Abstract
Prenatal stress in humans is associated with psychiatric problems in offspring such as anxiety, depression, and schizophrenia. These same illnesses are also associated with neuronal nicotinic acetylcholine receptor (nAChR) dysfunction. Despite the known associations between prenatal stress exposure and offspring mental illness, and between mental illness and nAChR dysfunction, it is not known whether prenatal stress exposure impacts neuronal nAChRs. Thus, we tested the hypothesis that maternal stress alters the development of hippocampal alpha4 beta2 (α4β2*) and alpha7 (α7*) nicotinic receptor levels in adult offspring. Female Sprague-Dawley rats experienced unpredictable variable stressors 2–3 times daily during the last week of gestation. At weaning (21days) the offspring of prenatally stressed (PS) and nonstressed (NS) dams were assigned to same-sex PS or NS groups. In young adulthood (56 days), the brains of offspring were collected and adjacent sections processed for quantitative autoradiography using [125I]-epibatidine (α4β2* nicotinic receptor-selective) and [125I]-α-bungarotoxin (α-BTX ; α7* nicotinic receptor-selective) ligands. We found that PS significantly increased hippocampal α4β2* nAChRs of males and females in all subfields analyzed. In contrast, only females showed a trend toward PS-induced increases in α7* nAChRs in the dentate gyrus. Interestingly, NS females displayed a significant left-biased lateralization of α7 nAChRs in the laconosum moleculare of area CA1, whereas PS females did not, suggesting that PS interfered with normal lateralization patterns of α7 nAChRs during development. Taken together, our results suggest that PS impacts the development of hippocampal nAChRs, which may be an important link between PS exposure and risk for neuropsychiatric illness.
Keywords: prenatal stress, gestation, maternal, stress, memory, nicotinic, acetylcholine, alpha4beta2, alpha7, epibatidine, alpha-bungarotoxin, asymmetry, lateralization, hippocampus, depression, anxiety, psychopathology
Introduction
Stress during pregnancy increases the risk for offspring to develop psychopathologies such as anxiety (Van den Bergh & Marcoen, 2004), depression (van den Bergh et al., 2008), and schizophrenia (Malaspina et al., 2008; for review see Schlotz & Phillips, 2009). These psychopathologies with etiological links to prenatal stress are also associated with brain cholinergic dysfunction. For example, nicotine addiction and rates of smoking are significantly higher among psychiatric patients than the general population (Poirier et al., 2002). Recent studies estimate smoking rates of approximately 64% in schizophrenia patients, and between 35–65% of patients with mood disorders such as major depression, as compared to 19–23% of smokers in the general population(Mineur & Picciotto, 2009; de Leon & Diaz, 2012; Dickerson et al., 2012). In addition, depressed patients show altered levels of the acetylcholine (ACh) precursor, choline, in the prefrontal cortex (Kumar et al., 2002), and choline levels increase in the hippocampus following electroconvulsive therapy, commensurate with an alleviation of depression symptoms (Ende et al., 2000).
Animal studies have increased our understanding of the role neuronal nicotinic acetylcholine receptors (nAchR) play in mediating depressive and anxiety-related behaviors. The two primary nAChRs expressed in the brain are the α4β2* and α7* (* denotes that the exact subunit composition of these receptors is not known; Gotti & Clementi, 2004; Gotti et al., 2006; Millar & Gotti, 2009). Drugs targeting either receptor subtype modulate depressive-like behavior in tests such as the forced swim, or tail suspension tests (Picciotto et al., 2002; Mineur & Picciotto, 2010). Similarly, hippocampal α7* nAChR activation alters rat anxiety-related behaviors in the social anxiety paradigm (File et al., 1998; File et al., 2000a).
Despite the known associations between prenatal stress and psychopathology in humans, and between psychopathology and cholinergic function, the relationship between prenatal stress and cholinergic function is largely unexplored. One rodent study found that prenatal stress increased corticosterone-induced Ach release in the hippocampus of rats (Day et al., 1998). However, whether prenatal stress impacts nAChR levels is not known. A handful of rodent studies have investigated the effects of stress or corticosterone exposure in adulthood, and found decreased levels of hippocampal α7* (Pauly & Collins, 1993; Grun et al., 1995; Stitzel et al., 1996; Stevens et al., 2001; Hunter et al., 2010) and α4β2* (Takita & Muramatsu, 1995) nAChRs. As such, we hypothesized that prenatal stress would also alter levels of α7* and α4β2* nAChRs in the hippocampus.
Methods
Animals
Twelve timed-pregnant Sprague-Dawley rats were ordered from Charles Rivers Laboratories (Portage, MI) and were 2 days pregnant upon arrival. Pregnant females were singly housed in static clear polycarbonate cages with wire bar lids and microisolator air filtration covers. All animals had ad libitum access to food and water. Bedding (Tekfresh, Harlan Laboratories Inc., Indianapolis, IN), food (2018 Teklad Global 18% Protein Rodent Diet, Harlan Laboratories Inc., Indianapolis, IN), and filtered water were changed weekly. One day prior to parturition, the females were transferred to larger cages (40. 6×30. 5×20. 3 cm) and extra bedding was provided as nesting material. Room conditions were maintained at 21°C with a 12:12 light/dark cycle. All animals were treated in accordance with NIH guidelines and all protocols were approved by the IACUC of the University of Colorado Anschutz Medical Campus.
Prenatal stress procedure
Half of the pregnant females were randomly selected to experience unpredictable variable stress 2–3 times daily during the last week of gestation (gestational days 14–21). The stressors were mild in nature and included restraint in cylindrical restrainers (60 min), swim in water at room temperature (15 min), exposure to a cold room at 4 °C (6 hours), social stress (5 rats/cage for 9 hours), and an overnight fast. We followed Koenig’s protocol (2005), with the exception of exposing animals to a reverse light schedule. All stressed animals received the same schedule of stressors. The remaining 6 females served as controls and were exposed to only routine animal husbandry.
Litters
All pups were born on gestation day 22. Food and water continued to be replaced weekly following parturition, but the bedding and nests were left undisturbed until weaning at 22 days of age to minimize stress (as detailed in Koenig et al., 2005) . Cage cleanliness was closely monitored during this time, and additional bedding was provided if necessary. Upon weaning, weekly cage changing resumed, and animals were housed 2 per cage with same-sex littermates. It was not feasible to completely prevent litter effects by using only one representative pup from each litter (Zorrilla, 1997), however, we tried to minimize litter effects by employing only two animals (of each sex) per litter (n=7–10/group; overall experiment N=37).
Tissue Collection
In early adulthood (56 days of age), animals were deeply anesthetized with isoflurane, decapitated, brains removed, frozen in dry ice snow and stored at −80 degrees Celsius. Two sets of adjacent sections (12 µM) were collected through the rostrocaudal extent of the hippocampus (bregma −2.30 to 4.52, Paxinos & Watson, 2007) onto gelatin-coated slides for processing with [125I]-α-BTX (α7* receptor selective) or [125I]-epibatidine (α4β2* receptor selective) autoradiography.
Autoradiography
α-bungarotoxin (α-BTX) Autoradiography
Brain tissue sections, subdivided into total- and nonspecific-binding groups, were incubated in a solution containing 50 mM Tris-HCl, 120 mM NaCl and 2 mg/ml bovine serum albumin (TBS/BSA buffer, pH 7.4) for 30 minutes at room temperature. The TBS/BSA buffer for the nonspecific tissue also contained 5 mM nicotine to define nonspecific binding. The two tissue sets were then incubated in the TBS/BSA buffer containing [125I]α-BTX (5 nM, specific activity 2200 Ci/mmol, Perkin Elmer, Waltham, Massachusetts) at 37oC for 3 hours. Following incubation with the ligand, the tissue was rinsed in the TBS/BSA buffer for 5 min, in TBS without BSA for 15 minutes and in PBS for 5 minutes, all at 37oC. After being stored for 1 day at 22 °C, sections were apposed to radiation-sensitive Hyperfilm for 2 days with 14C standards of known radioactivity (American Radiolabeled Chemicals, St. Louis, MO) for 72 hours (Adams et al., 2002).
Epibatidine Autoradiography
Brain tissue sections, subdivided into total- and nonspecific-binding groups, were incubated in a solution containing 144 mM NaCl, 1.5 mM KCl, 2 mM CaCl2 , 1 mM MgSO4 and 20 mM HEPES (isotonic buffer, pH 7.5) for 15 minutes at room temperature. The isotonic buffer for the nonspecific tissue also contained 300 nM nicotine to define nonspecific binding. The two tissue sets were then incubated in isotonic buffer containing [125I]epibatidine (0.5 nM, specific activity 2200 Ci/mmol, Perkin Elmer, Waltham, Massachusetts) at room temperature for 2 hours. Following incubation with the ligand, the tissue was rinsed in isotonic buffer for 2 X 10 seconds, in 0.1x isotonic buffer for 2 X 5 seconds and in 20 mM HEPES for 2 X 5 seconds, each rinse at 4oC. After being stored for 1 day at 22 °C, sections were apposed to radiation-sensitive Hyperfilm for 2 days with [14C] standards of known radioactivity for 7 days.
Quantification of Ligand Binding in the Hippocampus
Digital images were captured using a Chroma-Pro 45 light box and Retiga CCD camera (QImaging, Surrey, BC, Canada) using Simple PCI software (Hamamatsu Corp., Sewickley, PA). Autoradiograms were quantified with a computer-based image analysis system (ImageJ, NIH, Bethedsa, MD) using calibrated standards of reference (American Radiolabeled Chemicals, St. Lois, MO). Calibration curves of gray value against radioligand concentration (fmol/mg tissue) were constructed using [14C] standards of known radioactivity. Tribollet’s work (2004) served as an excellent reference for delineating subregions of the hippocampus in sections bound with epibatidine or α-BTX. Gray values were measured in subdivisions of the hippocampal formation for both epibatidine and α-BTX, and the corresponding radioactivity values were estimated from the calibration curve. Specific radioligand binding was calculated by subtracting values obtained in the presence of an excess of competing ligand (non-specific binding) from those in the absence (total binding), and are expressed as nCi/g tissue.
Statistics
Epibatidine (α4β2*) binding was analyzed by a mixed model 3-factor ANOVA where Sex and Stress Condition were the between-subjects variables, and Brain Hemisphere was the within-subjects variable. No significant effects of hemisphere were found, so the data were collapsed across hemisphere and analyzed by 2-factor between subjects ANOVA (Sex×Stress Condition). α-Bungarotoxin (α7*) binding was analyzed by a mixed model 2-factor ANOVA separately for males and females in which Stress Condition was the between-subjects variable, and Brain Hemisphere was the within-subjects variable.
Results
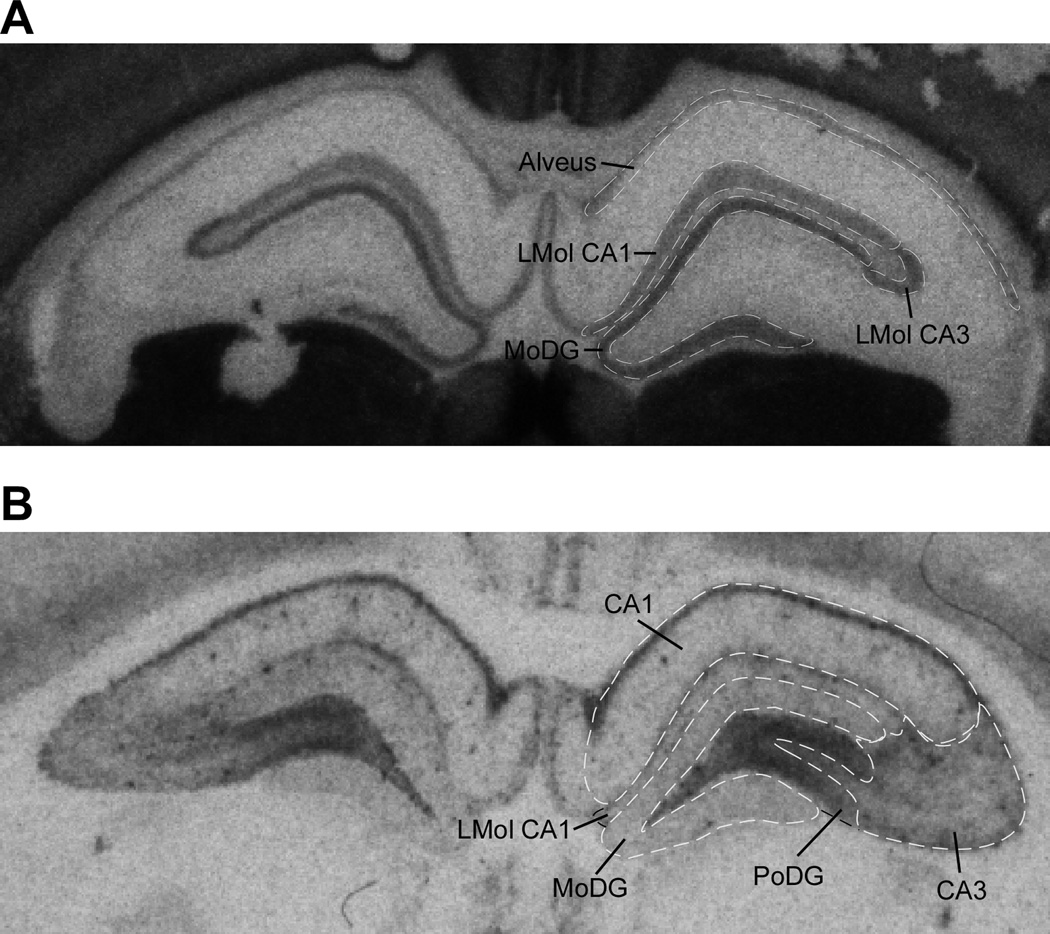
To investigate the effects of prenatal stress on levels of α7* and α4β2* nAChRs, we quantified the mean binding levels of α-BTX and epibatidine, respectively. The binding topography differed between epibatidine and α-BTX within the hippocampus (Tribollet et al., 2004). Epibatidine binding was present in the alveus, lacunosum moleculare of CA1 (LMol CA1), lacunosum moleculare of CA3 (LMol CA3), and the molecular layer of the dentate gyrus (MoDG). For α-BTX, the highest levels were seen in LMol CA1, the MoDG and the polymorphic layer of the dentate gyrus (PoDG), while lower levels of binding were seen in the remaining layers of CA1 and in area CA3 (Figure 1A and 1B).
Figure 1.
Representative photomicrograph of adjacent 12 µm hippocampal sections exposed to (A) I125-epibatadine for visualization of α4β2* nAChRs, and (B) I125-α-bungarotoxin for visualization of α7* nAChRs. (A) Epibatidine binding was found in the alveus, lacunosum moleculare of the CA1 (LMol CA1), lacunosum moleculare of the CA3 (LMol CA3), and the molecular layer of the dentate gyrus (MoDG). (B) a-BTX binding was highest in the CA1, lacunosum moleculare of the CA1 (LMol CA1), the molecular layer of the dentate gyrus (Mo DG), the polymorphic layer of the dentate gyrus (PoDG), and the CA3.
Epibatidine Binding
We examined hippocampal levels of epibatidine binding to determine the effects of prenatal stress and sex on α4β2* nAChRs levels. No significant effects of hemisphere were found on epibatidine binding for any hippocampal subfield, so the data were collapsed across hemisphere. Prenatal stress significantly increased epibatidine binding in the alveus [F(1,33)=7.023, p<0.02], LMol CA1 [F(1,33)=11.36, p<0.002], LMol CA3 [F(1,33)=10.75, p<0.003], and MoDG [F(1,33)=15.01, p<0.001] (Figures 1A and 2). No effects of sex or interactions between sex and stress condition were found for any of the subregions analyzed.
Figure 2.
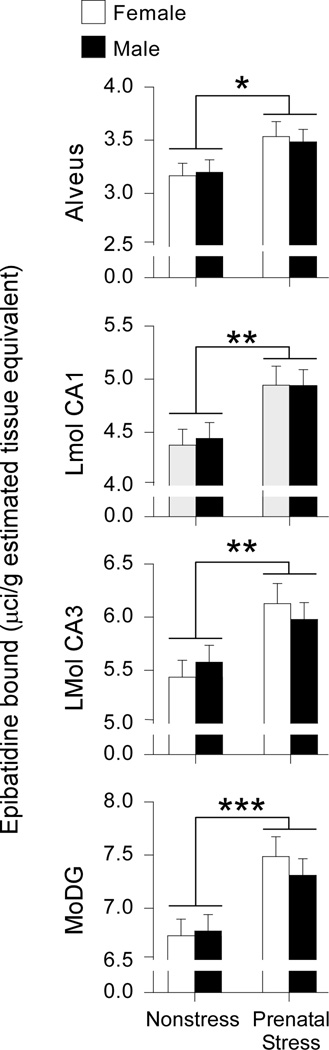
Prenatal stress increases α4β2* nicotinic acetylcholine receptor levels in the hippocampus. Prenatal stress significantly increased epibatidine binding in the following hippocampal subfields: lacunosum moleculare of the CA1 (LMol CA1), lacunosum moleculare of the CA3 (LMol CA3), and the molecular layer of the dentate gyrus (MoDG). No effects of sex or interactions between sex and stress condition were detected. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference between stressed and nonstressed groups (*p<0.05, **p<0.01, ***p<0.001).
α-BTX Binding
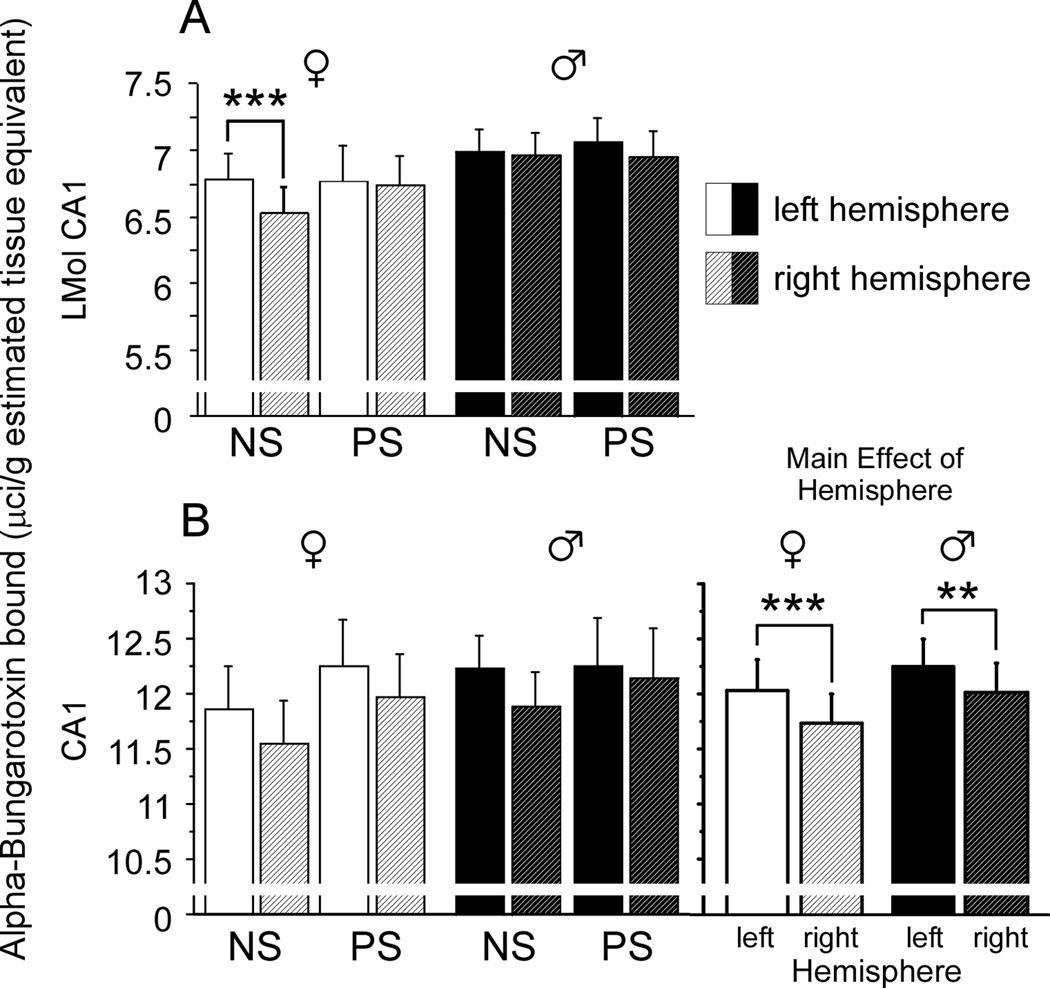
To determine the effects of prenatal stress on α7* nAChRs levels, we examined hippocampal levels of α-BTX binding separately in males and females. In the LMol CA1 of females, brain hemisphere significantly impacted α-BTX binding [Figure 4A; F(1,16)=7.90, p<0.01]. However, this main effect was qualified by a significant interaction between brain hemisphere and prenatal stress [F(1,16)=4.74, p<0.05]. Specifically, a left hemisphere bias in α-BTX binding was present in control [F(1,9)=49.13, p<0.0001] but not prenatally stressed females [F(1,9)=0.09, p=0.77], suggesting that prenatal stress disrupts a normative hemisphere difference in α7 receptor levels in the Lmol CA1 (Figure 4A). In contrast, no effects of stress condition or brain hemisphere were found in the LMol CA1 of males. In the remaining layers of the CA1 (Figure 4B), a significant left-biased lateralization of a-BTX binding was found in both females [Figure 4B, right panel; F(1,16)=17.15, p<0.001] and males [Figure 4B, right panel; F(1,18)=9.90, p<0.01]. Stress condition did not affect a-BTX binding in the remaining CA1 layers, nor did stress condition interact with brain hemisphere in either sex.
Figure 4.
Prenatal stress and brain hemisphere influence α7* nicotinic acetylcholine receptor levels in the CA1. (A) Lacunosum moleculare of the CA1 (LMol CA1). A significant interaction between brain hemisphere and stress condition was detected such that a significant left-biased lateralization of α-bungarotoxin binding was present in nonstressed (NS) but not prenatally stressed (PS) females. (B) CA1 remaining layers. Both males and females displayed a significant left-biased lateralization of α-bungarotoxin binding. No interactions between brain hemisphere and stress condition were detected for either sex. Data expressed as mean ± SEM. Asterisk (*) indicates a significant difference between left and right hemispheres (**p<0.01, ***p<0.001).
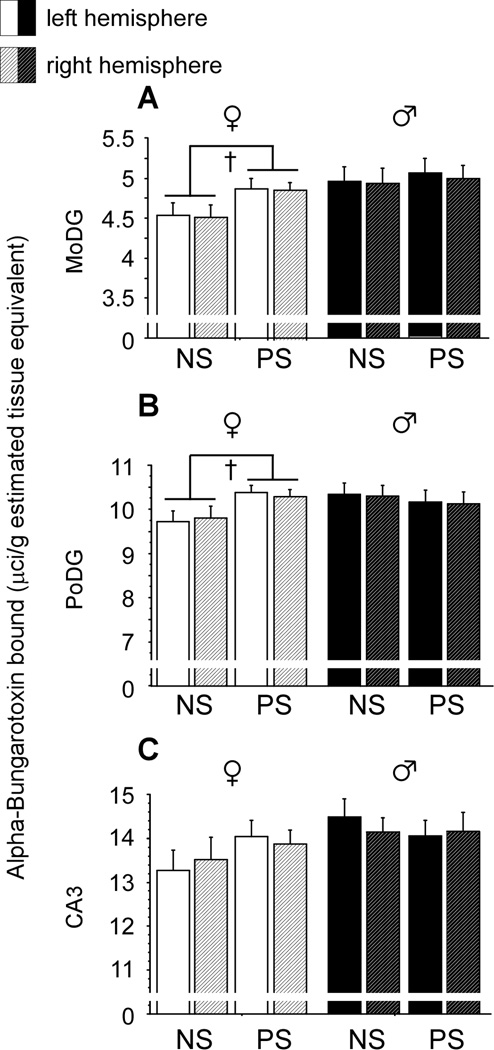
A trend toward a prenatal stress-induced increase in α-BTX binding was observed in the MoDG [Figure 5A; F(1,16)=3.23, p<0.1] and PoDG [Figure 5B; F(1,16)=3.31, p<0.1] of females but not males. Brain hemisphere did not impact α-BTX binding in the MoDG or PoDG of either sex, nor did hemisphere interact with stress condition. In the CA3, neither stress condition nor prenatal stress impacted α-BTX binding in males or females, and no interactions between these factors were observed in either sex (Figure 5C).
Figure 5.
Prenatal stress increases female α7* nicotinic acetylcholine receptor levels in the molecular layer of the dentate gyrus (MoDG) and polymorphic layer of the dentate gyrus (PoDG). (A&B) A trend toward a prenatal stress-induced increase in α-BTX binding was observed in the MoDG and PoDG of females but not males. No effects of brain hemisphere or interactions between brain hemisphere and stress condition were observed in either region. (C) Neither brain hemisphere nor stress condition influenced α-BTX binding in the CA3. Data expressed as mean ± SEM. Cross (†) indicates a difference between prenatally stressed (PS) and nonstressed (NS) females (p<0.1).
Discussion
Given that prenatal stress increases depressive- and anxiety-like behaviors in offspring (Vallee et al., 1997; Richardson et al., 2006; Markham & Koenig, 2011; Schulz et al., 2011; Weinstock, 2011), and that brain cholinergic activity also modulates depressive- and anxiety-like behaviors (Mineur & Picciotto, 2010), we hypothesized that prenatal stress would impact levels of hippocampal nAChRs. Our results support this hypothesis, as prenatal stress significantly increased adult α4β2* nicotinic receptor levels in the hippocampus. These increases in hippocampal α4β2* receptor levels may contribute to prenatal stress-induced increases in offspring anxiety- and depressive-like behaviors by increasing brain cholinergic activity. Indeed, the association between increased brain cholinergic activity and depressive symptoms was first recognized in humans when treatment with acetylcholinesterase inhibitors produced increased depressive symptoms (Janowsky et al., 1972; Janowsky et al., 1974). Similarly, given that nicotine activates and then rapidly desensitizes nAChRs, the anxiolytic effects of smoking may actually be due to decreased cholinergic signaling in the brain (Mineur & Picciotto, 2010). The “hypercholinergic” hypothesis of depression is further supported by numerous preclinical studies demonstrating that inhibiting the activity of α4β2* nAChRs alleviates depressive-like symptoms in rodents (Caldarone et al., 2004; Mineur et al., 2007; Rollema et al., 2009; Graef et al., 2011). Other studies also suggest that anxiety-related behaviors are modulated by nAChR activation in a dose- and time-dependent manner (File et al., 1998; File et al., 2000a; File et al., 2000b; File et al., 2001; File, 2003). Taken together, our data suggest that prenatal stress-induced increases in hippocampal α4β2* levels may contribute to increased depressive and anxiety-related behaviors in adult offspring.
In contrast to the across-the-board effects of prenatal stress on hippocampal α4β2* nAChRs in males and females, the effects of prenatal stress on α7* nAChRs were more subtle. In the dentate gyrus of females but not males, we observed a trend toward increased α7* nAChRs in both the MoDG and PoDG of females. Future studies with a larger sample size will decipher whether these interesting trends within the dentate gyrus are indeed meaningful. In the LMol CA1, prenatal stress and brain hemisphere interacted to impact α7* nAChRs, such that a large left-biased lateralization in α7* nAChRs was present in control but not prenatally stressed females. This finding suggests that prenatal stress disrupts a normative α7* nAChR asymmetry in the LMol CA1 of females. In addition, both males and females displayed a significant left-biased lateralization of α7* nAChRs in the remaining layers of the CA1, irrespective of prenatal stress exposure.
In humans, lateralization of brain structure and function is thought to reflect hemisphere specialization and increased efficiency (reviewed in Herve et al., 2013; Hou et al., 2013). In turn, mental illnesses such as schizophrenia, depression, and anxiety alter normative brain lateralization patterns (Shirakawa et al., 2001; Schore, 2002; Ribolsi et al., 2009; Buehlmann et al., 2010; Keune et al., 2011; Nusslock et al., 2011; Oertel-Knochel & Linden, 2011; Bruder et al., 2012; Nelson et al., 2012). For example, several hippocampal parameters that are lateralized in control subjects show reduced or exaggerated asymmetry in schizophrenia patients (Zaidel, 1999; Csernansky et al., 2002; Qiu et al., 2009). Thus, our finding that prenatal stress eliminates a left-biased asymmetry in the LMol CA1 may be analogous to the altered hippocampal lateralization patterns found in human mental illnesses such as schizophrenia. In addition, rodent studies also demonstrate hippocampal lateralization of structure and function, including a left-biased lateralization of high affinity choline uptake activity that is important for acetylcholine synthesis (Kristofikova et al., 2004). Thus, our finding that α-7* nAChR levels are left-biased in the CA1 contributes to our understanding of normative lateralization patterns in the hippocampal cholinergic system.
Although previous studies in rodents have investigated the effects of stress exposure in adulthood on hippocampal nAChRs (Pauly & Collins, 1993; Grun et al., 1995; Takita & Muramatsu, 1995; Stitzel et al., 1996; Stevens et al., 2001; Hunter et al., 2010), we are the first to report the effects of prenatal stress on nAChR development. As such, many questions remain. This study focused on the hippocampus, yet many other brain structures are significantly impacted by prenatal stress (Charil et al., 2010), several of which are rich in nAChRs. Thus, additional studies are needed to determine whether prenatal stress-induced alterations in nAChRs occur in regions such as the amygdala, and hypothalamus, and cortical areas. In addition, we do not know the time course for prenatal stress-induced increases in α4β2* nAChRs or for left-biased lateralization of α7* nAChRs. Understanding the time course for these changes is important for targeting potential timeframes for intervention. For example, does manipulation of α4β2* or α7* nAChRs during early development or in adulthood ameliorate the behavioral consequences of prenatal stress? The results of such experiments will provide better understanding of the relationships between prenatal stress, nAChR development, and adult behavioral outcomes, and will also inform future clinical studies.
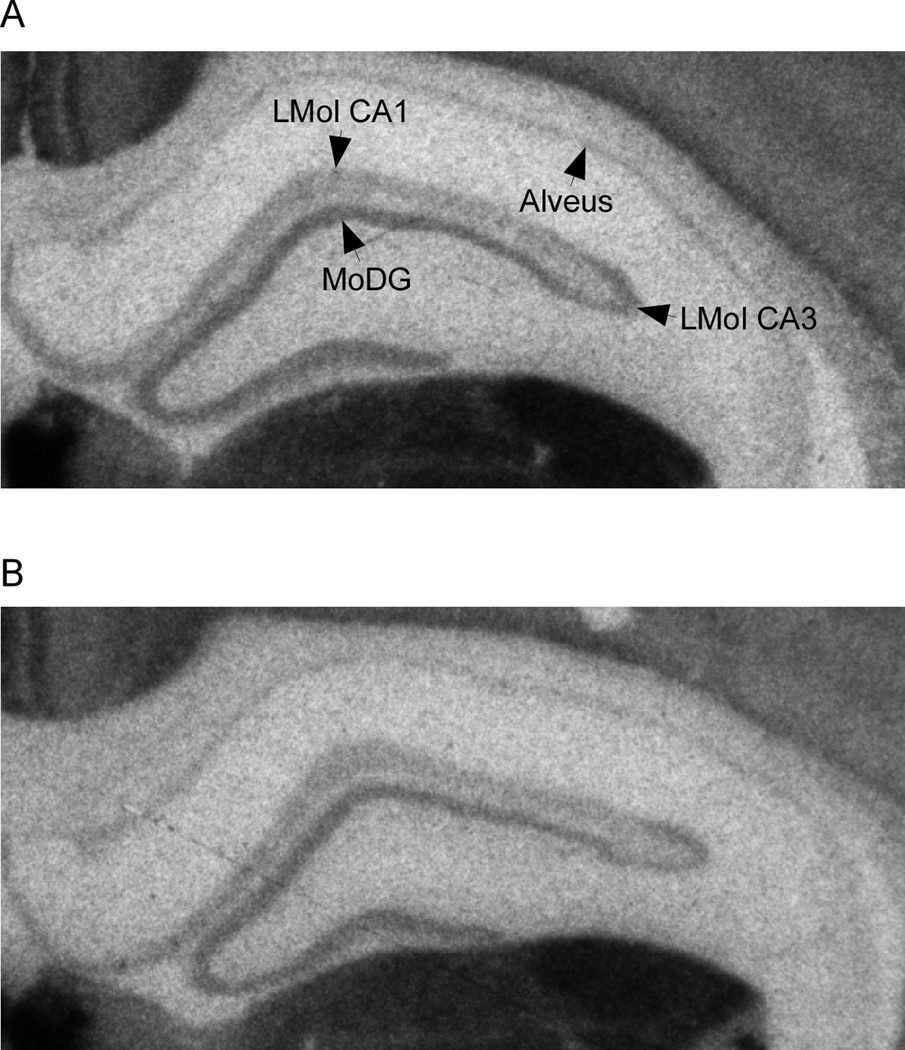
Figure 3.
Representative photomicrograph depicting the difference in hippocampal epibatadine binding intensity between prenatally stressed (A) and nonstressed (B) animals.
Acknowledgements
We thank Ariel Valdez for technical assistance and Mike Marks for providing guidance on epibatidine binding procedures. We also thank Melissa Sinkus, Clare Paterson, and Kristina McFadden for their helpful feedback on drafts of this manuscript.
Financial Support: VA Career Development Award (Schulz), VA Merit Awards (Stevens, Adams, and Leonard), and MH081177, MH073826, MH082999 (Leonard), NIH Training Grant NS097633 (Burke), and the Developmental Psychobiology Group Research Fund (Schulz).
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, Freedman R. Development of the [alpha]7 nicotinic cholinergic receptor in rat hippocampal formation. Developmental Brain Research. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Hellerstein D, Alvarenga JE, Alschuler D, McGrath PJ. Abnormal functional brain asymmetry in depression: Evidence of biologic commonality between major depression and dysthymia. Psychiatry Research. 2012;196:250–254. doi: 10.1016/j.psychres.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehlmann E, Berger GE, Aston J, Gschwandtner U, Pflueger MO, Borgwardt SJ, Radue E-W, Riecher-Roessler A. Hippocampus abnormalities in at risk mental states for psychosis? A cross-sectional high resolution region of interest magnetic resonance imaging study. Journal of Psychiatric Research. 2010;44:447–453. doi: 10.1016/j.jpsychires.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biological Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Research Reviews. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller JP, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- Day JC, Koehl M, Deroche V, Le Moal M, Maccari S. Prenatal stress enhances stress- and corticotropin-releasing factor-induced stimulation of hippocampal acetylcholine release in adult rats. Journal of Neuroscience. 1998;18:1886–1892. doi: 10.1523/JNEUROSCI.18-05-01886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz F. Genetics of schizophrenia and smoking: an approach to studying their comorbidity based on epidemiological findings. Human Genetics. 2012;131:877–901. doi: 10.1007/s00439-011-1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, Yolken RH. Cigarette Smoking Among Persons With Schizophrenia or Bipolar Disorder in Routine Clinical Settings, 1999–2011. Psychiatr Serv. 2012 doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. The hippocampus in patients treated with electroconvulsive therapy - A proton magnetic resonance spectroscopic imaging study. Archives of General Psychiatry. 2000;57:937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- File SE. Brain mechanisms mediating nicotine's effects on anxiety. European Journal of Pharmaceutical Sciences. 2003;19:S5–S5. [Google Scholar]
- File SE, Cheeta S, Kenny PJ. Neurobiological mechanisms by which nicotine mediates different types of anxiety. European Journal of Pharmacology. 2000a;393:231–236. doi: 10.1016/s0014-2999(99)00889-4. [DOI] [PubMed] [Google Scholar]
- File SE, Fluck E, Leahy A. Nicotine has calming effects on stress-induced mood changes in females, but enhances aggressive mood in males. International Journal of Neuropsychopharmacology. 2001;4:371–376. doi: 10.1017/S1461145701002577. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000b;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: Role of the dorsal hippocampus. Behavioral Neuroscience. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Progress in Neurobiology. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends in Pharmacological Sciences. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Graef S, Schoknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: An overview of preclinical and clinical findings. Psychopharmacology. 2011;215:205–229. doi: 10.1007/s00213-010-2153-8. [DOI] [PubMed] [Google Scholar]
- Grun EU, Pauly JR, Bullock AE, Collins AC. Corticosterone reversibly alters brain alpha-bungarotoxin binding and nicotine sensitivity. Pharmacology Biochemistry and Behavior. 1995;52:629–635. doi: 10.1016/0091-3057(95)00157-r. [DOI] [PubMed] [Google Scholar]
- Herve PY, Zago L, Petit L, Mazoyer B, Tzourio-Mazoyer N. Revisiting human hemispheric specialization with neuroimaging. Trends Cogn Sci. 2013;17:69–80. doi: 10.1016/j.tics.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Hou G, Yang X, Yuan TF. Hippocampal asymmetry: differences in structures and functions. Neurochem Res. 2013;38:453–460. doi: 10.1007/s11064-012-0954-3. [DOI] [PubMed] [Google Scholar]
- Hunter RG, Bloss EB, McCarthy KJ, McEwen BS. Regulation of the nicotinic receptor alpha7 subunit by chronic stress and corticosteroids. Brain Research. 2010;1325:141–146. doi: 10.1016/j.brainres.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Elyousef MK, Davis JM. ACETYLCHOLINE AND DEPRESSION. Psychosomatic Medicine. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Keune PM, Schoenenberg M, Wyckoff S, Mayer K, Riemann S, Hautzinger M, Strehl U. Frontal alpha-asymmetry in adults with attention deficit hyperactivity disorder: Replication and specification. Biological Psychology. 2011;87:306–310. doi: 10.1016/j.biopsycho.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behavioural Brain Research. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Kristofikova Z, St'astny F, Bubenikova V, Druga R, Klaschka J, Spaniel F. Age- and sex-dependent laterality of rat hippocampal cholinergic system in relation to animal models of neurodevelopmental and neurodegenerative disorders. Neurochemical Research. 2004;29:671–680. doi: 10.1023/b:nere.0000018837.27383.ff. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thomas A, Lavretsky H, Yue K, Huda A, Curran J, Venkatraman T, Estanol L, Mintz J, Mega M, Toga A. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. American Journal of Psychiatry. 2002;159:630–636. doi: 10.1176/appi.ajp.159.4.630. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S. Acute maternal stress in pregnancy and schizophrenia in offspring: A cohort prospective study. Bmc Psychiatry. 2008;8 doi: 10.1186/1471-244X-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl) 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Biological Basis for the Co-morbidity Between Smoking and Mood Disorders. Journal of Dual Diagnosis. 2009;5:122–130. doi: 10.1080/15504260902869964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends in Pharmacological Sciences. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BD, Sarapas C, Robison-Andrew EJ, Altman SE, Campbell ML, Shankman SA. Frontal Brain Asymmetry in Depression With Comorbid Anxiety: A Neuropsychological Investigation. Journal of Abnormal Psychology. 2012;121:579–591. doi: 10.1037/a0027587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive Vulnerability and Frontal Brain Asymmetry: Common Predictors of First Prospective Depressive Episode. Journal of Abnormal Psychology. 2011;120:497–503. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knochel V, Linden DE. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17:456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Collins AC. An autoradiographic analysis of alterations in nicotinic cholinergic receptors following 1 week of corticosterone supplementation. Neuroendocrinology. 1993;57:262–271. doi: 10.1159/000126368. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Elsevier Inc; 2007. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Poirier MF, Canceil O, Bayle F, Millet B, Bourdel MC, Moatti C, Olie JP, Attar-Levy D. Prevalence of smoking in psychiatric patients. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2002;26:529–537. doi: 10.1016/s0278-5846(01)00304-9. [DOI] [PubMed] [Google Scholar]
- Qiu A, Wang L, Younes L, Harms MP, Ratnanather JT, Miller MI, Csernansky JG. Neuroanatomical asymmetry patterns in individuals with schizophrenia and their non-psychotic siblings. Neuroimage. 2009;47:1221–1229. doi: 10.1016/j.neuroimage.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribolsi M, Koch G, Magni V, Di Lorenzo G, Rubino IA, Siracusano A, Centonze D. Abnormal brain lateralization and connectivity in schizophrenia. Rev Neurosci. 2009;20:61–70. doi: 10.1515/revneuro.2009.20.1.61. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147:2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, Picciotto MR. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. European Journal of Pharmacology. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Phillips DIW. Fetal origins of mental health: Evidence and mechanisms. Brain Behavior and Immunity. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Schore AN. Dysregulation of the right brain: a fundamental mechanism of traumatic attachment and the psychopathogenesis of posttraumatic stress disorder. Aust N Z J Psychiatry. 2002;36:9–30. doi: 10.1046/j.1440-1614.2002.00996.x. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiology & Behavior. 2011;104:340–347. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa O, Kitamura N, Lin XH, Hashimoto T, Maeda K. Abnormal neurochemical asymmetry in the temporal lobe of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:867–877. doi: 10.1016/s0278-5846(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Bullock AE, Collins AC. Chronic corticosterone treatment alters sensory gating in C3H mice. Pharmacology Biochemistry and Behavior. 2001;69:359–366. doi: 10.1016/s0091-3057(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Farnham DA, Collins AC. Chronic corticosterone treatment elicits dose-dependent changes in mouse brain alpha-bungarotoxin binding. Neuroscience. 1996;72:791–799. doi: 10.1016/0306-4522(95)00584-6. [DOI] [PubMed] [Google Scholar]
- Takita M, Muramatsu I. ALTERATION OF BRAIN NICOTINIC RECEPTORS INDUCED BY IMMOBILIZATION STRESS AND NICOTINE IN RATS. Brain Research. 1995;681:190–192. doi: 10.1016/0006-8993(95)00265-r. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124:405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vallee M, Mayo W, Dellu F, LeMoal M, Simon H, Maccari S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. Journal of Neuroscience. 1997;17:2626–2636. doi: 10.1523/JNEUROSCI.17-07-02626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8-and 9-year-olds. Child Development. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- van den Bergh BRH, van Calster B, Smits T, van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Weinstock M. Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: An update. Stress-the International Journal on the Biology of Stress. 2011;14:604–613. doi: 10.3109/10253890.2011.588294. [DOI] [PubMed] [Google Scholar]
- Zaidel DW. Regional differentiation of neuron morphology in human left and right hippocampus: comparing normal to schizophrenia. International Journal of Psychophysiology. 1999;34:187–196. doi: 10.1016/s0167-8760(99)00076-8. [DOI] [PubMed] [Google Scholar]