011; 80: 1222–1230 [DOI] [PubMed] [Google Scholar]
- 31.Brock A, Griffiths C, Rooney C. The impact of introducing ICD-10 on analysis of respiratory mortality trends in England and Wales. Health Stat Q. 2006; 29: 9–17 [PubMed] [Google Scholar]
- 32.NHS Employers. Quality and outcomes framework, 2012 http://www.nhsemployers.org/payandcontracts/generalmedicalservicescontract/qof/pages/qualityoutcomesframework.aspx (12 September 2012, date last accessed)