IFN-α can selectively inhibit cytokine-induced P-Akt as a potential mechanism to disrupt homeostasis of T lymphocytes.
Keywords: homeostatic proliferation, cytokines
Abstract
Persistent type I IFN production occurs during chronic viral infections, such as HIV disease. As type I IFNs have antiproliferative activity, it is possible that chronic exposure to these cytokines could adversely affect T cell homeostasis. We investigated the capacity of IFN-α to impair T cell proliferation induced by the homeostatic cytokine, IL-7, or another common γ-chain cytokine, IL-2, in cells from healthy human donors. We found that IL-7- or IL-2-induced proliferation of CD4+ T cells was partially inhibited in the presence of IFN-α. The CD4+ T cells that were exposed to IFN-α also displayed attenuated induction of IL-2 and CD40L following TCR stimulation. Analyses of signaling pathways indicated that IL-7 and IL-2 induced a delayed and sustained P-Akt signal that lasted for several days and was partially inhibited by IFN-α. In contrast, IL-7-induced P-STAT5 was not affected by IFN-α. Furthermore, IFN-α had no detectable effect on P-Akt that was induced by the chemokine SDF-1. Both inhibitors of P-Akt and P-STAT5 blocked IL-7-induced T cell proliferation, confirming that both signaling pathways are important for IL-7-induced T cell proliferation. These results demonstrate that IFN-α can selectively inhibit cytokine-induced P-Akt as a potential mechanism to disrupt homeostasis of T lymphocytes.
Introduction
Type I IFNs represent a key innate defense mechanism to fight viral infection. Among the various activities of type I IFNs, antiproliferative effects of these cytokines have been recognized as potentially important components of antiviral and -tumor defenses [1–3]. Although several molecular targets of cell-cycle regulation by type I IFNs have been identified [4–7], few studies have characterized these effects in primary T cells, and there is limited knowledge of the potential interactions of type I IFNs with homeostatic cytokines, such as IL-7 or IL-2, which are important for T cell survival and growth. Therefore, we performed studies to evaluate the effects of IFN-α on T cell proliferation, T cell function, and T cell signaling in a model of IL-7-induced homeostatic proliferation.
IL-7 is an important cytokine that is critical for maintenance of T cell numbers. Disruption of the IL-7/IL-7R axis results in severe lymphopenia [8, 9] and IL-7 is a critical factor in homeostatic T cell expansion that occurs in lymphopenic hosts [10, 11]. IL-7 administration in persons with HIV disease or other lymphopenic conditions results in T cell expansion and promotes T cell survival [12–15]. Thus, IL-7 is not only a key physiologic signal for T cell homeostasis but also, represents a developing tool for therapeutic interventions.
IL-7 mediates its effects by enhancing the expression of antiapoptotic molecules, such as B cell lymphoma 2 [10, 16, 17], and by inducing cellular proliferation through regulation of molecules that control cell-cycle progression, such as p27kip [18, 19]. IL-7 binds to a heterodimeric receptor comprised of an α-chain (CD127) and the common γ-chain (CD132). IL-7R signaling includes JAK/STAT and PI3K/P-Akt activation, which affect cellular survival and proliferation [20–22].
IL-2 is also an important growth factor for T cells that induces proliferation and uses similar signaling machinery as IL-7. IL-2 activates T cells by signaling through the shared common γ-chain and through the β-chain (CD122), whereas the α-chain of the IL-2R (CD25) provides for high-affinity interactions of this complex with the cytokine. IL-2R activation, like IL-7R activation, leads to activation of JAK/STAT signaling along with P-Akt activation. Previous studies suggest that IFN-α may lead to impairments of IL-2-induced STAT5 signaling that are demonstrable at the level of DNA binding [5].
Type I IFNs are produced at elevated levels in HIV disease, and although these cytokines play an important role in antiviral defenses, chronic exposure to these cytokines may have detrimental effects [23–25]. For example, as a result of chronic exposure, it is thought that type I IFNs could contribute to T cell death by regulating various apoptotic pathways [26–28]. An alternative, but not mutually exclusive, hypothesis is that type I IFNs could disrupt T cell homeostasis as a consequence of its antiproliferative effects. Here, we study the potential for IFN-α to inhibit T cell proliferation induced by the homeostatic cytokine, IL-7, and another T cell growth factor, IL-2. Our studies uncover novel aspects of IL-7 signaling kinetics in primary T cells and suggest that IFN-α may mediate antiproliferative activity by selectively regulating P-Akt in T cells stimulated with these cytokines.
MATERIALS AND METHODS
Cells and cell culture
Whole blood was collected from healthy adult volunteers who signed informed consent through a protocol approved by the University Hospitals of Cleveland Institutional Review Board. PBMCs were isolated over a Ficoll-Hypaque cushion. In some assays, PBMCs were labeled with CFSE. PBMCs were incubated in 0.25 μM CFSE at 37°C for 10 min and washed with PBS, supplemented with 10% FCS. CFSE-labeled cells were resuspended in X-VIVO serum-free medium and incubated in 24-well plates at a concentration of 2 million cells/ml. Cells were stimulated with rIL-7 (Cytheris; 5 ng/ml) to induce proliferation. IFN-α (PBL) was added at 500 U/ml or as otherwise indicated. Cells were allowed to incubate for 7 days and were then, in some cultures, additionally stimulated with SEB (2 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) for 2 h, followed by a 4 h incubation with Golgi plug reagent. Intracellular flow analyses were performed to measure expression of CD40L, IL-2, and IFN-γ. Additional experiments involved purified CD4+ cells that were separated from PBMC by negative selection (magnetic bead separation; Miltenyi Biotec, San Diego, CA, USA). Cells were >97% pure, as determined by flow cytometric analyses. Purified CD4 cells were CFSE labeled and incubated with or without IL-7 (5 ng/ml) or IL-2 (50 ng/ml; BD PharMingen, San Diego, CA, USA) ± IFN-α (500 U/ml or as indicated). After 3 or 7 days, cells were stimulated with CytoStim beads, which activate T cells by cross-linking TCRs (Miltenyi Biotec) for 2 h, followed by 3 h of Golgi plug (BD Biosciences, San Jose, CA, USA) treatment. Cells were assessed for CFSE dye dilution and for intracellular expression of CD40L. Some studies included IL-7-treated cells that were incubated additionally with wortmannin (500 nM; PI3K inhibitor; Sigma-Aldrich) or N′-[(4-Oxo-4H-chromen-3-yl)methylene]nicotinohydrazide (500 μM; P-STAT5 inhibitor; EMD Millipore, Billerica, MA, USA).
Flow cytometry
SEB-stimulated cells and cells incubated without stimulation were assessed for expression of CD40L, IL-2, and IFN-γ. Cells were surface stained with antibodies reactive to CD4, and dead cells were excluded from analyses by staining with Live/Dead Fixable Yellow Dead Cell Stain Kit (Invitrogen, Grand Island, NY, USA). Cells were fixed and permeabilized with the Cytofix/Cytopermeabilization Kit (BD PharMingen) and then stained with fluorochrome-labeled antibodies reactive with CD40L (BD PharMingen), IFN-γ (BioLegend, San Diego, CA, USA), IL-2 (BD Biosciences), or appropriate isotype controls. In some assays, cells were tested for expression of P-STAT5 and P-Akt by use of methods that we have described previously [29, 30]. In brief, cells were incubated with or without IL-7 and IFN-α for 15 min overnight (1 day) or for 2 or 3 days. Cells were treated with 100 μl 16% ultrapure methanol-free formaldehyde (Polysciences, Warrington, PA, USA) for 10 min at 37°C. Cells were then transferred to polystyrene tubes, washed with PBS, and resuspended in 500 μl cold 90% methanol for 30 min. Cells were washed and stained with anti-CD4 (BioLegend), anti-CD3 (BioLegend), anti-P-STAT5 (BD Biosciences; recognizing Tyr694), and anti-P-Akt antibodies (BD Biosciences; recognizing the Ser473 epitope) for 60 min on ice before analyses on a BD LSRII flow cytometer (BD Biosciences).
For experiments involving the assessment of apoptosis, CFSE-labeled PBMCs were incubated for 7 days under various conditions, washed, and surface stained with antibodies reactive to CD3 and CD4 (BioLegend) and additionally stained with Live/Dead Fixable Yellow Dead Cell Stain Kit (Invitrogen). Cells were washed twice with 1 ml ice-cold PBS and then stained with Annexin V-PE (BD PharMingen) in the presence of 1× Annexin V binding buffer (BD PharMingen) for 15 min. Cells were examined by flow cytometry.
In some studies, PBMCs were preincubated with IFN-α (1000 U/ml) for 2 days, washed, resuspended in 300 μl X-VIVO medium, placed in polystyrene tubes, incubated in a 37°C water bath for 10 min, and then stimulated with SDF-1 (10 ng/ml; R&D Systems, Minneapolis, MN, USA) for 1 min. Cells were treated with 400 μl BD Cytofix for 10 min, washed, and resuspended in 200 μl BD Phosflow Perm Buffer III. Cells were incubated on ice and in the dark for 30 min, washed, and stained with anti-CD3, anti-CD4, and anti-P-Akt fluorochrome-conjugated antibodies as above.
Statistical analyses
SPSS software was used for statistical analyses. Nonparametric tests were used to assess differences between cells incubated in different conditions (Kruskal-Wallis multigroup comparison and Mann-Whitney U-tests). Nonparametric tests (Wilcoxon signed-rank tests and sign tests) were used to assess differences in paired data
RESULTS
IFN-α impairs IL-7-induced proliferation responses and diminishes cellular function in CD4+ T cells
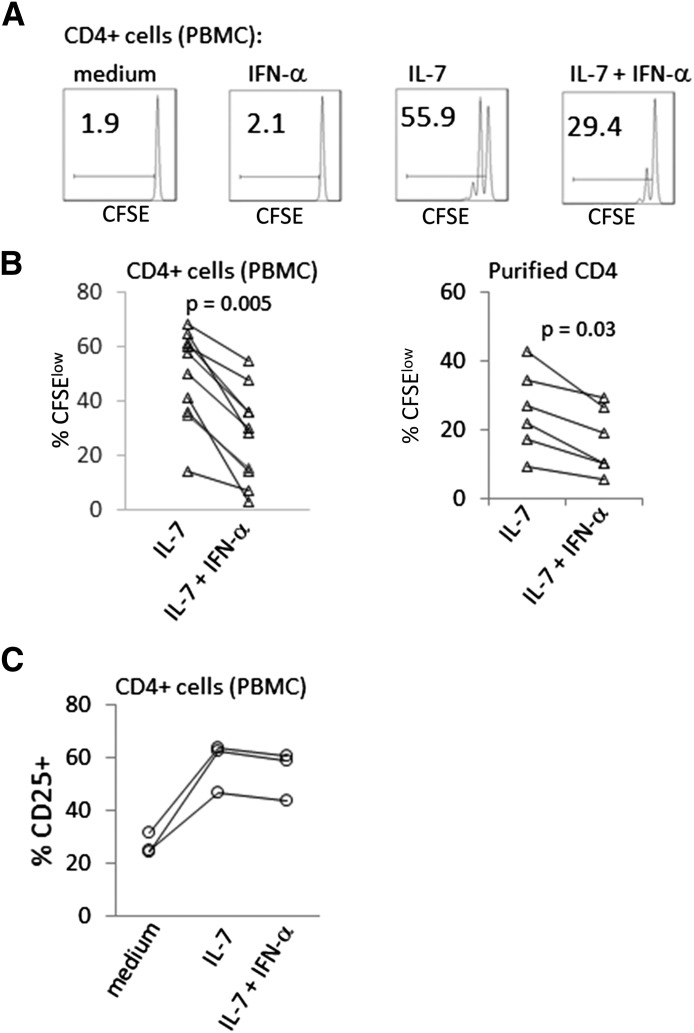
To assess the effects of IFN-α on IL-7-induced CD4+ T cell proliferation, CFSE-labeled PBMCs or purified CD4+ T cells were incubated with IL-7 for 7 days in the presence or absence of IFN-α. The addition of IFN-α to IL-7-treated cells reduced proliferation (CFSE dye dilution) among CD4+ T cells within PBMCs and also in the purified CD4+ T cell populations (Fig. 1). The magnitude of inhibition by IFN-α was dose dependent and still detectable at concentrations as low as 30 U/ml in PBMC assays (Supplemental Fig. 1). In contrast to the capacity of IFN-α to inhibit IL-7-induced T cell proliferation over 7 days, IFN-α had little effect on the induction of CD25 expression that was induced by IL-7 in CD4+ T cells after an overnight incubation (Fig. 1C). Thus, not all aspects of T cell responses to IL-7 were impaired by IFN-α.
Figure 1. IFN inhibits IL-7-induced proliferation.
PBMCs or negatively selected purified CD4+ T cells were CFSE labeled and incubated with IL-7 (5 ng/ml) ± IFN-α (500 U/ml). Flow cytometric analyses were performed after 7 days to assess cell proliferation. Cells were gated based on forward- and side-scatter characteristics to exclude debris and forward-scatter height versus area to exclude doublets. Viability dye stain was used to exclude dead cells. Histograms represent CD4+ lymphocytes. (A) CFSE dye dilution of gated CD4+ cells at 7 days is shown on the x-axis for the various conditions. (B) Summary data of CFSE dye dilution from studies of PBMC gated for CD4+ T cells (left) and of purified CD4+ T cell cultures (right). Each pair of connected symbols represents data from a different donor. (C) Assessment of CD25 expression in CD4+ T cells incubated overnight in medium alone, IL-7 (5 ng/ml) or IL-7 plus IFN-α (500 U/ml). Cells incubated in IFN-α alone showed no change in CD25 expression (not shown). Connected symbols represent data from a single donor. Results are shown for 3 different donors.
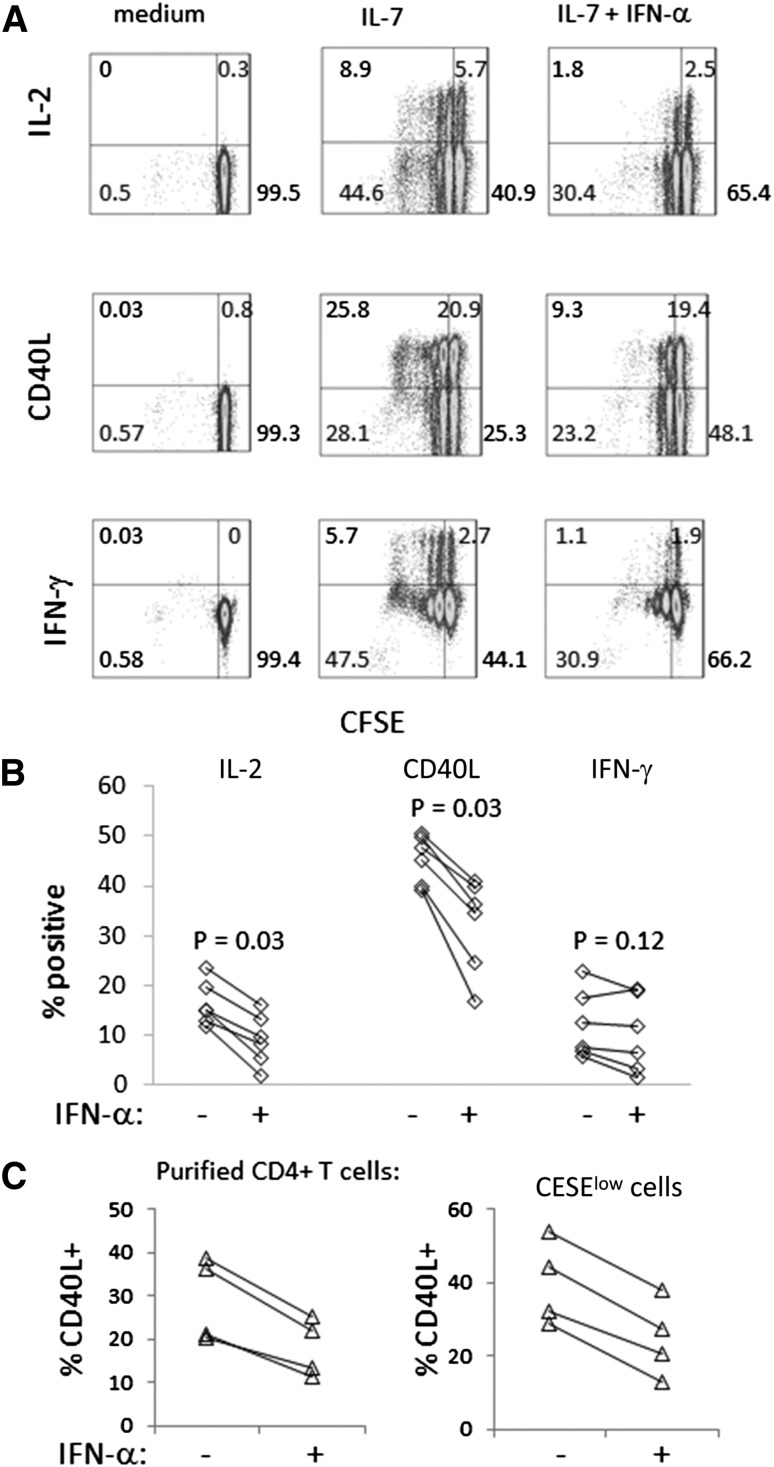
To test the functionality of CD4+ T cells that had been preincubated for 7 days with IL-7 or IL-7 + IFN-α, we stimulated cells with SEB and measured expression of intracellular CD40L, IL-2, or IFN-γ. The cells that were preincubated with IL-7 plus IFN-α were less able to express IL-2 and CD40L compared with cells incubated in IL-7 alone (Fig. 2). In contrast, the capacity of T cells to produce IFN-γ was not impaired significantly in T cells preincubated with IL-7 plus IFN-α. In 2 experiments, the cells were washed free of cytokine before SEB stimulation to determine if inhibition of SEB responsiveness was dependent on maintaining IFN-α in the cell culture. Even after washing the cells free of cytokine and replating before SEB stimulation, the cells that had been incubated previously with IFN-α + IL-7 demonstrated reduced SEB responses compared with cells incubated with IL-7 alone (percent inhibition of CD40L induction in 2 different donors = 56% and 50%, and percent inhibition of IL-2 induction = 30% and 61%, comparing cells preincubated with IL-7 + IFN-α with cells preincubated in IL-7 alone). Therefore, the magnitude of inhibition was similar whether IFN-α had been left in the culture for the duration of SEB stimulation or had been washed out of the culture before SEB stimulation.
Figure 2. Preincubation of CD4+ T cells in IFN-α plus IL-7 reduces T cell function compared with preincubation of cells in IL-7 alone.
PBMCs or purified CD4+ T cells were CFSE labeled and incubated with IL-7 (5 ng/ml) ± IFN-α (500 U/ml) for 7 days. Cells were then stimulated with SEB (PBMC) or CytoStim beads (purified CD4+ T cells) to induce intracellular cytokines or CD40L expression. Cells were gated based on forward- and side-scatter characteristics to exclude debris and forward-scatter height versus area to exclude doublets. Viability dye stain was used to exclude dead cells. CFSE dye dilution and intracellular CD40L expression among CD4+ cells were determined by flow cytometry. (A) Representative histograms depict IL-2, CD40L, and IFN-γ expression (y-axis), which were induced by SEB stimulation and CFSE dye dilution (x-axis). The CFSE dye dilution resulted from preincubation in IL-7 or IL-7 + IFN-α. The addition of SEB did not cause further proliferation, as the assay was performed over hours and the Golgi plug reagent had been added during the SEB incubation period (not shown). (B) Summary data of cytokine induction in PBMCs that had been incubated in IL-7 or IL-7 + IFN-α before SEB stimulation. (C) Summary data that use purified CD4+ T cells from 4 different donors, showing induction of CD40L expression in total and in CFSElow cells at day 7 following preincubation of cells in IL-7 or IL-7 + IFN-α (P = 0.068 in each experiment).
To ascertain if the effects of IFN-α on T cell functionality could be mediated through direct effects in T cells, purified CD4+ T cells that had been preincubated for 7 days with IL-7 or IL-7 + IFN-α were stimulated with CytoStim beads, as examined for intracellular induction of CD40L. Similar to our observations in PBMCs, the addition of IFN-α to IL-7 during the 7 day preincubation resulted in diminished induction of CD40L following TCR stimulation. Notably, the defects in CD40L induction among cells that were preincubated with IL-7 plus IFN-α were observed, even in cells that had proliferated during the preincubation period (Fig. 2C).
IFN-α diminishes T cell viability
To assess the potential for IFN-α to affect cell viability, we incubated PBMCs from 14 healthy control donors in medium alone or medium supplemented with IFN-α, IL-7, or both cytokines. After 7 days, CD4+ T cells were examined with viability dye stain to assess cell death (Supplemental Fig. 2). Analyses indicated that there was an increase in cell death among CD4+ T cells cultured for 7 days with IFN-α (mean = 18.7%; n = 14) compared with death in cells incubated in medium alone (mean = 11%; P = 0.002). There was also an increase in cell death in cells incubated with IL-7 plus IFN-α (mean = 9.1%) compared with cells incubated in IL-7 alone (mean = 6.4%; P = 0.039). Thus, IFN diminished CD4+ T cell viability in 7 day cultures.
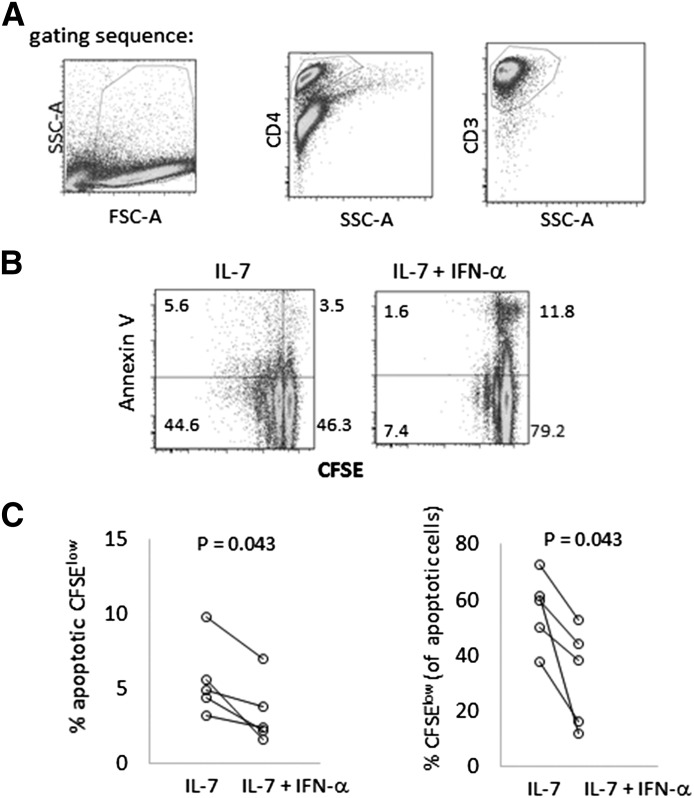
Although this observation suggested an adverse effect of IFN-α on T cell viability, it did not address the possibility that cells treated with IL-7 + IFN-α might proliferate normally but then die after cell division. To address this possibility, we assessed cell viability in PBMCs from 5 additional donors by use of Annexin V to identify early and late apoptotic cells (Fig. 3). Analyses of CD3+CD4+ cells demonstrated that among these 5 subjects, the addition of IFN-α to IL-7-treated cells resulted in reduced frequencies of live and apoptotic CFSE dim cells (divided cells). Moreover, among the apoptotic cells, the proportion that had proliferated and then died during the culture period was actually reduced by IFN-α. These results are most consistent with a failure of cells to divide rather than division followed by death in cells exposed to IL-7 + IFN-α.
Figure 3. IFN-α causes cell death, primarily in nondividing T cells, in IL-7-treated cell cultures.
PBMCs were CFSE labeled and incubated with IL-7 (5 ng/ml) ± IFN-α (500 U/ml) for 7 days. (A) Apoptosis was assessed by first gating out debris, then gating on CD4+ cells, and then CD3+ cells. SSC-A, Side-scatter-area; FSC-A, forward-scatter-area. (B) Histograms depict cell proliferation by CFSE dye dilution (x-axis) and apoptosis by Annexin V binding (y-axis) in IL-7 and IL-7 + IFN-α-treated cells. (C) Data summarize the percentages of apoptotic CFSElow cells among the CD4+CD3+ cells (left) and the percentage of CFSElow cells among only the apoptotic CD4+CD3+ cells (right).
IFN-α inhibits P-Akt but not P-STAT5 in IL-7-treated cells
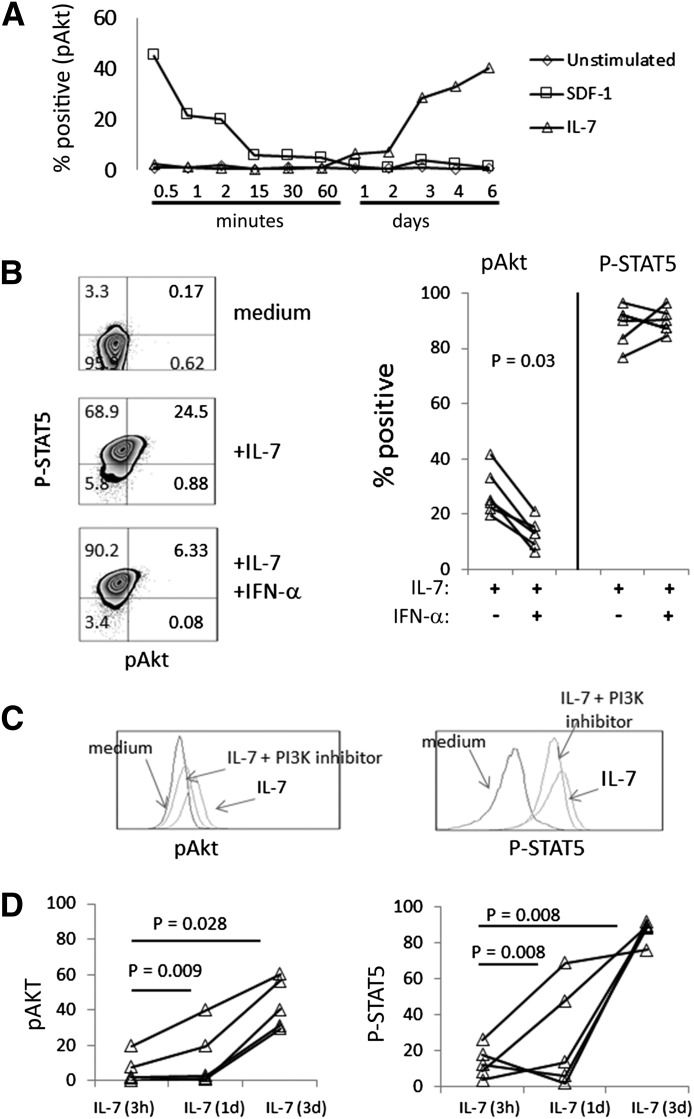
To understand the effects of IFN-α on cell signaling, we compared P-Akt with P-STAT5 expression in PBMCs that had been incubated with IL-7 or with IL-7 plus IFN-α. Cell signaling was assessed at various time-points. After IL-7 stimulation, a P-STAT5 signal was rapidly detected (1–15 min; not shown) and remained high through 3 days of cell culture (not shown). In contrast, P-Akt was not consistently detected at early time-points, ranging from minutes up through 1 day poststimulation, but became consistently discernible 2–3 days poststimulation with IL-7 and remained detectable over several days (Fig. 4A). The delayed induction of P-Akt that was mediated by IL-7 was in contrast to a rapid and transient induction of P-Akt that was induced by the chemokine SDF-1 (Fig. 4A). Importantly, in the presence of IFN-α, P-Akt, which was induced by IL-7, was blunted, as assessed at day 2 (not shown) and day 3 poststimulation (Fig. 4B). In contrast, there was no appreciable effect of IFN-α on P-STAT5 expression (Fig. 4B). To confirm the specificity of our P-Akt antibody, some cells were treated with the PI3K inhibitor wortmannin on day 2, and P-Akt was assessed on day 3. The blocking of PI3K resulted in blunted P-Akt expression but did not affect P-STAT5 (Fig. 4C).
Figure 4. IFN-α impairs P-Akt but not P-STAT5 signaling in IL-7-treated cells.
(A) PBMCs were incubated in medium alone, medium + IL-7 (5 ng/ml), or medium + SDF-1 (10 ng/ml) for various periods of time as indicated, and then cells were assessed for expression of P-Akt by intracellular flow cytometry. (B) Flow cytometry histograms (left) in CD3+CD4+ cells, showing induction of P-STAT5 (y-axis) and P-Akt (x-axis) at 3 days of incubation in PBMC treated with medium alone, medium + IL-7 (5 ng/ml), or medium + IL-7 and IFN-α (500 U/ml). No induction of P-Akt was observed in cells incubated with IFN-α alone (not shown). Summary data from 6 donors are provided (right). (C) PBMCs were incubated with IL-7 for 2 days, and then some cells were treated with an inhibitor of PI3K signaling (wortmannin). After an additional overnight incubation, cells were assessed for P-Akt expression. (D) PBMCs from 5 different donors were treated with IL-7 for 3 days and assessed for P-Akt expression by intracellular flow cytometry (labeled “IL-7”). In comparison, some cells were incubated with IL-7 for 3 h (IL-7, 3 h) or 1 day (IL-7, 1 day), washed, and then returned to culture in the absence of IL-7 until analysis for P-Akt at Day 3.
To determine if the expression of P-Akt and P-STAT5, which we detected at day 3 poststimulation, was dependent on prolonged IL-7 exposure, we incubated cells with IL-7 for 3 h or 1 day, washed the cells, and replated the cells in medium alone until day 3. We compared the P-Akt expression and P-STAT5 signal at day 3 in these cells to signals detected in cells that had been left in IL-7 for 3 days continuously. The washing of IL-7 from the cell cultures after 3 h or 1 day of incubation in the absence of IFN-α resulted in diminished P-Akt and P-STAT5 detection on day 3 (Fig. 4D). These observations suggest that prolonged and continuous IL-7 exposure are required to observe optimal, late signaling events.
To ascertain if IFN-α might adversely affect expression of CD127, which provides a docking site for PI3K upstream of Akt activation [32], we incubated PBMCs with IFN-α for various periods of time (1–3 days). We found no evidence of diminished CD127 expression after incubating CD4+ cells in IFN-α (not shown). This observation, together with the lack of an effect on P-STAT5 by IFN-α, suggests that the signaling deficiencies induced by IFN-α are more likely mediated by a postreceptor signaling mechanism rather than by modulation of CD127 expression.
IFN-α inhibits P-Akt signaling that is induced by IL-2 but not by SDF-1
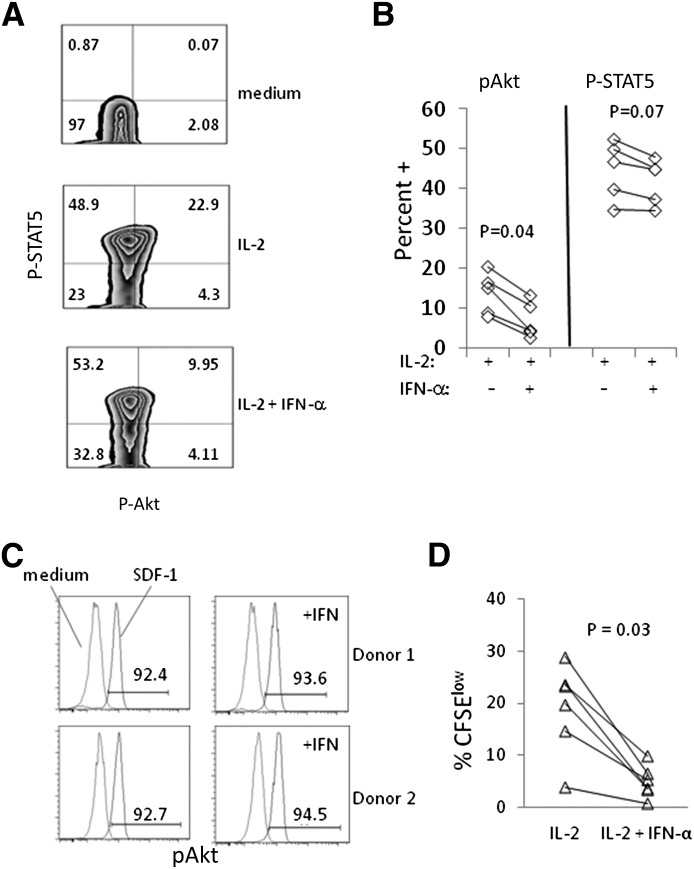
We next considered the potential for IFN-α to inhibit P-Akt upon activation of T cells with other stimuli. PBMCs were stimulated with IL-2 or SDF-1 during or after exposure to IFN-α. For IL-2 studies, PBMCs were incubated with IL-2, plus or minus IFN-α, for 3 days and assessed for P-STAT5 and P-Akt expression. Similar to the effects of IFN-α on IL-7 signaling, induction of P-Akt after 3 days of stimulation by IL-2 was impaired by IFN-α, but there was little effect on P-STAT5 (Fig. 5A and B). To examine further the specificity of the IFN-α effect, PBMCs were preincubated with IFN-α for 2 days and then stimulated with SDF-1, the chemokine ligand for CXCR4. In contrast to our observations with IL-7 or IL-2 stimulation, IFN-α had no detectable effect on P-Akt induction by SDF-1 (Fig. 5C). These data suggest that IFN-α may affect P-Akt signaling from cytokine receptors more readily than from chemokine receptors.
Figure 5. Impaired responses to IL-2 but not SDF-1 in CD4 cells exposed to IFN-α.
(A) PBMCs were CFSE labeled and incubated in medium alone, medium + IL-2 (50 ng/ml), or medium + IL-2 + IFN-α (500 U/ml). Histograms show expression of P-STAT5 (y-axis) and P-Akt (x-axis) after 3 days of incubation among CD3+CD4+ cells, and cells incubated in IFN-α looked similar to cells incubated in medium alone (not shown). (B) Results are shown for cells from 5 different donors comparing P-Akt and P-STAT5 induction in CD3+CD4+ cells incubated for 3 days in the presence of IL-2 or IL-2 + IFN-α. (C) PBMCs were preincubated in medium alone or in medium + IFN-α (1000 U/ml) for 2 days before stimulating cells with SDF-1 (10 ng/ml) for 1 min. The percent of P-Akt+ cells is indicated for cells stimulated with SDF-1. (D) PBMCs were incubated for 7 days with IL-2 or IL-2 + IFN-α. The percentage of CFSElow cells was determined by flow cytometry (debris, doublets, and dead cells were removed from the analysis). Each pair of connected symbols represents cells from a different donor (n = 6).
As IFN-α impaired P-Akt expression in IL-2-stimulated cells, we also asked if proliferation of these cells was impaired at 7 days poststimulation. In 6 different donors, we found clear evidence that IFN-α reduced the proliferation of CD4+ T cells in response to IL-2 stimulation (Fig. 5D). These data indicate that IFN-α inhibited P-Akt induction and proliferation but only marginally reduced P-STAT5 induction in IL-2-stimulated T cells.
Inhibition of P-Akt or P-STAT5 blocks cellular proliferation and function
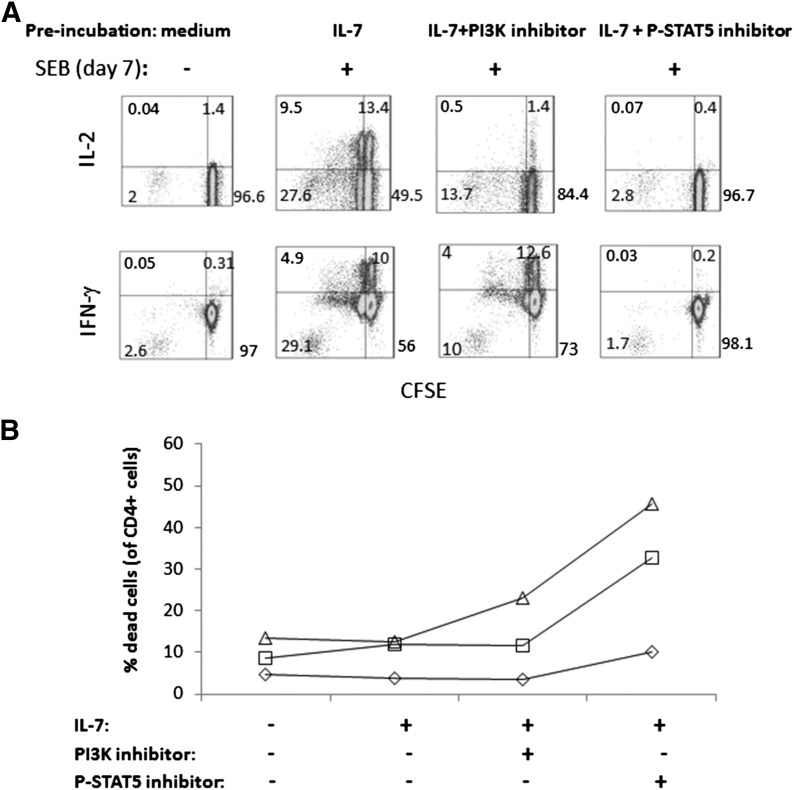
To confirm that Akt and STAT5 signaling are important for cellular proliferation and to ascertain if disruption of either pathway results in functional impairments among cells that survive 7 day incubations, CFSE-labeled PBMCs were stimulated with IL-7 and incubated with or without a PI3K inhibitor (wortmannin) or a P-STAT5 inhibitor. After 7 days, CFSE dye dilution and responses to SEB stimulation were measured. Incubation of IL-7-stimulated cells with an inhibitor of PI3K reduced cellular proliferation and diminished the ability of cells to respond to TCR activation. Similar to the effects of IFN-α on T cell function, IL-2 production appeared to be more severely affected than IFN-γ production in surviving cells after TCR stimulation (Fig. 6A). The blocking of P-STAT5 manifested in even more striking impairments that rendered cells completely dysfunctional. Inhibition of P-STAT5 also caused enhanced cell death in IL-7-stimulated cells after 7 days of culture (Fig. 6B). Therefore, T cells that have been stimulated with IL-7 demonstrate impaired functional responses if signaling through P-STAT5 or P-Akt is blocked.
Figure 6. Inhibition of PI3K or STAT5 reduced cell proliferation, decreased functionality, and enhanced cell death in cells treated with IL-7.
(A) CFSE-labeled PBMCs were incubated in medium alone, medium + IL-7, medium + IL-7 + PI3K inhibitor, and IL-7 + STAT5 inhibitor and then assessed 7 days later for proliferation (CFSE dye dilution, x-axis) and response to SEB stimulation (CD40L induction, y-axis). Data are representative of results from 3 different donors. (B) Viability was assessed in the above cultures by gating on forward- and side-scatter to eliminate debris and monocytes, followed by forward-scatter-height versus forward-scatter-area to exclude doublets (not shown). Then, cells were gated on CD4 cells to assess viability dye stain. The percentage of dead CD4+ cells is shown on the y-axis under the various conditions (x-axis). Connected symbols represent data from a single donor. Data from 3 different donors are shown as different symbols.
DISCUSSION
Our studies demonstrate that IFN-α mediates inhibition of IL-7- or IL-2-induced T cell proliferation. In IL-7-stimulated cells, we found evidence of enhanced cell death when IFN-α was also added to cultures, making it difficult to tease apart the potential role for cell death and the potential antimitotic effects of IFN-α. Analyses of apoptosis among cells that had divided during the 7 day incubation, however, suggested that IFN-α primarily caused cell death among cells that had failed to divide. Importantly, this observation does not exclude the possibility that the IL-7-treated cells, which died in the presence of IFN-α, may have entered cell-cycle originally but died before their first mitotic division.
Along with inhibition of T cell proliferation, our results indicate that the addition of IFN-α to IL-7-stimulated cells leads to decreased P-Akt but has no detectable effect on P-STAT5 expression compared with cells stimulated with IL-7 alone. Although we measured P-STAT5 at a site that is important for DNA binding (Tyr694 [32]), our studies do not rule out the possibility that IFN-α may impair P-STAT5 DNA binding downstream of phosphorylation [5]. Nonetheless, we found that IFN-α had little effect on the induction of CD25 expression by IL-7 during an overnight incubation. As the CD25 gene promoter region has a well-defined STAT5 binding site, which promotes transcriptional activation [33, 34], this observation suggests that P-STAT5 signaling is not impaired downstream of phosphorylation when cells are treated with IFN-α, at least at the time-point and under the conditions used here. The sustained capacity of cells to signal via P-STAT5 in the presence of IFN-α, despite reductions in P-AKT signaling, is also consistent with reports by others that IFN-α impairs IL-7-induced T cell proliferation but does not inhibit P-STAT5 signaling in primary T cells [35].
Our results indicate that P-Akt can be inhibited by IFN-α when cells are activated by IL-7 or IL-2; however, we did not find evidence that IFN-α could inhibit P-Akt induced by SDF-1. This raises the possibility that IFN-α mediates its effects on signaling machinery that is not shared between SDF-1 and common γ-chain cytokines, such as IL-7 or IL-2. Possibilities include PI3K subunits, adaptor molecules, or perhaps receptor components that may be differentially regulated by IFN-α. For the latter possibility, we found no evidence, at least for CD127, that cytokine receptor expression was disrupted by IFN-α (not shown). Recent studies by others also indicate that IFN-α does not impair CD127 expression in T cells and may actually enhance expression in long-term cultures [35]. The delay in P-Akt that occurs after IL-7 stimulation is consistent with the possibility that IL-7 may induce a secondary factor that leads to enhancement of the signal. This raises the possibility that IFN-α could inhibit the induction of this unknown secondary signal. Further studies that address these possibilities will be needed to clarify the mechanism of P-Akt inhibition by IFN-α.
The kinetics of Akt activation that we have defined here add to our understanding of IL-7- mediated signaling. Previous studies indicate that PI3K signaling is rapid in thymocytes exposed to IL-7 but delayed in mature T cells [22, 36]. Studies in primary bulk T cells suggest that IL-7-induced P-Akt is delayed but detectable within 3–6 h after IL-7 exposure [22]. Our results suggest that these signals are delayed for 1–2 days but then sustained for several days after IL-7 stimulation. Although we have focused our analyses on CD4+ T cells, we noted somewhat stronger but transient P-Akt expression in CD4−CD3+ cells (mostly CD8+ T cells) at earlier time-points (not shown). This may explain why we find a longer delay than the previous studies that used bulk T cells for analyses. Alternatively, the studies used different methods of detection (Western blot in previous studies and flow cytometry in our study) that could also contribute to these differences [22]. In either case, both studies confirm that IL-7-induced Akt signaling is delayed in mature T cells.
Our experiments also demonstrate a requirement for prolonged IL-7 exposure for optimal induction of P-Akt (Fig. 4D). This observation may have important implications for mechanisms of homeostatic proliferation. Although it is difficult to extrapolate in vitro observations to in vivo conditions, our findings suggest that to induce homeostatic division, T cells likely need to experience sustained high levels of systemic IL-7 exposure or dwell for prolonged periods close to an IL-7 source, such as the fibroblastic reticular network within lymph nodes or stromal cells of the bone marrow. It is interesting to speculate that T cells with the potential to arrest their migration within IL-7-producing niches may be more likely to undergo homeostatic proliferation in vivo and that cells may alter their migratory properties in lymphopenic conditions to promote their exposure to IL-7.
Our observations also have implications for HIV disease where sustained IFN-α exposure has been implicated in pathogenesis. In particular, IFN-α is increased in plasmas of HIV-infected persons, and IFN-α levels are correlated directly with plasma viremia and inversely with CD4 cell counts [23]. Furthermore, gene signatures indicative of type I IFN activation are observed in CD4+ T cells from HIV patients who experience relatively poor CD4 T cell reconstitution during the administration of antiretroviral therapy [37]. Thus, the antiproliferative activity of type I IFN and particularly, the impairment of IL-7 responsiveness could conceivably play an important role in limiting T cell reconstitution in HIV-infected persons receiving antiretroviral therapy or in persons with uncontrolled, chronic disease. Although strategies for neutralizing IFN are being considered to ameliorate the immunopathology of chronic, treated HIV infection, an alternative strategy may be to circumvent the antiproliferative activity of this cytokine while leaving antiviral and innate-immune activities intact.
AUTHORSHIP
S.F.S., M.M.L., G.A.H., C.V.H., and T.P.N. designed the studies. T.P.N., D.A.B., and J.C.M. performed experiments. All authors contributed to writing the manuscript.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (Grant AI-104480), the Center for Aids Research at Case Western Reserve University (NIH Grant AI-036219), and a grant from the James B. Pendleton Charitable Trust.
Glossary
- CD40L
cluster of differentiation 40 ligand
- P
phosphorylation
- SDF-1
stromal cell-derived factor 1
- SEB
Staphylococcus enterotoxin B
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Blomgren H., Strander H., Cantell K. (1974) Effect of human leukocyte interferon on the response of lymphocytes to mitogenic stimuli in vitro. Scand. J. Immunol. 3, 697–705. [DOI] [PubMed] [Google Scholar]
- 2.Nederman T., Benediktsson G. (1982) Effects of interferon on growth rate and radiation sensitivity of cultured, human glioma cells. Acta Radiol. Oncol. 21, 231–234. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn S., Blomgren H., Einhorn N., Strander H. (1983) In vitro and in vivo effects of interferon on the response of human lymphocytes to mitogens. Clin. Exp. Immunol. 51, 369–374. [PMC free article] [PubMed] [Google Scholar]
- 4.Einat M., Resnitzky D., Kimchi A. (1985) Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature 313, 597–600. [DOI] [PubMed] [Google Scholar]
- 5.Erickson S., Matikainen S., Thyrell L., Sangfelt O., Julkunen I., Einhorn S., Grandér D. (2002) Interferon-alpha inhibits Stat5 DNA-binding in IL-2 stimulated primary T-lymphocytes. Eur. J. Biochem. 269, 29–37. [DOI] [PubMed] [Google Scholar]
- 6.Sangfelt O., Erickson S., Einhorn S., Grandér D. (1997) Induction of Cip/Kip and Ink4 cyclin dependent kinase inhibitors by interferon-alpha in hematopoietic cell lines. Oncogene 14, 415–423. [DOI] [PubMed] [Google Scholar]
- 7.Tiefenbrun N., Melamed D., Levy N., Resnitzky D., Hoffman I., Reed S. I., Kimchi A. (1996) Alpha interferon suppresses the cyclin D3 and cdc25A genes, leading to a reversible G0-like arrest. Mol. Cell. Biol. 16, 3934–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giliani S., Mori L., de Saint Basile G., Le Deist F., Rodriguez-Perez C., Forino C., Mazzolari E., Dupuis S., Elhasid R., Kessel A., Galambrun C., Gil J., Fischer A., Etzioni A., Notarangelo L. D. (2005) Interleukin-7 receptor alpha (IL-7Ralpha) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol. Rev. 203, 110–126. [DOI] [PubMed] [Google Scholar]
- 9.Von Freeden-Jeffry U., Vieira P., Lucian L. A., McNeil T., Burdach S. E., Murray R. (1995) Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schluns K. S., Kieper W. C., Jameson S. C., Lefrançois L. (2000) Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432. [DOI] [PubMed] [Google Scholar]
- 11.Seddon B., Zamoyska R. (2002) TCR and IL-7 receptor signals can operate independently or synergize to promote lymphopenia-induced expansion of naive T cells. J. Immunol. 169, 3752–3759. [DOI] [PubMed] [Google Scholar]
- 12.Lévy Y., Sereti I., Tambussi G., Routy J. P., Lelièvre J. D., Delfraissy J. F., Molina J. M., Fischl M., Goujard C., Rodriguez B., Rouzioux C., Avettand-Fenoël V., Croughs T., Beq S., Morre M., Poulin J. F., Sekaly R. P., Thiebaut R., Lederman M. M. (2012) Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin. Infect. Dis. 55, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sereti I., Dunham R. M., Spritzler J., Aga E., Proschan M. A., Medvik K., Battaglia C. A., Landay A. L., Pahwa S., Fischl M. A., Asmuth D. M., Tenorio A. R., Altman J. D., Fox L., Moir S., Malaspina A., Morre M., Buffet R., Silvestri G., Lederman M. M.; ACTG 5214 Study Team (2009) IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sportès C., Babb R. R., Krumlauf M. C., Hakim F. T., Steinberg S. M., Chow C. K., Brown M. R., Fleisher T. A., Noel P., Maric I., Stetler-Stevenson M., Engel J., Buffet R., Morre M., Amato R. J., Pecora A., Mackall C. L., Gress R. E. (2010) Phase I study of recombinant human interleukin-7 administration in subjects with refractory malignancy. Clin. Cancer Res. 16, 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sportès C., Hakim F. T., Memon S. A., Zhang H., Chua K. S., Brown M. R., Fleisher T. A., Krumlauf M. C., Babb R. R., Chow C. K., Fry T. J., Engels J., Buffet R., Morre M., Amato R. J., Venzon D. J., Korngold R., Pecora A., Gress R. E., Mackall C. L. (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amos C. L., Woetmann A., Nielsen M., Geisler C., Odum N., Brown B. L., Dobson P. R. (1998) The role of caspase 3 and BclxL in the action of interleukin 7 (IL-7): a survival factor in activated human T cells. Cytokine 10, 662–668. [DOI] [PubMed] [Google Scholar]
- 17.Hassan J., Reen D. J. (1998) IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur. J. Immunol. 28, 3057–3065. [DOI] [PubMed] [Google Scholar]
- 18.Barata J. T., Cardoso A. A., Nadler L. M., Boussiotis V. A. (2001) Interleukin-7 promotes survival and cell cycle progression of T-cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1). Blood 98, 1524–1531. [DOI] [PubMed] [Google Scholar]
- 19.Li W. Q., Jiang Q., Aleem E., Kaldis P., Khaled A. R., Durum S. K. (2006) IL-7 promotes T cell proliferation through destabilization of p27Kip1. J. Exp. Med. 203, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadi H., Ke S., Roifman C. M. (1994) Activation of phosphatidylinositol-3 kinase by ligation of the interleukin-7 receptor is dependent on protein tyrosine kinase activity. Blood 84, 1579–1586. [PubMed] [Google Scholar]
- 21.Foxwell B. M., Beadling C., Guschin D., Kerr I., Cantrell D. (1995) Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur. J. Immunol. 25, 3041–3046. [DOI] [PubMed] [Google Scholar]
- 22.Lali F. V., Crawley J., McCulloch D. A., Foxwell B. M. (2004) A late, prolonged activation of the phosphatidylinositol 3-kinase pathway is required for T cell proliferation. J. Immunol. 172, 3527–3534. [DOI] [PubMed] [Google Scholar]
- 23.Hardy G. A., Sieg S., Rodriguez B., Anthony D., Asaad R., Jiang W., Mudd J., Schacker T., Funderburg N. T., Pilch-Cooper H. A., Debernardo R., Rabin R. L., Lederman M. M., Harding C. V. (2013) Interferon-α is the primary plasma type-I IFN in HIV-1 infection and correlates with immune activation and disease markers. PLoS ONE 8, e56527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy G. A., Sieg S. F., Rodriguez B., Jiang W., Asaad R., Lederman M. M., Harding C. V. (2009) Desensitization to type I interferon in HIV-1 infection correlates with markers of immune activation and disease progression. Blood 113, 5497–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbeuval J. P., Shearer G. M. (2007) HIV-1 immunopathogenesis: how good interferon turns bad. Clin. Immunol. 123, 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraietta J. A., Mueller Y. M., Yang G., Boesteanu A. C., Gracias D. T., Do D. H., Hope J. L., Kathuria N., McGettigan S. E., Lewis M. G., Giavedoni L. D., Jacobson J. M., Katsikis P. D. (2013) Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog. 9, e1003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbeuval J. P., Grivel J. C., Boasso A., Hardy A. W., Chougnet C., Dolan M. J., Yagita H., Lifson J. D., Shearer G. M. (2005) CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood 106, 3524–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herbeuval J. P., Hardy A. W., Boasso A., Anderson S. A., Dolan M. J., Dy M., Shearer G. M. (2005) Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type I IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 102, 13974–13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bazdar D. A., Kalinowska M., Sieg S. F. (2009) Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J. Infect. Dis. 199, 1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mudd J. C., Murphy P., Manion M., Debernardo R., Hardacre J., Ammori J., Hardy G. A., Harding C. V., Mahabaleshwar G. H., Jain M. K., Jacobson J. M., Brooks A. D., Lewis S., Schacker T. W., Anderson J., Haddad E. K., Cubas R. A., Rodriguez B., Sieg S. F., Lederman M. M. (2013) Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood 121, 2914–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkitaraman A. R., Cowling R. J. (1994) Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur. J. Immunol. 24, 2168–2174. [DOI] [PubMed] [Google Scholar]
- 32.Gouilleux F., Wakao H., Mundt M., Groner B. (1994) Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 13, 4361–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John S., Robbins C. M., Leonard W. J. (1996) An IL-2 response element in the human IL-2 receptor alpha chain promoter is a composite element that binds Stat5, Elf-1, HMG-I(Y) and a GATA family protein. EMBO J. 15, 5627–5635. [PMC free article] [PubMed] [Google Scholar]
- 34.Lécine P., Algarté M., Rameil P., Beadling C., Bucher P., Nabholz M., Imbert J. (1996) Elf-1 and Stat5 bind to a critical element in a new enhancer of the human interleukin-2 receptor alpha gene. Mol. Cell. Biol. 16, 6829–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cha L., de Jong E., French M. A., Fernandez S. (2014) IFN-α exerts opposing effects on activation-induced and IL-7-induced proliferation of T cells that may impair homeostatic maintenance of CD4+ T cell numbers in treated HIV infection. J. Immunol. 193, 2178–2186. [DOI] [PubMed] [Google Scholar]
- 36.Dadi H. K., Roifman C. M. (1993) Activation of phosphatidylinositol-3 kinase by ligation of the interleukin-7 receptor on human thymocytes. J. Clin. Invest. 92, 1559–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez S., Tanaskovic S., Helbig K., Rajasuriar R., Kramski M., Murray J. M., Beard M., Purcell D., Lewin S. R., Price P., French M. A. (2011) CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J. Infect. Dis. 204, 1927–1935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.