Mycobacterium tuberculosis-induced iNOS expression in human macrophages is NOD2-dependent via the transcription factor NF-κB.
Keywords: NLR, nitric oxide, NF-κB
Abstract
M.tb, which causes TB, is a host-adapted intracellular pathogen of macrophages. Macrophage intracellular PRRs, such as NOD proteins, regulate proinflammatory cytokine production in response to various pathogenic organisms. We demonstrated previously that NOD2 plays an important role in controlling the inflammatory response and viability of M.tb and Mycobacterium bovis BCG in human macrophages. Various inflammatory mediators, such as cytokines, ROS, and RNS, such as NO, can mediate this control. iNOS (or NOS2) is a key enzyme for NO production and M.tb control during infection of mouse macrophages; however, the role of NO during infection of human macrophages remains unclear, in part, as a result of the low amounts of NO produced in these cells. Here, we tested the hypothesis that activation of NOD2 by its ligands (MDP and GMDP, the latter from M.tb) plays an important role in the expression and activity of iNOS and NO production in human macrophages. We demonstrate that M.tb or M. bovis BCG infection enhances iNOS expression in human macrophages. The M.tb-induced iNOS expression and NO production are dependent on NOD2 expression during M.tb infection. Finally, NF-κB activation is required for NOD2-dependent expression of iNOS in human macrophages. Our data provide evidence for a new molecular pathway that links activation of NOD2, an important intracellular PRR, and iNOS expression and activity during M.tb infection of human macrophages.
Introduction
M.tb is the causative agent of TB, which afflicts ∼ ⅓ of the world’s population and was responsible for 1.3 million deaths in 2012 [1]. The high incidence of death is attributed to an increase in HIV infection, increasing drug resistance, and the length/cost of drug treatment [2]. It is imperative to understand the mechanisms that underlie the survival of M.tb in humans to treat patients effectively for this disease, as well as create useful vaccines. Human macrophages serve as the host cell niche for M.tb but also aid in its clearance through finely tuned mechanisms. They use myriad defense mechanisms to protect the host, including the production of ROS/RNS, inflammatory cytokines and proteases, and phagosome acidification during activation [3].
NLRs, a group of intracellular PRRs, are recognized by the peptidoglycan layer of Gram-negative and -positive bacterial cell walls [4] and activate cellular signaling events that induce the production of inflammatory mediators [5, 6]. NOD2 belongs to the NLR family and was originally found to recognize bacterial MDP. M.tb produces GMDP, and this change in a functional group (acetyl to glycolyl) enhances NOD2-mediated immune functions [7]. We showed recently that NOD2 plays an important role in regulating the inflammatory response and intracellular growth of M.tb in primary human macrophages [8].
The contribution of NOD2 to the range of macrophage host defense mechanisms remains unclear; in particular, it is not known whether NOD2 activation mediates NO production, an effector of the innate immune system [9]. Resting immune cells lack iNOS (NOS2 gene product) required for NO production. A variety of extracellular stimuli from bacterial and fungal cell-wall components can activate the cells to induce iNOS expression. TLR4 activation by LPS induces iNOS expression through the NF-κB-dependent pathway [10], and cytokines, such as TNF, IL-1β, and IFN-γ, activate iNOS expression [11]. NO and superoxide radicals are potent inhibitors of pathogen replication in the host environment [12] and also play a role in signaling during inflammation [13, 14]. Furthermore, IFN-γ-activated mouse macrophages costimulated with MDP induce NO production [15], and MDP can induce iNOS expression in mouse macrophages [16].
Little is known regarding the relationship of NOD2 and iNOS during M.tb infection. A study that uses IFN-γ-activated, NOD2-deficient mouse macrophages showed a decrease in NO production in response to M.tb [17]. In the mouse model of TB, NO plays an important role in controlling M.tb growth [18]. However, it has been difficult to reproduce this response in human macrophages, which may be a result of known differences in the amount of NO produced by mouse and human macrophages, as well as a lack of sensitivity in the assays used [19]. There are also differences in the upstream signaling pathways of NO production between human and mouse macrophages [20–22]. Recent studies indicate that whereas low compared with mouse macrophages, human monocytes produce NO in response to M.tb [23]. Furthermore, clinical studies indicate that patients with TB have increased iNOS expression in the inflammatory zone of granulomas [24], and alveolar macrophages from patients with TB have enhanced iNOS protein expression [25].
Our recent finding that NOD2 controls M.tb growth in human macrophages [8] led us to ask whether NOD2 activation regulates iNOS expression and NO production in these cells. In support of this possibility, our previous study demonstrated that NOD2-deficient MDMs infected with M.tb have reduced TNF and IL-1β production. Such cytokines produced by various stimuli play a role in iNOS expression [26]. Here, we show that NOD2 agonists increase the expression of iNOS mRNA and protein, as well as NO production, in human macrophages. In concert with previous reports, we show that M.tb induces iNOS expression and NO production; however, we show for the first time in human macrophages that this phenomenon is NOD2 and NF-κB dependent. Together, these findings support a new role for NOD2 and further highlight its importance in the human macrophage response to M.tb infection.
MATERIALS AND METHODS
Buffers and reagents
D-PBS, TRIzol, and RPMI-1640 medium with l-glutamine containing phenol red or phenol red deficient were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). RHH medium (Invitrogen Life Technologies and CSL Behring, Kankakee, IL, USA) was used for cell-culture experiments. MDP (Sigma-Aldrich, St. Louis, MO, USA), GMDP (InvivoGen, San Diego, CA, USA), and IFN-γ (BD Biosciences, Mountain View, CA, USA) were used for assays involving iNOS expression and NO production. DAF-FM diacetate was purchased from Invitrogen Life Technologies for NO production assays. Accell scramble siRNA and NOD2 siRNA were purchased from Dharmacon RNAi Technologies (GE Healthcare; Lafayette, CO, USA). NF-κB inhibitor BAY 11-7085 was purchased from Cayman Chemical (Ann Arbor, MI, USA). 7H11 Agar was prepared with Bacto Middlebrook 7H11 agar, oleic acid-albumin-dextrose-catalase enrichment medium, and glycerol (Difco Laboratories, Detroit, MI, USA).
Antibodies
Unconjugated rabbit anti-human NOD2 was purchased from ProSci (Poway, CA, USA) and used under optimized conditions, as we have reported previously [8]. β-Αctin, goat anti-rabbit IgG-HRP, and donkey anti-goat IgG-HRP antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies specific for iNOS and NF-κB-p65 were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-rabbit Alexa Flour -488 antibody was purchased from Invitrogen Life Technologies.
Bacterial strains and culture
Lyophilized M.tb H37Rv (ATCC #25618) and M. bovis BCG (ATCC #35734) were obtained from the American Tissue Culture Collection (Manassas, VA, USA), reconstituted, and used as described [27]. The concentration of bacteria (1–2 × 108 bacteria/ml) and degree of clumping (≤10%) were determined by counting in a Petroff-Hausser chamber. Bacteria prepared in this fashion are ≥90% viable by CFU assay.
Isolation and culture of human macrophages
Blood obtained from healthy purified protein derivative-negative human volunteers by use of an approved protocol by The Ohio State University Institutional Review Board was processed as described [28]. In brief, PBMCs were isolated from heparinized blood on a Ficoll-Paque cushion (Amersham Biosciences/GE Healthcare, Pittsburgh, PA, USA) and cultured in Teflon wells (Savillex, Eden Praire, MN, USA) for 1 (to isolate monocytes)–5 (to isolate MDMs) days in the presence of RPMI-1640 medium containing 20% autologous serum (2.0 × 106 PBMCs/ml) at 37°C/5% CO2 [29]. The cells were harvested and plated in tissue-culture plates with 10% autologous serum for 2 h. Nonadherent cells were washed away, and the monocyte/MDM monolayers were maintained in RPMI containing 10% autologous serum. MDMs generated in this fashion are 99% positive for HLA-DR and 83% positive for CD14 [28].
RNA isolation
Day 5 PBMCs in Teflon wells were harvested and mononuclear phagocytes adhered to tissue-culture plates with 10% autologous serum (4 × 106 PBMCs/ml). After washing away lymphocytes, the MDMs (4 × 105 cells) were incubated with 10% autologous serum overnight and stimulated with NOD2 agonists or repleted in 20% autologous serum until day 12. At different time-points, cells were lysed in TRIzol, and total RNA was isolated by use of the RNeasy column method (Qiagen, Valenica, CA, USA).
Gene expression studies by qRT-PCR
RNA (100 ng) was reverse transcribed to cDNA by RT enzyme (SuperScript II; Invitrogen Life Technologies), and qRT-PCR was performed by use of a human iNOS TaqMan gene-expression kit (Applied Biosystems, Life Technologies, Thermo Fisher Scientific, Grand Island, NY, USA). iNOS amplification was normalized to β-actin as a housekeeping gene (ΔCt). RCN and fold change were determined. RCN was calculated as follows: RCN = E−ΔCt × 100, where E is the efficiency (2 = 100% efficiency), and Ct = Ct (target) − Ct (reference) [30]. Fold change was calculated from RCN compared with the resting group for NOS2 mRNA experiments. Triplicate samples were analyzed in duplicate wells in each experiment.
Transfection of MDMs
Day 5 PBMCs were transfected with Accell scramble siRNA or NOD2 siRNA (target sequence CUUUAGGAUGUACAGUUA ; 368 nM) by use of the Amaxa Nucleofector (Lonza Group, Gaithersburg, MD, USA). Transfected cells were used for subsequent experiments, such as qRT-PCR, confocal microscopy, and Western blotting. Assessment of the NOD2 siRNA specificity and effects on monolayer density/viability were assessed, as we described previously [8].
Macrophage stimulation/infection, cell lysis, and Western blotting
Day 12 MDMs (for experiments without transfection) or day 5 MDMs transfected with scramble or NOD2 siRNA (4.0 × 105) for 72 h were incubated with M.tb or BCG (MOI 5:1; triplicate wells) in RHH for 30 min at 37°C in 5% CO2 on a platform shaker for equal dispersion of bacteria, followed by an additional incubation for 90 min without shaking. The cells were then washed 3 times with warm RPMI, repleted with RPMI containing 1–2% human autologous serum, and incubated for 24 h. For NF-κB inhibitor experiments, MDMs were pretreated with BAY 11-7085 (10 µM) for 1 h and stimulated with MDP, GMDP, or IFN-γ for 24 h. The monolayers were washed once with PBS, lysed in TN-1 lysis buffer [50 mM Tris (pH 8.0), 10 mM EDTA, 10 mM Na4PO7, 10 mM NaF, 1% Triton X-100, 125 mM NaCl, 10 mM Na3VO4, 10 µg/ml aprotinin, and 10 µg/ml leupeptin], placed on ice for 10 min, and then centrifuged at 17,949 g for 10 min at 4°C to remove cell debris [31], or cells were lysed by use of TRIzol for RNA isolation. Pharmacological inhibition of NF-κB was confirmed by stimulating the cells with LPS (100 ng/ml) for 24 h, and cell-free culture supernatants were harvested and analyzed for TNF production by ELISA. For experiments that use the NOD2 agonists MDP, GMDP, or IFN-γ, 4.0 × 105 day 5 MDMs were adhered to a 12-well plate for 2 h at 37°C in 5% CO2, washed to remove lymphocytes, and then incubated with MDP (5 µg/ml), GMDP (5 µg/ml), or IFN-γ (10 ng/ml) for 24 h in RPMI containing 1% autologous serum. Cells were then lysed with TN-1 for Western blotting or TRIzol for RNA isolation to study iNOS expression. For NOD2 knockdown assessment, lysates were collected as above at 72 h or 96 h post-transfection of siRNA. Protein concentrations of the cleared lysates were measured by use of the bicinchoninic acid protein assay kit (Pierce, Life Technologies, Thermo Fisher Scientific). Proteins were separated by SDS-PAGE and analyzed by Western blot by probing with the primary and secondary antibodies of interest and development by use of ECL (Amersham Biosciences/GE Healthcare). All experiments were performed in triplicate with 3 or more donors.
NO assay by DAF-FM diacetate staining
MDMs (2 × 105) were adhered to glass coverslips in a 24-well, tissue-culture plate for 2 h at 37°C and washed to remove lymphocytes. The cells were treated with GMDP, IFN-γ, or M.tb for 24 h in phenol red-free RPMI containing 1% autologous serum. At 24 h, cells were washed 1 time with warm phenol red-free RPMI and repleted with 5 μM/ml DAF-FM diacetate for 30 min at 37°C. Next, cells were washed with warm phenol red-free RPMI, 3 times, and left in the 3rd wash for 15 min at 37°C to allow for de-esterification of the dye. Finally, cells were fixed with 10% formalin (10 min at room temperature), washed with PBS, and mounted on glass slides, which were viewed by use of an Olympus FluoView FV1000 laser-scanning confocal microscope at an excitation/emissions maxima of 495/515 nm. Consecutive macrophages/test group (150–300) were counted, and MFI was calculated by use of Olympus FluoView FV1000 software.
Nitrite assay
MDMs (4 × 105) were plated in a 12-well, tissue-culture plate for 2 h at 37°C and washed to remove lymphocytes. The cells were treated with GMDP or IFN-γ for 24 h in phenol red-free RPMI containing 1% autologous serum. After 24 h, cell culture supernatants were centrifuged to remove debris and used for the DAN assay, which was performed according to the method of Misko et al. [32]. In brief, DAN was dissolved in 0.062 N HCL at a concentration of 0.05 mg/ml. Cell culture supernatants (100 µl) were placed in 96-well plates in triplicate, and 10 µl DAN was added to each well and incubated for 10 min at room temperature. The reaction was stopped by adding 5 µl 2.8 N NaOH, and the plate was read on a Perkin-Elmer luminescence spectrometer (360/440 nm). Standard curves were generated by use of sodium nitrite from 0.04 to 10 µM in phenol red-free RPMI.
NF-κB-p65 translocation studies
Day 5 MDMs were transfected with scramble siRNA or NOD2 siRNA, incubated with 20% autologous serum for 72 h, and subsequently stimulated with MDP or GMDP for 2 h. MDM monolayers formed on coverslips were washed with PBS, fixed with 2% paraformaldehyde, and permeabilized with 100% methanol for 5 min at room temperature [33]. The cells were blocked overnight at 4°C in blocking buffer (D-PBS + 5 mg/ml BSA + 10% heat-inactivated FBS), incubated with antibodies against the NF-κB-p65 subunit, followed by incubation with the secondary rabbit antibody conjugated to Alexa Fluor 488. The permeabilized MDMs were incubated with rabbit IgG as the isotype control. MDM nuclei were labeled with 0.1 μg/ml DNA stain DAPI (Molecular Probes, Carlsbad, CA, USA) in PBS for 5 min at room temperature. After extensive washing in blocking buffer, the coverslips were dried and mounted on glass slides. NF-κB-p65 translocation was examined by confocal microscopy (Olympus FluoView FV1000 laser-scanning confocal microscope).
Statistics
In all cases, the number of independent experiments equals the number of different donors used (i.e., each experiment is performed with a different donor). A paired 1-tailed Student’s t-test was used to analyze the differences between 2 groups in figures showing a representative experiment. An unpaired 1-tailed Student’s t-test was used to analyze differences between 2 groups in figures showing cumulative data from independent experiments. Significance was defined as *P < 0.05; **P < 0.005; and ***P < 0.0005.
RESULTS
NOD2 agonists increase iNOS expression in human macrophages
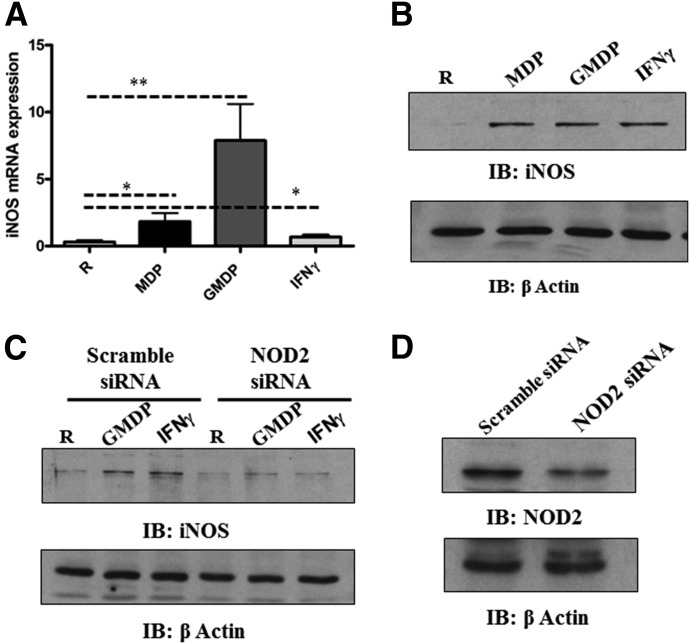
We demonstrated previously that M.tb activates NOD2 and subsequent inflammatory cytokine responses and M.tb growth in macrophages—effects that were reversed following NOD2 knockdown [8]. These studies indicated that NOD2 is required for optimizing antimycobactericidal activity in human macrophages. NO production is an important innate immune response against M.tb. The link between NOD2 and RNS is suggested from the literature, and a microarray study of M.tb-infected control and NOD2 knockdown human macrophages performed in the laboratory suggested that iNOS expression is down-regulated following NOD2 knockdown (data not shown). Thus, we sought to determine whether NOD2 agonists induce iNOS mRNA in human macrophages. Results showed that MDP induces iNOS mRNA expression, and as predicted, GMDP did so to a greater extent (Fig. 1A). IFN-γ treatment induced a lower level of iNOS expression at the time-point studied. We next determined the amount of protein produced during MDM stimulation with NOD2 agonists. MDP and GMDP induced similar amounts of protein at the time-point studied (Fig. 1B). IFN-γ was used as a positive control for the ability of MDMs to induce iNOS protein [34].We next investigated whether the GMDP and IFN-γ-mediated up-regulation of iNOS protein is NOD2 dependent. Scramble and NOD2 siRNA-transfected MDM monolayers were incubated for 72 h to achieve maximum NOD2 knockdown and then incubated with GMDP or IFN-γ for 24 h. We determined that NOD2 deficiency leads to a marked reduction in iNOS protein by GMDP and IFN-γ (Fig. 1C). The efficiency of NOD2 knockdown by siRNA was confirmed by Western blot (Fig. 1D). Thus, iNOS expression and protein production are NOD2 dependent in human macrophages.
Figure 1. NOD2 activation regulates iNOS expression in human macrophages.
MDM monolayers were treated with MDP (5 μg/ml), GMDP (5 μg/ml), or IFN-γ (10 ng/ml) for 24 h; then, monolayers were lysed with TRIzol for extraction of total RNA or lysed with TN-1 lysis buffer for Western blot. (A) qRT-PCR was used to determine iNOS mRNA levels. Data were normalized to the β-actin gene, and RCN was determined. Shown are cumulative data from 5 independent experiments (mean ± sem; *P < 0.05; **P < 0.005). R, Resting group. (B) Equal amounts of cell lysates were analyzed for iNOS expression or loading control β-actin by Western blot. Shown is a representative experiment from 3 independent experiments. IB, Immunoblot. (C) Scramble or NOD2 siRNA-transfected MDM monolayers were incubated with GMDP (5 μg/ml) or IFN-γ (10 ng/ml) in 1% autologous serum for 24 h. Cells were lysed with TN-1 buffer, and cell lysates were analyzed for iNOS production by Western blot and reprobed for β-actin as a loading control. Shown is a representative experiment of 3 independent experiments. NOD2 knockdown was confirmed by Western blot (D). Shown is a representative experiment of 3 experiments.
NOD2 activation regulates NO production in human macrophages
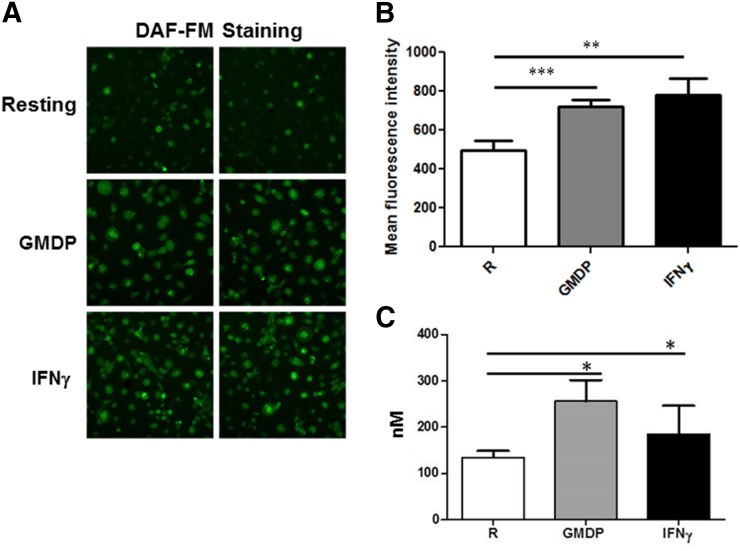
Next, we sought to confirm whether the iNOS expression that we observed in response to NOD2 agonists in MDMs resulted in NO production. It was observed previously that IFN-γ-activated mouse macrophages stimulated with MDP led to NO production in a NOD2-dependent manner [17]. We used a membrane-permeable, NO-sensitive fluorescent dye, DAF-FM diacetate, to measure NO production in human macrophages [35]. Our results demonstrate that GMDP and IFN-γ stimulation enhances NO production in human macrophages (Fig. 2). Figure 2A shows 2 representative microscopic images from MDMs stimulated with GMDP or IFN-γ, and Fig. 2B shows that exposure of MDMs to GMDP or IFN-γ for 24 h leads to a small but consistent and significant increase in NO production. We also determined the amount of nitrite produced in human macrophages during GMDP NOD2 activation by the DAN assay. Consistent with NO production, stimulation of MDMs with GMDP and IFN-γ significantly enhanced the production of nitrite to 256.7 ± 26.87 nM and 183.0 ± 36.59 nM, respectively (Fig. 2C). The result with IFN-γ stimulation is similar to data reported previously by use of primary human alveolar macrophages [34].
Figure 2. NOD2 agonists induce NO production in human macrophages.
Day 5 MDM monolayers on coverslips were treated with GMDP or IFN-γ for 24 h, and then cells were incubated with DAF-FM diacetate in RPMI containing phenol red-free for 30 min. Cells were washed 3 times with warm RPMI containing phenol red-free and then allowed to sit for 15 min for de-esterification to occur. Next, cells were fixed, mounted on slides, and examined by confocal microscopy with excitation/emission maxima of 495/515 nm (Olympus FluoView FV1000). (A) Two representative micrographs (40×) are shown of DAF-FM diacetate staining for each test group of 3 independent experiments performed on duplicate coverslips. (B) MFI of macrophages (150–300)/test group was calculated by use of Olympus FluoView FV1000 software. (C) The amount nitrite produced during GMDP- and IFN-γ-treated MDMs was determined by the DAN assay. Day 5 MDMs were washed and replenished with RPMI media without phenol red and subsequently incubated with GMDP or IFN-γ for 24 h, and the culture supernatants were centrifuged to remove all cell debris. The cell-free culture supernatants were used to determine the level of nitrite produced. (B and C) Cumulative data of the MFIs for each test group obtained from 3 independent experiments (mean ± sem; *P < 0.05; **P < 0.005; ***P < 0.0005).
M.tb infection up-regulates iNOS expression in human macrophages
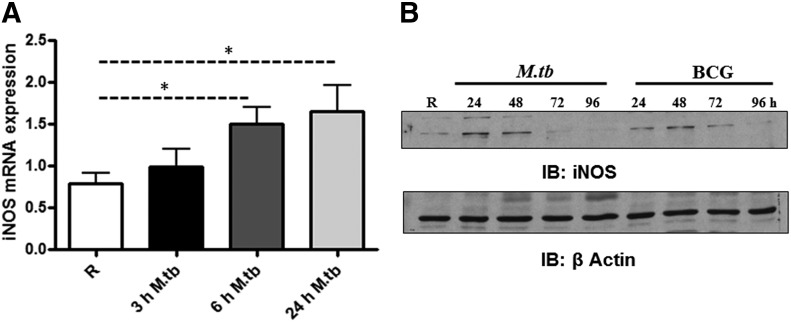
The attenuated M.tb strain H37Ra enhances iNOS mRNA and protein expression, as well as NO production, in human alveolar macrophages of TB patients [36], and the virulent strain Erdman induces NO production in human monocytes and monocytic cell lines [23]. We next determined whether virulent M.tb is able to induce iNOS expression in human macrophages. MDM monolayers were incubated with M.tb strain H37Rv for 3, 6, or 24 h and then lysed for analysis of iNOS mRNA expression and protein production. Results from qRT-PCR show that M.tb induces iNOS mRNA expression at 6 and 24 h (Fig. 3A). To determine the specificity of the response, we compared iNOS protein production following incubation of MDMs with M.tb H37RV or the attenuated vaccine strain M. bovis BCG (MOI 5:1) for 24, 48, 72, and 96 h. Western blots were performed on cell lysates. Our results indicate that iNOS protein expression is enhanced at 24 and 48 h for both bacteria and decreased at 72 h (Fig. 3B). A notable difference between the virulent and attenuated strain was seen at 24 h, where M.tb increased iNOS expression to a level greater than BCG.
Figure 3. M.tb-mediated induction of iNOS mRNA and protein in human macrophages.
Day 12 MDMs were incubated with M.tb (MOI 5:1) for 2 h, washed 3 times, and repleted with 2% autologous serum for 3, 6, and 24 h. Monolayers were lysed with TRIzol for extraction of total RNA or lysed with TN-1 buffer for whole-cell lysate at indicated time-points. (A) qRT-PCR was used to determine iNOS mRNA levels. Data were normalized to the β-actin gene, and RCN was determined. Shown are cumulative data from 5 independent experiments (mean ± sem; *P < 0.05). (B) Equal amounts of cell lysates were used to determine iNOS protein levels by Western blotting by use of iNOS antibody or β-actin antibody as a loading control. Shown is a representative experiment of 3 independent experiments.
M.tb-mediated iNOS expression is dependent on NOD2
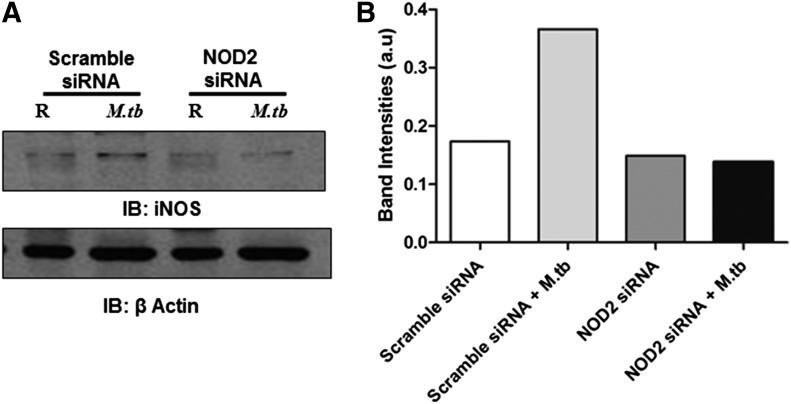
As we observed a decrease in iNOS expression upon stimulation of NOD2-deficient MDMs with the component of peptidoglycan found in M.tb (GMDP), we sought to determine whether NOD2 is responsible for the M.tb-induced iNOS expression. MDMs were transfected with scramble or NOD2 siRNA, and after 72 h of transfection, they were incubated with M.tb for 24 h. Cell lysates were generated for analysis of iNOS protein production. Our results show that NOD2 deficiency in human macrophages leads to a decrease in iNOS protein production (Fig. 4A) during M.tb infection. Band intensities were determined by use of NIH ImageJ software and presented in Fig. 4B. Thus, our results indicate that NOD2 is an important mediator of iNOS expression during M.tb infection of human macrophages.
Figure 4. M.tb-mediated induction of iNOS is NOD2 dependent in human macrophages.
(A) Scramble or NOD2 siRNA-transfected MDM monolayers were incubated with M.tb (MOI 5:1) for 24 h, and the monolayers were incubated with M.tb (MOI 5:1) for 2 h, washed 3 times, and repleted with 1% autologous serum for 24 h. Cells were lysed with TN-1 buffer, and iNOS or β-actin protein levels were examined by Western blot. Shown is a representative Western blot of 3 independent experiments. (B) Graph showing the densitometry analysis of Western blot bands by use of NIH ImageJ software.
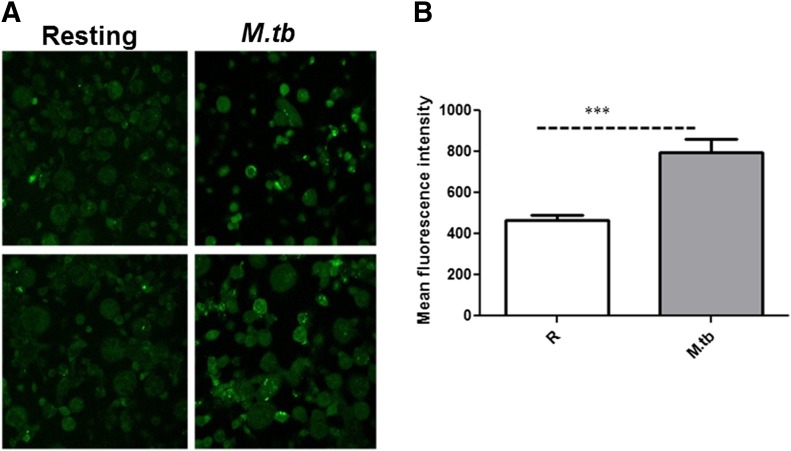
We next determined whether M.tb-mediated induction of iNOS resulted in increased NO production in human macrophages. MDM monolayers were incubated with M.tb for 24 h and then exposed to DAF-FM diacetate to detect NO production. Our results show a significant increase in NO production in MDMs infected with M.tb (Fig. 5).
Figure 5. M.tb induces NO production in human macrophages.
(A) Day 12 MDM monolayers on coverslips were incubated with M.tb, as described previously for 24 h, and then cells were incubated with DAF-FM diacetate, fixed, and examined by confocal microscopy with excitation/emission maxima of 495/515 nm (Olympus FluoView FV1000). Two representative micrographs (40×) of DAF-FM diacetate staining are shown for each test group of 3 independent experiments performed on duplicate coverslips. (B) Shown are cumulative data of the MFIs for each test group obtained from 3 independent experiments (mean ± sem; ***P < 0.0005).
NOD2-mediated up-regulation of iNOS requires NF-κB activation
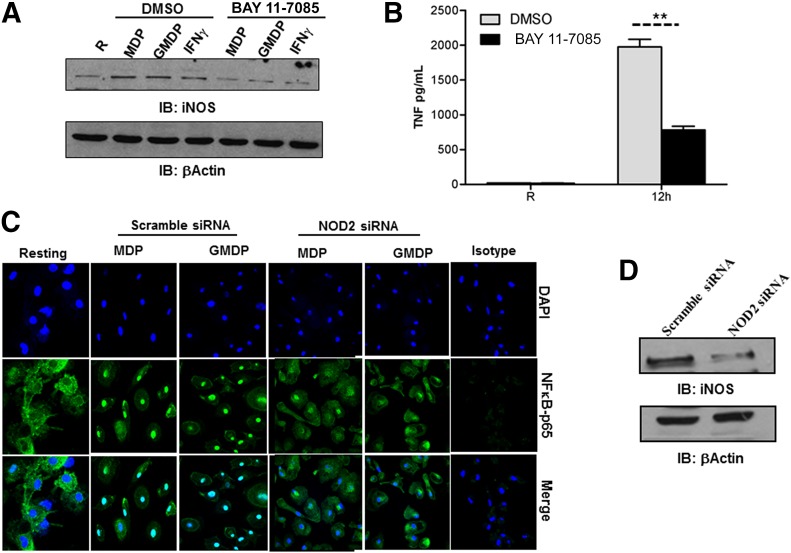
Human iNOS gene expression is regulated by several transcription factors [37], and which one(s) is used is dependent on the nature of the stimulus. There are 3 NF-κB binding sites in the human iNOS promoter. NOD2 activation by M.tb or its ligands MDP or GMDP enhances NF-κB transcription in human macrophages [8]. Thus, we next sought to determine whether NF-κB has a role in NOD2-mediated iNOS expression in our model. We pretreated MDMs with the IκB kinase/NF-κB inhibitor BAY 11-7085 and subsequently incubated cells with the NOD2 ligands MDP or GMDP or IFN-γ for 24 h. Cell lysates were analyzed for iNOS protein levels. Our results show that inhibition of NF-κB significantly reduces iNOS expression in human macrophages (Fig. 6A). Pharmacological inhibition of NF-κB was confirmed by stimulating the cells with LPS, and cell-free culture supernatants were analyzed for TNF production by ELISA. The NF-κB inhibitor significantly decreased LPS-mediated TNF production (**P < 0.005; Fig. 6B).
Figure 6. NOD2-mediated NF-κB activation is required for iNOS expression in human macrophages.
Day 5 MDMs were pretreated with the NF-κB inhibitor BAY 11-7085 for 30 min and subsequently stimulated with MDP (5 μg/ml), GMDP (5 μg/ml), or IFN-γ (10 ng/ml) for 24 h. (A) Cells were lysed with TN-1 buffer, and whole-cell lysates were analyzed for iNOS protein levels by Western blot by use of iNOS antibody or β-actin antibody as a loading control. Shown is a representative experiment of 2 independent experiments. (B) In parallel, NF-κB inhibitor-treated cells were stimulated with LPS (50 ng/ml) for 12 h. Cell culture supernatants were used to measure TNF production by ELISA. Shown is a representative experiment (mean ± sd of triplicate samples, n = 2, **P < 0.005). (C) Day 5 MDMs were transfected with scramble or NOD2 siRNA and plated on coverslips after 72 h. Cells were stimulated with MDP, GMDP, or left untreated for 6 h, fixed and permeabilized, and stained with NF-κB-p65 antibody or isotype control, followed by Alexa Flour 488-conjugated secondary antibody. The nuclei were stained with DAPI. Cells were then examined by confocal microscopy for NF-κB p-65 translocation. Shown are representative images from 2 independent experiments. NOD2 knockdown was confirmed by Western blot (D). Shown is a representative experiment of 2 experiments.
We confirmed NF-κB activation by NOD2 by use of confocal microscopy to assess NF-κB-p65 translocation to the nucleus. The results show that NOD2 deficiency in MDMs significantly reduces the translocation of NF-κB in response to MDP and GMDP (Fig. 6C). This result is consistent with those shown in Fig. 1C. We also assessed NOD2-mediated activation of C/EBPβ in our human macrophage model. The result indicated that NOD2 activation by MDP or GMDP does not increase expression or phosphorylation of C/EBPβ (result not shown).
DISCUSSION
Whereas NOD2 is one of the most well-characterized members of the NLR family, there are limited reports on the functions of NOD2 in innate immune responses besides cytokine production. Recent reports indicate a role for NOD2 in autophagy and MHC class I and class II presentation [38–40]. We demonstrated previously that M.tb growth is controlled by NOD2 at an early time-point [8], which led us to question whether other early innate-immune responses were being affected by NOD2. NO plays an important role in the innate-immune response to many bacterial infections. As NO production in macrophages relies on expression of iNOS, we elected to study the role of NOD2 in the iNOS signaling pathway as a result of its relevance to M.tb infection. Our studies show that NF-κB-mediated iNOS expression is dependent on NOD2 in human macrophages during M.tb infection and in turn, regulates NO production.
In the mouse model, iNOS is identified as a protective locus against TB. Mice deficient in iNOS succumb to infection within 33–45 days compared with wild-type mice that survive an average of 160 days [18]. Large, granulomatous lesions were found in the lung, liver, and spleens at 30 days postinfection, whereas few were found in wild-type mice at this time-point. Similar results of mortality and tissue damage were found by use of the iNOS inhibitors, aminoguanidine and NG-monomethyl-l-arginine, in the mouse model [41]. The role of NO during M.tb pathogenesis in humans was a controversial topic in the past; however, there is now more evidence in the literature that supports its impact. Initial reports testing human cells for the production of NO were negative, as a result of the low amounts of NO produced by these cells and the use of insensitive assays. The use of a more sensitive assay with the fluorescent probe DAN, which detects as little as 10 nM nitrite, allowed researchers to detect nitrite in human monocytes incubated with M.tb [32]. Jagannath et al. [23] detected nitrite by use of the DAN assay and reported that M.tb is able to induce NO production in human monocytes and that the NO produced is bacteriostatic. Whether NO is bacteriostatic or bactericidal is dependent on the specificity of the RNS and strain of mycobacteria [42–44]. Although the mechanism has not been identified, the ability of M.tb to resist the effects of RNS is a result of a novel gene, noxR1 [45]. It is interesting to note that iNOS-deficient mice treated with the same drugs that cure M.tb-infected, immunocompetent mice are ineffective. This suggests that the effectiveness of tuberculocidal drugs in vivo may be dependent on iNOS-derived NO [12].
The precise mechanism(s) underlying how NO antagonizes M.tb are unknown; however, it may include disruption of bacterial DNA, proteins, signaling, or induction of apoptosis. As we found recently that NOD2 is an important modulator of the immune response to M.tb and is necessary to control its growth in primary human macrophages, and the literature in the mouse model indicates that NOD2 may play a role in NO production in response to M.tb, we studied whether NOD2 plays a role in the expression of the necessary mediator of NO production—iNOS. Our results provide evidence that MDP induces iNOS mRNA at 24 h, and the M.tb-produced agonist GMDP induces more robust mRNA expression. This was predicted, as comparative studies of MDP and GMDP indicate that GMDP is a more robust stimulator of the immune response [7].
mRNA expression values do not often translate to the amount of protein synthesized in eukaryotic cells, so we explored the ability of NOD2 agonists to induce iNOS protein expression in human macrophages. Our findings demonstrate that NOD2 agonists are effective at inducing iNOS protein expression and at the same level as a known inducer of iNOS in human alveolar macrophages and IFN-γ-stimulated cells [34]. These NOD2 agonists are known to induce the production of the proinflammatory cytokines, TNF and IL-1β. Our laboratory has shown that the M.tb-induced cytokine production is NOD2 dependent in human macrophages [8]. Alveolar macrophages from TB patients produce TNF and IL-1β at a higher level than healthy patients, and the use of an inhibitor of NO led to a reduction in TNF and IL-1β [46]. It is known that iNOS expression can be induced by proinflammatory cytokines [47, 48]. The lack of these cytokines being produced is a possible reason why iNOS is not expressed when NOD2 is deficient.
To evaluate directly the role of NOD2 in regulating iNOS expression, we used a well-established method in our laboratory to knock down NOD2 in human macrophages [8]. To date, the role of NOD2 in iNOS and NO production has relied solely on the use of primary mouse macrophages or the iNOS knockout mouse model [15–17]. Our results show that NOD2 is necessary to induce protein expression of iNOS and subsequent NO production in human macrophages. NO is an effective host-defense molecule against microbial pathogens. NO can damage DNA as well as interact with accessory protein targets and result in enzymatic inactivation [49]. With the use of a compound that is nonfluorescent until it binds NO to form a fluorescent benzotrizole, we found that the GMDP-induced iNOS expression consequently results in NO production. As GMDP is a metabolite of the M.tb cell wall, it is possible that it is an important mediator of M.tb-induced iNOS expression and NO production.
Our results are consistent with previous findings that M.tb enhances iNOS expression. However, here, we compare virulent M.tb to the attenuated vaccine strain, BCG. We observed that M.tb and BCG induce iNOS at 24 h, but M.tb enhances iNOS expression at a greater level when compared with BCG. However, the levels decrease at 72 h for both strains. In this study, we were able to confirm that the M.tb-induced iNOS expression results in NO production by use of a different technique than published previously (DAF-FM diacetate). This NO could potentially be bacteriostatic or bactericidal, as mentioned previously. Human alveolar macrophages isolated from patients with fibrosis and challenged with BCG for 24 h displayed enhanced iNOS expression. This led to peroxynitrate production and the subsequent killing of BCG [50].
Previous reports have shown that human iNOS gene expression is regulated by several transcription factors, such as NF-κB, AP-1, C/EBPβ, and Stat1 [37]. Our results provide evidence that NOD2 activation induces iNOS expression through NF-κB. Inhibition of NF-κB in human macrophages significantly reduces iNOS protein expression in response to the NOD2 agonists MDP and GMDP. This was confirmed further by knockdown of NOD2, which impairs NF-κB-p65 translocation, required for iNOS expression. Our results indicate that C/EBPβ is not activated by MDP or GMDP in human macrophages.
We show for, the first time, that iNOS induction by M.tb in human macrophages is NOD2 dependent. This cytosolic PRR is an important protein in the iNOS signaling pathway, which leads to NO production. The role of a PRR in the induction of an inflammatory mediator, such as iNOS, is not reported in human macrophages. In mouse macrophages, iNOS expression is mediated by TLR4 in MyD88-dependent and -independent manners [51]; however, this pathway for iNOS has not been reported in human macrophages. For example, in mouse macrophages, bacterial lipoprotein activation of TLR2 leads to NO-dependent killing of M.tb, but in human alveolar macrophages, and monocytes, the TLR2-activated antimicrobial pathway was found to be NO independent [52]. Our finding is important, as it identifies a cytosolic PRR potentially involved in the antimicrobial killing effects of NO. NOD2 is thought to be a secondary line of defense in macrophages to fight bacteria that are able to avoid the initial host-defense mechanisms at the surface, but now, these data suggest an even more important role for this receptor in inducing a pathway that results in bacterial killing. This is of particular importance during M.tb infection, where the bacteria are able to modulate the host-immune response and reside in a unique phagosome. Clinical samples have high levels of iNOS expression in the inflammatory zone surrounding tuberculous granulomas, and NOD2 could be the mechanism for this expression [24].
Patients with Crohn’s disease have inflamed mucosa. They are homozygous for the 3020insC NOD2 mutation (loss of function) and are reported to have enhanced iNOS expression and NO production in the active mucosa [53]. This appears to be somewhat contradictory to the results in this manuscript, as NOD2 knockdown led to decreased iNOS expression. However, the enhanced iNOS expression and NO production in Crohn’s disease patients are likely a result of other infiltrating cells, such as neutrophils or epithelial cells, found in the area. It has been shown that elicited but not circulating neutrophils produce larger amounts of NO [54, 55]. Furthermore, we show here that NOD2 is important in iNOS expression. However, we did not study endothelial NOS, which is expressed in cultured human bronchiolar epithelium and also responsible for NO production [56]. These reasons could explain why patients with Crohn’s disease have increased iNOS and NO levels.
In summary, we provide further evidence that human macrophages are able to produce NO and show, for the first time, that NO production is dependent on NOD2-induced expression of iNOS. The transcription factor NF-κB is required for NOD2-mediated iNOS expression in human macrophages. Finally, we provide evidence that the cytosolic PRR, NOD2, is a mediator of iNOS production in response to M.tb. These findings uncover a new mechanism for how NOD2 plays a role during M.tb pathogenesis and point toward GMDP as being clinically useful for controlling M.tb infection in an NO-dependent manner.
AUTHORSHIP
M.B.L., M.V.S.R., and L.S.S. designed the experiments and wrote the manuscript. M.B.L., M.V.S.R., and H.N. performed the experiments.
Acknowledgments
This work was supported, in part, by U.S. National Institutes of Health (NIH) Grant AI059639 (to L.S.S.) and a T32 NIH Training Grant (to M.B.L.). The authors thank the Campus Microscopy and Imaging Facility at The Ohio State University (Columbus, OH, USA).
Glossary
- ΔCt
change in comparative threshold
- BCG
bacillus Calmette-Guérin
- D-PBS
Dulbecco’s PBS
- DAF-FM
4-amino-5-methylamino-2,7-difluorofluorescein
- DAN
diaminonapthalene
- GMDP
N-glycolyl-muramyl dipeptide
- M.tb
Mycobacterium tuberculosis
- MDM
monocyte-derived macrophage
- MDP
N-acetylmuramyl-l-alanyl-d-isoglutamine hydrate
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- NLR
nucleotide-binding oligomerization domain-like receptor
- NOD2
nucleotide-binding oligomerization domain 2
- PRR
pattern recognition receptor
- qRT-PCR
quantitative RT-PCR
- RCN
relative copy number
- RHH
RPMI 1640 plus 10 mM HEPES plus 0.4% human serum albumin
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- TB
tuberculosis
DISCLOSURES
The authors do not have a conflict of interest.
REFERENCES
- 1.WHO (2013) WHO Global Tuberculosis Report 2013 Factsheet. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.WHO (2010) Multidrug and Extensively Drug-Resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. World Health Organization, Geneva, Switzerland, 1–72. [Google Scholar]
- 3.Guirado E., Schlesinger L. S. (2013) Modeling the Mycobacterium tuberculosis granuloma—the critical battlefield in host immunity and disease. Front. Immunol. 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inohara N., Ogura Y., Fontalba A., Gutierrez O., Pons F., Crespo J., Fukase K., Inamura S., Kusumoto S., Hashimoto M., Foster S. J., Moran A. P., Fernandez-Luna J. L., Nuñez G. (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 278, 5509–5512. [DOI] [PubMed] [Google Scholar]
- 5.Viala J., Chaput C., Boneca I. G., Cardona A., Girardin S. E., Moran A. P., Athman R., Mémet S., Huerre M. R., Coyle A. J., DiStefano P. S., Sansonetti P. J., Labigne A., Bertin J., Philpott D. J., Ferrero R. L. (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5, 1166–1174. [DOI] [PubMed] [Google Scholar]
- 6.Chaput C., Sander L. E., Suttorp N., Opitz B. (2013) NOD-like receptors in lung diseases. Front. Immunol. 4, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulombe F., Divangahi M., Veyrier F., de Léséleuc L., Gleason J. L., Yang Y., Kelliher M. A., Pandey A. K., Sassetti C. M., Reed M. B., Behr M. A. (2009) Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J. Exp. Med. 206, 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks M. N., Rajaram M. V., Azad A. K., Amer A. O., Valdivia-Arenas M. A., Park J. H., Núñez G., Schlesinger L. S. (2011) NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell. Microbiol. 13, 402–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdan C. (2001) Nitric oxide and the immune response. Nat. Immunol. 2, 907–916. [DOI] [PubMed] [Google Scholar]
- 10.Xie Q. W., Kashiwabara Y., Nathan C. (1994) Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269, 4705–4708. [PubMed] [Google Scholar]
- 11.Lowenstein C. J., Padalko E. (2004) iNOS (NOS2) at a glance. J. Cell Sci. 117, 2865–2867. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C., Shiloh M. U. (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97, 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korhonen R., Lahti A., Kankaanranta H., Moilanen E. (2005) Nitric oxide production and signaling in inflammation. Curr. Drug Targets Inflamm. Allergy 4, 471–479. [DOI] [PubMed] [Google Scholar]
- 14.Laroux F. S., Lefer D. J., Kawachi S., Scalia R., Cockrell A. S., Gray L., Van der Heyde H., Hoffman J. M., Grisham M. B. (2000) Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid. Redox Signal. 2, 391–396. [DOI] [PubMed] [Google Scholar]
- 15.Tötemeyer S., Sheppard M., Lloyd A., Roper D., Dowson C., Underhill D., Murray P., Maskell D., Bryant C. (2006) IFN-gamma enhances production of nitric oxide from macrophages via a mechanism that depends on nucleotide oligomerization domain-2. J. Immunol. 176, 4804–4810. [DOI] [PubMed] [Google Scholar]
- 16.Bansal K., Balaji K. N. (2011) Intracellular pathogen sensor NOD2 programs macrophages to trigger Notch1 activation. J. Biol. Chem. 286, 5823–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandotra S., Jang S., Murray P. J., Salgame P., Ehrt S. (2007) Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect. Immun. 75, 5127–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMicking J. D., North R. J., LaCourse R., Mudgett J. S., Shah S. K., Nathan C. F. (1997) Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94, 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nims R. W., Cook J. C., Krishna M. C., Christodoulou D., Poore C. M., Miles A. M., Grisham M. B., Wink D. A. (1996) Colorimetric assays for nitric oxide and nitrogen oxide species formed from nitric oxide stock solutions and donor compounds. Methods Enzymol. 268, 93–105. [DOI] [PubMed] [Google Scholar]
- 20.Cameron M. L., Granger D. L., Weinberg J. B., Kozumbo W. J., Koren H. S. (1990) Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am. Rev. Respir. Dis. 142, 1313–1319. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg J. B., Misukonis M. A., Shami P. J., Mason S. N., Sauls D. L., Dittman W. A., Wood E. R., Smith G. K., McDonald B., Bachus K. E. (1995) Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 86, 1184–1195. [PubMed] [Google Scholar]
- 22.Sakai N., Milstien S. (1993) Availability of tetrahydrobiopterin is not a factor in the inability to detect nitric oxide production by human macrophages. Biochem. Biophys. Res. Commun. 193, 378–383. [DOI] [PubMed] [Google Scholar]
- 23.Jagannath C., Actor J. K., Hunter R. L. Jr. (1998) Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide 2, 174–186. [DOI] [PubMed] [Google Scholar]
- 24.Choi H. S., Rai P. R., Chu H. W., Cool C., Chan E. D. (2002) Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166, 178–186. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson S., Bonecini-Almeida Mda. G., Lapa e Silva J. R., Nathan C., Xie Q. W., Mumford R., Weidner J. R., Calaycay J., Geng J., Boechat N., Linhares C., Rom W., Ho J. L. (1996) Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J. Exp. Med. 183, 2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geller D. A., de Vera M. E., Russell D. A., Shapiro R. A., Nussler A. K., Simmons R. L., Billiar T. R. (1995) A central role for IL-1 beta in the in vitro and in vivo regulation of hepatic inducible nitric oxide synthase. IL-1 beta induces hepatic nitric oxide synthesis. J. Immunol. 155, 4890–4898. [PubMed] [Google Scholar]
- 27.Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. (1990) Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144, 2771–2780. [PubMed] [Google Scholar]
- 28.Schlesinger L. S., Horwitz M. A. (1991) Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147, 1983–1994. [PubMed] [Google Scholar]
- 29.Beharka A. A., Gaynor C. D., Kang B. K., Voelker D. R., McCormack F. X., Schlesinger L. S. (2002) Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J. Immunol. 169, 3565–3573. [DOI] [PubMed] [Google Scholar]
- 30.Gavrilin M. A., Bouakl I. J., Knatz N. L., Duncan M. D., Hall M. W., Gunn J. S., Wewers M. D. (2006) Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc. Natl. Acad. Sci. USA 103, 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajaram M. V., Ganesan L. P., Parsa K. V., Butchar J. P., Gunn J. S., Tridandapani S. (2006) Akt/Protein kinase B modulates macrophage inflammatory response to Francisella infection and confers a survival advantage in mice. J. Immunol. 177, 6317–6324. [DOI] [PubMed] [Google Scholar]
- 32.Misko T. P., Schilling R. J., Salvemini D., Moore W. M., Currie M. G. (1993) A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 214, 11–16. [DOI] [PubMed] [Google Scholar]
- 33.Rajaram M. V., Brooks M. N., Morris J. D., Torrelles J. B., Azad A. K., Schlesinger L. S. (2010) Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J. Immunol. 185, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howard M., Roux J., Lee H., Miyazawa B., Lee J. W., Gartland B., Howard A. J., Matthay M. A., Carles M., Pittet J. F. (2010) Activation of the stress protein response inhibits the STAT1 signalling pathway and iNOS function in alveolar macrophages: role of Hsp90 and Hsp70. Thorax 65, 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng J. Z., Wang D., Braun A. P. (2005) DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein) diacetate detects impairment of agonist-stimulated nitric oxide synthesis by elevated glucose in human vascular endothelial cells: reversal by vitamin C and L-sepiapterin. J. Pharmacol. Exp. Ther. 315, 931–940. [DOI] [PubMed] [Google Scholar]
- 36.Rich E. A., Torres M., Sada E., Finegan C. K., Hamilton B. D., Toossi Z. (1997) Mycobacterium tuberculosis (MTB)-stimulated production of nitric oxide by human alveolar macrophages and relationship of nitric oxide production to growth inhibition of MTB. Tuber. Lung Dis. 78, 247–255. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z., Shao L., Du Q., Park K. S., Geller D. A. (2007) Identification of a classic cytokine-induced enhancer upstream in the human iNOS promoter. FASEB J. 21, 535–542. [DOI] [PubMed] [Google Scholar]
- 38.Cooney R., Baker J., Brain O., Danis B., Pichulik T., Allan P., Ferguson D. J., Campbell B. J., Jewell D., Simmons A. (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16, 90–97. [DOI] [PubMed] [Google Scholar]
- 39.Travassos L. H., Carneiro L. A., Ramjeet M., Hussey S., Kim Y. G., Magalhães J. G., Yuan L., Soares F., Chea E., Le Bourhis L., Boneca I. G., Allaoui A., Jones N. L., Nuñez G., Girardin S. E., Philpott D. J. (2010) Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62. [DOI] [PubMed] [Google Scholar]
- 40.Homer C. R., Richmond A. L., Rebert N. A., Achkar J. P., McDonald C. (2010) ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn's disease pathogenesis. Gastroenterology 139, 1630–1641, 1641.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan J., Tanaka K., Carroll D., Flynn J., Bloom B. R. (1995) Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63, 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhoades E. R., Orme I. M. (1997) Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect. Immun. 65, 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu K., Mitchell C., Xing Y., Magliozzo R. S., Bloom B. R., Chan J. (1999) Toxicity of nitrogen oxides and related oxidants on mycobacteria: M. tuberculosis is resistant to peroxynitrite anion. Tuber. Lung Dis. 79, 191–198. [DOI] [PubMed] [Google Scholar]
- 44.Bryk R., Griffin P., Nathan C. (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407, 211–215. [DOI] [PubMed] [Google Scholar]
- 45.Ehrt S., Shiloh M. U., Ruan J., Choi M., Gunzburg S., Nathan C., Xie Q., Riley L. W. (1997) A novel antioxidant gene from Mycobacterium tuberculosis. J. Exp. Med. 186, 1885–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuo H. P., Wang C. H., Huang K. S., Lin H. C., Yu C. T., Liu C. Y., Lu L. C. (2000) Nitric oxide modulates interleukin-1beta and tumor necrosis factor-alpha synthesis by alveolar macrophages in pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 161, 192–199. [DOI] [PubMed] [Google Scholar]
- 47.Balligand J. L., Ungureanu-Longrois D., Simmons W. W., Pimental D., Malinski T. A., Kapturczak M., Taha Z., Lowenstein C. J., Davidoff A. J., Kelly R. A. (1994) Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J. Biol. Chem. 269, 27580–27588. [PubMed] [Google Scholar]
- 48.Adams V., Nehrhoff B., Späte U., Linke A., Schulze P. C., Baur A., Gielen S., Hambrecht R., Schuler G. (2002) Induction of iNOS expression in skeletal muscle by IL-1beta and NFkappaB activation: an in vitro and in vivo study. Cardiovasc. Res. 54, 95–104. [DOI] [PubMed] [Google Scholar]
- 49.Chan E. D., Chan J., Schluger N. W. (2001) What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am. J. Respir. Cell Mol. Biol. 25, 606–612. [DOI] [PubMed] [Google Scholar]
- 50.Nozaki Y., Hasegawa Y., Ichiyama S., Nakashima I., Shimokata K. (1997) Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 65, 3644–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toshchakov V., Jones B. W., Perera P. Y., Thomas K., Cody M. J., Zhang S., Williams B. R., Major J., Hamilton T. A., Fenton M. J., Vogel S. N. (2002) TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 3, 392–398. [DOI] [PubMed] [Google Scholar]
- 52.Thoma-Uszynski S., Stenger S., Takeuchi O., Ochoa M. T., Engele M., Sieling P. A., Barnes P. F., Rollinghoff M., Bolcskei P. L., Wagner M., Akira S., Norgard M. V., Belisle J. T., Godowski P. J., Bloom B. R., Modlin R. L. (2001) Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291, 1544–1547. [DOI] [PubMed] [Google Scholar]
- 53.Kimura H., Miura S., Shigematsu T., Ohkubo N., Tsuzuki Y., Kurose I., Higuchi H., Akiba Y., Hokari R., Hirokawa M., Serizawa H., Ishii H. (1997) Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn’s disease. Dig. Dis. Sci. 42, 1047–1054. [DOI] [PubMed] [Google Scholar]
- 54.Grisham M. B., Ware K., Gilleland H. E. Jr., Gilleland L. B., Abell C. L., Yamada T. (1992) Neutrophil-mediated nitrosamine formation: role of nitric oxide in rats. Gastroenterology 103, 1260–1266. [DOI] [PubMed] [Google Scholar]
- 55.Grisham M. B., Specian R. D., Zimmerman T. E. (1994) Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J. Pharmacol. Exp. Ther. 271, 1114–1121. [PubMed] [Google Scholar]
- 56.Shaul P. W., North A. J., Wu L. C., Wells L. B., Brannon T. S., Lau K. S., Michel T., Margraf L. R., Star R. A. (1994) Endothelial nitric oxide synthase is expressed in cultured human bronchiolar epithelium. J. Clin. Invest. 94, 2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]