Abstract
Background
Home health care (HHC) has been the fastest growing health care sector for the past 3 decades. The uncontrolled home environment, increased use of indwelling devices, and the complexity of illnesses among HHC patients lead to increased risk for infections.
Methods
A systematic review of studies evaluating infection prevalence and risk factors among adult patients who received HHC services was conducted and guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Literature was searched using Medline, PubMed, and the Cumulative Index to Nursing and Allied Health as well as hand searching. Two reviewers independently assessed study quality using validated quality assessment checklists.
Results
Twenty-five studies met the inclusion criteria and were reviewed. The infection rates and identified risk factors for infections varied dramatically between studies. In general, patients receiving home parental nutrition treatments had higher infection rates than patients receiving home infusion therapy. The identified risk factors were limited by small sample sizes and other methodologic flaws.
Conclusions
Establishing a surveillance system for HHC infections, identifying patients at high risk for infections, tailoring HHC and patient education based on patient living conditions, and facilitating communication between different health care facilities will enhance infection control in HHC settings. Future studies should use a nationally representative sample and multivariate analysis for the identification of risk factors for infections.
Keywords: Infectious disease, Infection rate, Increased risk, Home environment, Home infusion, Hospice
Health care delivery systems in developed countries have undergone a dramatic change since the 1970s, with many acutely ill patients moving out of hospitals to their homes to reduce hospital and nursing home stays, improve patient outcomes, and subsequently cut health care costs.1,2 As a result, home health care (HHC), defined as “care provided by professionals to a person in his/her own home,”3 has become 1 of fastest growing health care sectors. In the United States, more than $72.2 billion was spent on HHC during 2009 alone4 and approximately 12 million Americans, most (69%) older than age 65 years,5 received care from more than 33,000 home health care providers nationwide during 2010.4 The demand for HHC is expected to increase as the population continues to age, with an estimate that 20% of Americans will be older than age 65 years in 2030.6 The increase in HHC services is not a phenomenon unique to the United States. In other developed countries, HHC is also expanding due to a combination of demographic shifts, changes in the epidemiologic landscape of diseases, and advances in technological support.7
Receiving health care in the home has many advantages for patients. It provides them with necessary care and services in the comfort of their own home and maintains their dignity and independence. However, it also poses special challenges and health hazards, one of which is infection control. Although patients making use of home care are less acutely ill than patients in hospitals or long-term-care facilities, they are exposed to potential hazards that are not experienced by hospitalized patients or long-term facility residents. These hazards put HHC patients at high risk for infections. For example, although HHC is overseen by health professionals, much of the actual care is provided by the patients themselves, family members, or close friends who do not have formal training. Unlike hospitals or long-term-care facilities, the home environment is usually limited by space and lacks sufficient supplies or resources, a situation that poses unintentional sanitary hazards. In addition, the increasing use of indwelling devices in HHC can further expose patients to risk for infections.8
During the 1990s, 3 infection outbreaks that captured national attention were reported in patients receiving HHC, and all were related to indwelling catheters.9-11 Since then, researchers have sporadically studied infection rates and risk factors in home care patients, focusing primarily on patients receiving home parental nutrition (HPN) treatment.12-16 Although individual studies aid in understanding, a systematic analysis provides a more complete picture of infections in home care settings, guiding clinical practices in HHC and identifying gaps in knowledge that need to be addressed in future research. A search of the literature revealed that no systematic review of infection in HHC settings has been published. To address the gap in knowledge, we conducted a systematic review to critically review and synthesize published evidence on infection prevalence and risk factors among adult patients who received HHC services and to evaluate the methodologic quality of these studies. The questions that guided our systemic review were, What are the infection rates among the HHC patients? and, What are the known risk factors for infections among patients receiving HHC services at home? The information presented in our systematic review is the critical first step for HHC professionals to develop guidelines to prevent and control infections in HHC.
METHODS
Our systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.17 PRISMA is a 27-item checklist that is used to improve the reporting of systematic reviews and meta-analyses and has been endorsed by major biomedical journals for publication of systematic reviews.18
A comprehensive search of the literature was conducted independently by 2 reviewers (JS and CM). Three electronic databases (Medline, PubMed, and Cumulative Index to Nursing and Allied Health Literature [CINAHL]) were used and the search terms included home care, home health care, hospice, and home infusion in various combinations with infection, sepsis, pneumonia, infectious disease, and communicable diseases. Hand searching of reference lists was also conducted to identify relevant citations.
The following inclusion criteria were used to identify relevant studies: original research that primarily examined the infection rates and/or identified risk factors of infections in adult patients receiving HHC services, written in English, and published through May 2013. Furthermore, patients in these studies must have been receiving health or supportive care, including hospice, infusion treatment, or total parenteral nutrition at home. Researchers could use either experimental or nonexperimental designs. The primary outcome measures for this review were infection rates and risk factors related to infections. This review was not limited to a specific type of infection given the dearth of studies on HHC related infections. Editorials, commentaries, studies with very small sample sizes (ie, <20), or studies that focused on infections among HHC workers or family members were excluded. We also excluded studies related to outbreaks because these can inflate the actual infection rates occurring in the HHC settings, and risk factors examined during the outbreak period usually focused on very specific factors such as 1 specific type of needleless device.9
The following data were extracted from each study by 2 researchers (JS and CM): research objectives, design, sample size, target population, infection type(s), infection rate, and identified risk factors. Study quality was assessed by using 2 observational research checklists, respectively, 1 for studies only describing infection rates, the other for studies examining risk factors. Published by Agency of Healthcare Research and Quality, these 2 checklists were specifically designed for observational studies that examine incidence or prevalence, or identify risk factors of chronic diseases and have been well tested.19 The checklists do not yield a composite score like some quality assessment tools,20 but summarize the major threats to the study's internal validity and external validity.19 To meet the needs for our systematic review, the original checklists, which contain a primary epidemiologic focus, were carefully reviewed and certain items that are not applicable in our systematic review such as subgroup definition, symptom severity and frequency of chronic diseases, and study follow-up, were removed. The modified checklists consisted of 4 main components: study description, interval validity, external validity, and overall writing. Using these modified checklists, we developed a list of strengths and weaknesses for each of the reviewed studies.
All included studies were reviewed by 2 of the authors (JS and CM). To ensure consistency, at the beginning of the review process each of the 2 reviewers independently assessed 2 studies and compared the results. Differences between the reviewers were discussed to ensure the same interpretation of criteria. Following the first process, the reviewers met for discussion after finishing every 3 studies and resolved all discrepancies.
RESULTS
Study selection
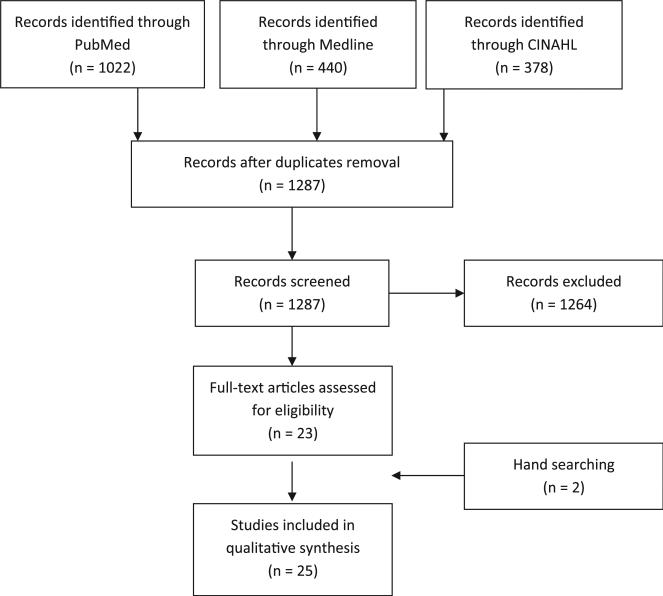
The Medline search yielded 440 articles, the PubMed search yielded 1,022 articles, whereas the CINAHL search yielded 378 articles. After removal of duplicates, titles and abstracts of 1,287 articles were screened. One thousand two hundred sixty-four articles were excluded for the following reasons: 1,239 did not include HHC patients or focused on outcomes not related to infections; 13 were not research studies; 3 had very small sample sizes (ie, <20); 6 focused on pediatric patients; and 3 were outbreak studies. Hand searching of reference lists of retrieved articles added 2 additional eligible articles for review. This resulted in 25 studies included in our systematic review (Fig 1).
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for article selection.17 CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Characteristics of studies
Table 1 describes the characteristics of the 25 reviewed studies. A majority (n = 13; 52%) was conducted in the United States. Nine studies (36%) were conducted in Canada and European countries, 1 study (4%) included both American and Canadian sites, 1 study (4%) was conducted in South Africa, and 1 was from Japan (4%). More than half of the reviewed studies (n = 14; 56%) focused on patients receiving HPN treatments, 4 (16%) focused on general HHC patients, 3 (12%) focused on home infusion patients, 3 (12%) on patients with indwelling devices (2 with urinary catheter only and 1 with both urinary catheter and intravenous device), and 1 (4%) on HHC patients with mechanical ventilators. Most studies (n = 17; 68%) were conducted in a single HHC site; 8 (32.0%) were multisite studies, including those which used national representative samples from the United States.21,22 Most researchers examined a single type of device-related infection such as intravenous (IV) line-associated infections (n = 19; 76%),12-16,21,23-36 urinary catheter-related infections (n = 4; 16%),25,26,31,37 or ventilator-associated pneumonia (n = 1; 4%)38; only 3 (12%) studies 22,39,40 described all types of infections in general HHC patients.
Infection rates
Table 2 summarizes the infection rates that were reported in 24 of the reviewed articles; 1 study did not report infection rates and was excluded from our summary.23 Various definitions were used to calculate infection rates. Researchers who focused on device-associated infections often reported infection rates as the number of infections per 1,000 device days12-14,16,21,25,26,28-32,35,38; with some exceptions such as the number of infections per catheter year,15,24,33 the number of infections per 100 catheter insertions,37 the number of infections per 1,000 HPN days,30 and the number of infections during the 31-month study period.34 Researchers who studied various types of infections (eg, sepsis, urinary tract infection, respiratory infections, and skin infections) usually reported the proportion of infected patients out of the total number of patients.22,39,40 In 8 studies, researchers adopted multiple definitions.15,16,24,26,33,35,37,38
Using the most commonly employed definition—the number of infections per 1,000 device days—the highest infection rate reported was 10.04 for central line-associated bloodstream infection (CLABSI) among 31 patients receiving HPN therapy, which was reduced to 6.48 after the HHC agency implemented ethanol lock therapy.14 The next highest infection rate was found among 43 HHC patients with long-term indwelling urinary catheter with a self-reported symptomatic urinary tract infection (UTI) rate of 8.4 per 1,000 catheter days.26 In general, patients receiving HPN treatment had higher rates of catheter-associated infections per 1,000 catheter days (ranging from 0.44 to 10.04 with average of 3.89) than patients in the home infusion center (ranging from 0.45 to 1.25 with average of 0.90). In 2 studies, researchers examined infections among the general HHC patients and reported CLABSI rates as 0.2225 and 1.1,31 respectively. The lowest infection rate was reported by researchers who studied the infection rates over an 11-year period in a single HHC agency in North Carolina, reporting an average of 0 CLABSIs for hospice patients per 1,000 catheter days.25
Using the definition of proportion of infected patients out of total patients, the highest infection rate reported by researchers was 80.9% in 1 study of 47 HPN patients.15 This is followed by 70% in a study that included 527 HPN patients.33 In 1 of 8 studies in which multiple infection definitions were reported, researchers found 70% of patients (ie, 30 out of 43) with long-term indwelling urinary catheters self-reported a history of symptomatic UTI with antibiotic treatment.26 In that study, researchers also reported the number of catheter-associated UTIs per 1,000 catheter days as 8.4, which is considered high by this definition. This demonstrates that these 2 different types of infection definitions were consistent in terms of reporting infection frequency.
Among the 25 reviewed articles, only 1 study focused on HHC patients receiving home mechanical ventilation, and the researchers reported 47% of patients developed ventilator-associated pneumonia.38 Using the definition of proportion of infected patients out of total patients, researchers reported infection rates for all types of infections as 5.1%,40 6.1%,39 and 11.5%22 for general HHC patients and 10.4% 22 for hospice patients.
Risk factors of infections
Researchers in 14 studies examined and identified different risk factors for infections among HHC patients (Table 3). Eight studies12,16,23,24,28,30,32,35 identified the risk factors for IV catheter-associated infections as patient characteristics or medical history, including younger age (reported by 2 studies),35,40 underlying diseases,16,30 bone marrow transplant history,32 receiving total parenteral nutrition treatment,32 and history of bloodstream infection32; treatment-related factors, including receiving infusion therapy outside the home,32 in HPN treatment <5 years,12 or family members with HPN training12; catheter-related factors, including multilumen catheter32 or central venous access salvage23; and patient social or economic factors such as being a part-time student or recipient of social welfare.12 Although most researchers found that catheter types were associated with infection, these findings were not consistent and sometimes even conflicted. One group of researchers reported patients with an implanted port were less likely to get infections than those with a tunneled noncuffed catheter,23 whereas another found that patients with a port were more likely to get infections than patients with a tunneled catheter.16 In addition, researchers also identified a peripherally inserted central catheter as a risk factor.28
In studies with outcomes other than IV catheter-associated infections, researchers identified being a woman,26 catheter change interval <4 weeks,37 and number of nurses changing catheters as risk factors for catheter-associated UTI.37 Daily duration of ventilation was a risk factor for ventilator-associated pneumonia.38 Researchers in 3 studies22,39,40 examined risk factors for nonspecific infections in general HHC patients and identified indwelling devices as a commonly reported risk factor for infection.39,40 One group of researchers examined how characteristics of the HHC agency affects patients’ risk for infection and found the large HHC agencies and those offering paid sick leave to staff were more likely to have patients with infections.22 These researchers argued that better infection screening systems in large HHC agencies might contribute to the increased number of infections observed.
Methodologic quality
The quality of reviewed studies varied. Studies mainly used objective measurements of infections based on clinical symptoms and laboratory confirmations, with some indicating that they used the Centers for Disease Control and Prevention definitions of infections.14,25,35,38,39 However, in 3 studies researchers relied on patients’ self-reported information in identifying infections.12,13,26 A major threat to the internal validity was related to the small sample sizes, which may limit the ability to detect significant associations.12,23,24,26,30,35,38 Also, bivariate analysis was frequently used to identify risk factors without adjusting for major confounding variables.12,14,22,28,30,35,38,40 Furthermore, researchers in 1 study used multivariate logistic analysis when the outcome variable was the continuous measure of the number of infections.16 The major threat to external validity was the sampling methods. The majority of researchers used convenience samples and examined infections in a single HHC agency, which limits the generalizability of results.14-16,23-26,28,30,33,35-40
DISCUSSION
Identifying the underlying prevalence and risk of developing infections in HHC populations is important because it is the first critical step to developing infection control strategies in HHC settings. Here, we report the findings of the first systematic review to our knowledge that examines infection rates and risk factors in HHC settings. We found that estimations of infection rates varied substantially among the reviewed studies. Overall, HHC patients receiving HPN had higher CLABSI rates than patients receiving home infusion therapy. Patients receiving parental nutrition in the home are a special group of the HHC population who are unable to obtain nutrition orally or through enteral routes and often receive prolonged therapy (from 405 days24-44 months30). Because there is a limited number of central venous catheters a person can have in a lifetime,23 patients receiving prolonged parental nutrition usually have to keep 1 catheter in place for as long as possible. In addition, the nutritional solution that is infused through the catheter provides a perfect environment for bacteria to grow if infection control practices are not strictly followed. Finally, when parental nutrition is given in the home, line care is often provided by the family members who usually have very little formal training. All of these reasons place these patients at a high risk for infections. Patients with home infusion treatments who had lower infection rates have diverse diagnoses and receive infusion treatments for many different reasons, such as chemotherapy or IV antibiotic treatment. Compared with HPN patients, home infusion patients usually have IV catheters for a shorter period and the IV antibiotic treatment may provide them with protection from infection. Our review also found that general HHC patients had the lowest infection rates compared with HPN or home infusion patients. This may be related to the underlying characteristics of the general HHC patient population who are less likely to be immune compromised or have invasive devices, which have been found to increase patients risk for infection.32
We found a large variance in identified risk factors. For example, researchers in many studies identified patients’ underlying medical conditions related to infection risk; however, the reported medical conditions varied between studies. For example, motor disorder was found related to infection in 1 study,30 whereas bone marrow transplantation was reported in another study.32 This could be due to the fact that different studies focused on patient populations with different comorbidities. In addition, many researchers investigated how catheter types affected the risk of infections. Among different types of catheters used in HHC, the implantable port may pose high risk because its membranes have a finite number of times for perforation before losing their properties and leaking. Therefore, during the HPN therapy, the needle is not changed with each nutrition bag, which can cause a permanent portal of germ entry.23 Peripherally inserted central lines that are used commonly because of ease of bedside placement were also reported to be related to increasing the risk of infection; it was suggested that this may be due to the arm where the peripherally inserted central catheter is placed having more exposure to contamination than the chest where other types of catheters are usually placed.28 However, this study failed to control for potential covariates; therefore, the validity of the study findings is questionable. Tunneled catheters are considered to have lower risk because of the subcutaneous tunneled track (~5-10 cm) between the external skin and bloodstream that can reduce the chance for germs to enter.16 However, in 1 study patients receiving parental nutrition with a noncuffed tunneled catheter were found more likely to have recurrent infections than patients with implanted ports. The authors explained that the discrepancy may have been due to the sample sizes and underlying risks of the patients.23
Unexpected findings were also identified. For example, younger age was reported as a risk factor in 2 studies,35,40 which is in contradiction to the fact that the elderly people are more vulnerable to infections because of their immune dysfunction, limited cognitive function, and multiple comorbidities.41 In those 2 studies,35,40 younger patients might have had underlying medical conditions that placed them at higher risk for infections; however, the bivariate analysis without controlling for covariance that was used in both studies was not able to examine this possibility. In another study, researchers reported that noncancer patients were more likely to have infections than cancer patients after controlling for other covariates, but failed to explain the reason for this result.24 Finally, some researchers reported that patients with the family member having parental nutrition training,12 or patients with shorter catheter change intervals,37 were at higher risk for infection, which is not consistent with what we would expect to observe in practice. One explanation is that by nature, the cross-sectional study design cannot detect a causal relationship. It may be a problem of “reverse causality” and the increasing number of infections led to the family member becoming more involved in HPN training, or the catheter being changed more often. However, none of these possibilities were further explored in the reviewed studies.
Suggestions for future research
As indicated by our systematic review, the quality of some of the reviewed studies is limited by significant methodologic flaws. The existing evidence primarily focused on patients receiving parental nutrition therapy in the home, with only few other HHC patient populations included. The majority of studies were conducted in a single-site HHC setting, which limits generalizability. To produce new evidence regarding these important patient care and safety issues, future studies should use a nationally representative sample, focus more on HHC patients other than those receiving parental nutrition, include large sample sizes, and use multivariate analysis for risk factors identification.
In addition, when patients receive HHC, usually it is augmented by the care provided by family members or friends in their own home over a prolonged period of time. Therefore, the risk of infection is likely to be influenced by many other socioecologic factors, such as the safety and sanitation hazards of the home environments and the caregiver's capability to deliver the needed care services. In 2008, the Association for Professionals in Infection Control and Epidemiology, Inc, and the Centers for Disease Control and Prevention Healthcare Infection Control Practices Advisory Committee published surveillance definitions for HHC and home hospice infections,8 emphasizing the potential influence of the environment and caregiver on home care patients’ risk for infection. However, only 1 study examined how socioeconomic factors may affect infections at home and found that patients receiving social welfare were more likely to get infections.12 Future research is needed to further explore this area.
Clinical implications
Our review demonstrates that infection is prevalent in HHC settings. As hospital stays are getting shorter and HHC service expands, many acutely ill and vulnerable patients will receive more invasive and advanced care at home, and patients’ risk for infections will continue to increase. Infection prevention and control, which is critical in assuring high-quality, safe HHC services, was recognized by different organizations, including The Joint Commission, as 1 of its national patient safety goals for home care.42 To reach this national patient safety goal, the first and critical step is to establish a surveillance system in home care to identify home care-associated infections and capture the associated risk factors. As suggested by our review, there was substantial variability in terms of defining and measuring infection rates across the studies. Adopting approaches commonly used in hospital settings such as defining infection rates as the number of infections per 1,000 device days may not be applicable because the data on when the devices are placed and removed are usually not available in HHC settings. Simple and practical approaches have been called for by researchers,43 such as using the days in infusion treatment as a proxy for the catheter days for home infusion patients when calculating infection rate or using the proportion of infected patients out of total number of HHC patients to calculate infection rates. These approaches will simplify the measurement of infection in HHC settings and provide the possibility for HHC agencies to begin benchmarking quality of care.
To develop effective home care-associated infection prevention and control practice, it is important to identify HHC patients who are at high risk for infection. Special attention should be given to patients receiving parental nutrition treatment in the home because they experienced the highest infection rates when compared with other home care patient populations. Our review also suggests that home care patients are heterogeneous, and identified risk factors varied dramatically between studies, with some even contradicting each other. Therefore, trying to pinpoint a 1-size-fits-all approach is not appropriate in HHC settings. A group of researchers developed analytic models and utilized them to successfully identify HHC patients at high risk for hospitalization44; a similar approach could be applied in infection control to identify HHC patients who have high risk for infections. Because infection control is limited in home care, a tailored care plan targeted at high-risk patient populations can ensure a patient safety in a cost-effective way.
HHC is provided in a special home environment that is usually limited by space and resources. Even though it is overseen by health care professionals, care in this setting is primarily provided by patients or family members who have very limited training.3,8 All of these factors pose special challenges to infection control in HHC settings. Other issues that were not fully addressed by our reviewed studies include the home environment and caregivers’ readiness for providing the care, which may be essential for successful implementation of infection control in home care. During the first home visit, the home environment should be assessed from an infection control perspective such as examining for general cleanliness, utility system, availability of running water and toilet facilities, and presence of pets or pests, and an infection control plan should be developed accordingly.8 For example, appropriate hand hygiene supplies should be provided to patients without hand-washing facilities. Educating patients and caregivers on appropriate infection control strategies should be given during the first home visit and reemphasized throughout the HHC service periods. Education should be tailored according to patients’ and caregivers’ literacy levels to ensure complete understanding.
Finally, home care infection control is not limited to HHC settings only. Because patients frequently transfer between home care, hospitals, and long-term facilities, it is also important to facilitate an efficient and effective communication between these health care facilities. For example, when a patient is discharged from an acute care hospital to a home care agency with any indwelling devices, the health care professional in the hospital should notify the home care physician or nurses of the status of the indwelling devices, the associated risk, and any special care procedures. Ineffective communication among various clinicians involved in patient care and in the transition may significantly jeopardize patient safety and long-term recovery.
Limitations
Our systematic review has several limitations. First, we only used 3 database searching engines and hand searching. Even though we believe that PubMed, Medline, and CINAHL are comprehensive and inclusive in terms of health care research evidence, there is a chance we may have missed some relevant studies. Second, we only included studies published in English and did not reach out for grey literature, which may introduce publication bias. Third, we considered conducting a meta-analysis. However, the majority of the reviewed studies did not report the prerequisite statistics for the calculation. Therefore, this systematic review only provides narrative review of the existing evidence. Finally, we included several studies conducted outside the United States in the review. Despite the differences in health care systems and care delivery between the United States and other countries, patients’ risk factors such as underlying conditions, characteristics, and infection control strategies remain similar across countries. Therefore, all reviewed studies have international relevance.
Supplementary Material
Acknowledgments
The authors thank the librarians at Columbia University for their assistance in data searching, and Kristine Kulage and the Columbia University School of Nursing writing workshop for their assistance with review of the manuscript.
Footnotes
Conflicts of interest: None to report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.ajic.2013.12.018.
References
- 1.Home-based long-term care. World Health Organization; Geneva (Switzerland): 2000. [Google Scholar]
- 2.Jarvis WR. Infection control and changing health-care delivery systems. Emerg Infect Dis. 2001;7:170–3. doi: 10.3201/eid0702.010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thome B, Dykes AK, Hallberg IR. Home care with regard to definition, care recipients, content and outcome: systematic literature review. J Clin Nurs. 2003;12:860–72. doi: 10.1046/j.1365-2702.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Basic statistics about home care. The National Association for Home Care & Hospice; Washington (DC): 2010. [Google Scholar]
- 5.Caffrey C, Sengupta M, Moss A, Harris-Kojetin L, Valverde R. Home health care and discharged hospice care patients: United States, 2000 and 2007. Natl Health Stat Rep. 2011:1–27. [PubMed] [Google Scholar]
- 6.Vincent G. The Older Population in the United States: 2010 to 2050. US Census Bureau; Washington (DC): 2010. [Google Scholar]
- 7.Tarricone R, Tsouros AD. The solid facts: home care in Europe. World Health Organization; Geneva (Switzerland): 2008. [Google Scholar]
- 8.APIC [October 23, 2013];APIC-HICPAC Surveillance Definitions for Home Health Care and Home Hospice Infections. 2008 Available from: http://www.apic.org/Resource_/TinyMceFileManager/Practice_Guidance/HH-Surv-Def.pdf.
- 9.Do AN, Ray BJ, Banerjee SN, Illian AF, Barnett BJ, Pham MH, et al. Bloodstream infection associated with needleless device use and the importance of infection-control practices in the home health care setting. J Infect Dis. 1999;179:442–8. doi: 10.1086/314592. [DOI] [PubMed] [Google Scholar]
- 10.Danzig LE, Short LJ, Collins K, Mahoney M, Sepe S, Bland L, et al. Bloodstream infections associated with a needleless intravenous infusion system in patients receiving home infusion therapy. JAMA. 1995;273:1862–4. [PubMed] [Google Scholar]
- 11.Kellerman S, Shay DK, Howard J, Goes C, Feusner J, Rosenberg J, et al. Bloodstream infections in home infusion patients: the influence of race and needleless intravascular access devices. J Pediatr. 1996;129:711–7. doi: 10.1016/s0022-3476(96)70154-3. [DOI] [PubMed] [Google Scholar]
- 12.Chang A, Enns R, Saqui O, Chatur N, Whittaker S, Allard JP. Line sepsis in home parenteral nutrition patients: are there socioeconomic risk factors? A Canadian study. JPEN J Parenter Enter Nutr. 2005;29:408–12. doi: 10.1177/0148607105029006408. [DOI] [PubMed] [Google Scholar]
- 13.Crispin A, Thul P, Arnold D, Schild S, Weimann A. Central venous catheter complications during home parenteral nutrition: a prospective pilot study of 481 patients with more than 30,000 catheter days. Onkologie. 2008;31:605–9. doi: 10.1159/000162286. [DOI] [PubMed] [Google Scholar]
- 14.John BK, Khan MA, Speerhas R, Rhoda K, Hamilton C, Dechicco R, et al. Ethanol lock therapy in reducing catheter-related bloodstream infections in adult home parenteral nutrition patients: results of a retrospective study. JPEN J Parenter Enter Nutr. 2012;36:603–10. doi: 10.1177/0148607111428452. [DOI] [PubMed] [Google Scholar]
- 15.Marra AR, Opilla M, Edmond MB, Kirby DF. Epidemiology of bloodstream infections in patients receiving long-term total parenteral nutrition. J Clin Gastroenterol. 2007;41:19–28. doi: 10.1097/01.mcg.0000212606.13348.f7. [DOI] [PubMed] [Google Scholar]
- 16.Santarpia L, Pasanisi F, Alfonsi L, Violante G, Tiseo D, De Simone G, et al. Prevention and treatment of implanted central venous catheter (CVC)-related sepsis: a report after six years of home parenteral nutrition (HPN). Clin Nutr. 2002;21:207–11. doi: 10.1054/clnu.2002.0541. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson EL, Cortazal M. Publication guidelines need widespread adoption. J Clin Epidemiol. 2012;65:239–46. doi: 10.1016/j.jclinepi.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Shamliyan T KR, Ansari MT, Raman G, Berkman ND, Grant M, Janes G, et al. Development of the quality criteria to evaluate nontherapeutic studies of incidence, prevalence, or risk factors of chronic diseases: pilot study of new checklists. [December 27, 2013];Agency for Healthcare Research and Quality. 2011 AHRQ Publication No. 11-EHC008-EF. Available from: http://effectivehealthcare.ahrq.gov/. [PubMed]
- 20.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moureau N, Poole S, Murdock MA, Gray SM, Semba CP. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Intervent Radiol. 2002;13:1009–16. doi: 10.1016/s1051-0443(07)61865-x. [DOI] [PubMed] [Google Scholar]
- 22.Dwyer LL, Harris-Kojetin LD, Valverde RH, Frazier JM, Simon AE, Stone ND, et al. Infections in long-term care populations in the United States. J Am Geriatr Soc. 2013;61:342–9. doi: 10.1111/jgs.12153. [DOI] [PubMed] [Google Scholar]
- 23.Beraud G, Seguy D, Alfandari S, Lenne X, Leburgue F, Faure K, et al. Factors associated with recurrence of catheter-related bloodstream infections in home parenteral nutrition patients. Eur J Clin Microbiol Infec Dis. 2012;31:2929–33. doi: 10.1007/s10096-012-1643-5. [DOI] [PubMed] [Google Scholar]
- 24.Shirotani N, Iino T, Numata K, Kameoka S. Complications of central venous catheters in patients on home parenteral nutrition: an analysis of 68 patients over 16 years. Surg Today. 2006;36:420–4. doi: 10.1007/s00595-005-3179-0. [DOI] [PubMed] [Google Scholar]
- 25.Weber DJ, Brown V, Huslage K, Sickbert-Bennett E, Rutala WA. Device-related infections in home health care and hospice: infection rates, 1998-2008. Infect Control Hosp Epidemiol. 2009;30:1022–4. doi: 10.1086/605641. [DOI] [PubMed] [Google Scholar]
- 26.Wilde MH, Brasch J, Getliffe K, Brown KA, McMahon JM, Smith JA, et al. Study on the use of long-term urinary catheters in community-dwelling individuals. J Wound Ostomy Continence Nurs. 2010;37:301–10. doi: 10.1097/WON.0b013e3181d73ac4. [DOI] [PubMed] [Google Scholar]
- 27.Bozzetti F. Central venous catheter complications in 447 patients on home parenteral nutrition: an analysis of over 100,000 catheter days. Clin Nutr. 2002;21:475–85. doi: 10.1054/clnu.2002.0578. [DOI] [PubMed] [Google Scholar]
- 28.DeLegge MH, Borak G, Moore N. Central venous access in the home parenteral nutrition population-you PICC. JPEN J Parenter Enter Nutr. 2005;29:425–8. doi: 10.1177/0148607105029006425. [DOI] [PubMed] [Google Scholar]
- 29.Ireton-Jones C, DeLegge M. Home parenteral nutrition registry: a five-year retrospective evaluation of outcomes of patients receiving home parenteral nutrition support. Nutrition. 2005;21:156–60. doi: 10.1016/j.nut.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Reimund JM, Arondel Y, Finck G, Zimmermann F, Duclos B, Baumann R. Catheter-related infection in patients on home parenteral nutrition: results of a prospective survey. Clin Nutr. 2002;21:33–8. doi: 10.1054/clnu.2001.0500. [DOI] [PubMed] [Google Scholar]
- 31.Rosenheimer L, Embry FC, Sanford J, Silver SR. Infection surveillance in home care: device-related incidence rates. Am J Infection Control. 1998;26:359–63. doi: 10.1016/s0196-6553(98)80018-7. [DOI] [PubMed] [Google Scholar]
- 32.Tokars JI, Cookson ST, McArthur MA, Boyer CL, McGeer AJ, Jarvis WR. Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy. Ann Intern Med. 1999;131:340–7. doi: 10.7326/0003-4819-131-5-199909070-00004. [DOI] [PubMed] [Google Scholar]
- 33.Buchman AL, Moukarzel A, Goodson B, Herzog F, Pollack P, Reyen L, et al. Catheter-related infections associated with home parenteral nutrition and predictive factors for the need for catheter removal in their treatment. JPEN J Parenter Enter Nutr. 1994;18:297–302. doi: 10.1177/014860719401800403. [DOI] [PubMed] [Google Scholar]
- 34.O'Keefe SJ, Burnes JU, Thompson RL. Recurrent sepsis in home parenteral nutrition patients: an analysis of risk factors. JPEN J Parenter Enter Nutr. 1994;18:256–63. doi: 10.1177/0148607194018003256. [DOI] [PubMed] [Google Scholar]
- 35.White MC, Ragland KE. Surveillance of intravenous catheter-related infections among home care clients. Am J Infect Control. 1994;22:231–5. doi: 10.1016/0196-6553(94)99002-6. [DOI] [PubMed] [Google Scholar]
- 36.Williams N, Carlson GL, Scott NA, Irving MH. Incidence and management of catheter-related sepsis in patients receiving home parenteral nutrition. Br J Surg. 1994;81:392–4. doi: 10.1002/bjs.1800810324. [DOI] [PubMed] [Google Scholar]
- 37.White MC, Ragland KE. Urinary catheter-related infections among home care patients. J Wound Ostomy Continence Nurs. 1995;22:286–90. doi: 10.1097/00152192-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Chenoweth CE, Washer LL, Obeyesekera K, Friedman C, Brewer K, Fugitt GE, et al. Ventilator-associated pneumonia in the home care setting. Infect Control Hosp Epidemiol. 2007;28:910–5. doi: 10.1086/519179. [DOI] [PubMed] [Google Scholar]
- 39.Patte R, Drouvot V, Quenon JL, Denic L, Briand V, Patris S. Prevalence of hospital-acquired infections in a home care setting. J Hosp Infect. 2005;59:148–51. doi: 10.1016/j.jhin.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 40.White MC. Infections and infection risks in home care settings. Infect Control Hosp Epidemiol. 1993;13:535–9. doi: 10.1086/646593. [DOI] [PubMed] [Google Scholar]
- 41. [December 27, 2013];Older Americans 2010: Key indicators of well-being. 2010 Available from: http://www.agingstats.gov/Main_Site/Data/2010_Documents/docs/OA_2010.pdf.
- 42.Commission TJ. Home care national patient safety goals. The Joint Commission Accreditation Home Care; Oak Brook (IL): 2014. [Google Scholar]
- 43.Manangan LP, Pearson ML, Tokars JI, Miller E, Jarvis WR. Feasibility of national surveillance of health-care-associated infections in home-care settings. Emerg Infect Dis. 2002;8:233–6. doi: 10.3201/eid0803.010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosati RJ, Huang L. Development and testing of an analytic model to identify home healthcare patients at risk for a hospitalization within the first 60 days of care. Home Health Care Serv Q. 2007;26:21–36. doi: 10.1300/J027v26n04_03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.