Abstract
Psychopathic personality is characterized by Interpersonal dominance, Impulsivity, sensation seeking, poor planning, and aggressiveness. Studies have shown that the Multidimensional Personality Question-naire (MPQ) can be used to estimate scores on the fearless-dominant (FD) and the Impulsive-antisocial (IA) dimensions of the Psychopathic Personality Inventory (PPI), the best validated self-report measure of psychopathic personality traits. Prior behavior genetic studies reported roughly equal genetic and nonshared environmental influences for both FD and IA, which remained stable from adolescence to young adulthood. However, no prior studies address genetic and environmental influences on these dimensions beyond early adulthood. We utilized the classic twin method to examine genetic and environmental influences on variance in FD and IA in a sample of middle-aged male twins. Biometric modeling indicated that the variance In both factors Is best explained by additive genetic and nonshared environmental influences. FD showed roughly equal contributions from genetic and environmental factors, whereas IA showed greater contributions from environmental than genetic factors. Additionally, the small phenotypic correlation between FD and IA was explained entirely by nonshared environmental factors.
Psychopathy is a personality disorder whose etiology is not well understood. Recent behavior genetic investigations have explicated the degree to which the expression of psychopathic personality traits through early adulthood is influenced by genetic and environmental factors (e.g., Blonigen, Hicks, Erueger, Patrick, & lacono, 2006), but relatively little is known about the extent of these influences on psychopathic traits beyond early adulthood, A better understanding of genetic and environmental architecture of psychopathic personality dimensions throughout the lifetime may have important implications for the development of psychotherapeutic interventions and molecular genetic research. To this extent, we utilized the classic twin method to estimate genetic and environmental contributions to variance in the two psychopathic personality dimensions assessed via normative personality in a community sample of middle-aged twins.
ASSESSMENT OF PSYCHOPATHIC TRAITS IN NONINSTITUTIONALIZED POPULATIONS
Various self-report measures have been used by researchers to examine psychopathic personality traits in the general population. The Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996) has emerged as one of the best validated and widely used. PPI scores are positively correlated with scores on other self-report measures of psychopathy (Benning, Patrick, Salekin, & Leistico, 2005; Salekin, Trobst, & Krioukova, 2001) as well as known external correlates of psychopathy, Including institutional infractions (Edens, Poythress, & Watkins, 2001), narcissism (Lilienfeld & Andrews, 1996), and reduced skin conductance (Verschuere, Crombez, De Clercq, & Koster, 2005). Moderate correlations have been reported between PPI total scores and scores on the Psychopathy Checklist-Revised (PCL-R; Hare, 2003; Poythress, Edens, & Lilienfeld, 1998), the best validated measure of psychopathy. Factor analyses of the PPI have yielded two largely orthogonal dimensions, one tapping fearless-dominant (FD) features of psychopathic personality such as low trait anxiety, interpersonal dominance, and sensation seeking, and the other indexing impulsive-antisocial (IA) features such as unconventionality, impulsivity, poor planning, aggressiveness, and alienation (Benning, Patrick, Hicks, Blonigen, & Kmeger, 2003; Patrick, Edens, Poythress, Lilienfeld, Benning, 2006; Wilson, Frick, & Clements, 1999) however, see (Neumann, Malterer, & Newman, 2008). Significant yet modest correlations have been reported between scores on the interpersonal/affective factor of the PCL-R and the FD factor of the PPI, and between PCL-R lifestyle/antisocial factor and IA scores (Benning, Patrick, Blonigen, Hicks, & lacono, 2005).1
Recently, research on the self-report assessment of psychopathy has demonstrated that normative personality measures such as the Multidimensional Personality Questionnaire (MPQ; Tellegen, 1982), can be used to estimate FD and IA scores. In their study of the factor structure of the PPI, Benning and colleagues (2003) found that by utilizing the MPQ primary-trait scales they were able to account for substantial variance in both the FD and IA. In that study, regression weights from a large sample of community adults who were administered both the MPQ and PPI were applied to all eleven MPQ primary scale scores to yield standardized score estimates for FD and IA, In the original factor analysis, MPQ scores accounted for 79% and 71% of the variance In FD and IA scores, respectively, after being statistically disattenuated to correct for the fact that the MPQ and PPI were administered several years apart (Benning et al., 2003), Without statistical disattenuation, the proportions of FD and IA variance accounted for by MPQ scores were 49% and 45%, respectively. Given that the original multiple Rs were likely attenuated by the test-retest unreliability of repeated personality measurements over time and that disattenuated estimates may be artificially inflated by the disattenuation procedure, the actual proportion of the variance in the PPI factors captured by the MPQ likely lies between the two estimates reported, suggesting that MPQ-estimated PPI scores appear to represent at least reasonable proxies for actual PPI scores. This method of estimating FD and IA scores based on MPQ scores has since been replicated in several independent samples (Benning, Patrick, Blonigen, et al., 2005; Benning, Patrick, Salekin, et al., 2005; Blonigen, Hicks, Kmeger, Patrick, & Iacono, 2005; Blonigen et al., 2006; Witt & Donnellan, 2008; Witt, Donnellan, Blonigen, Krueger, & Conger, 2009), with results yielding generally similar patterns of convergent and divergent validity as has been observed with psychopathy-specific self-report measures. In addition, one recent study used item response theory analyses to compare the assessment of the latent trait of psychopathy via PPI scores versus MPQ-estimated PPI scores, finding comparable precision between the two measures (Walton, Roberts, Krueger, Blonigen, & Hicks, 2008). Taken together these studies suggest that psychopathic personality traits can be reliably and validly assessed in noninstltutlonalized populations using MPQ-estimated FD and IA factor scores.2
PSYCHOPATHIC PERSONALITY DIMENSIONS AS A FUNCTION OF AGE
Substantial research corroborates age-related changes in personality traits (Colligen & Offord, 1992). Limited evidence from the psychopathy literature suggests that the two psychopathic dimensions may exhibit divergent developmental trajectories across adulthood. A cross-sectional analysis of PCL-R scores in a sample of Incarcerated adult males reported that, unlike Interpersonal/affective factor scores which showed relative temporal stability, lifestyle/antisocial factor scores decreased consistently with age (Harpur & Hare, 1994). Notably, visual inspection of the findings suggests that the sharpest drop in F2 occurred in the group of middle-aged and older men (Harpur & Hare, 1994). Likewise, Blonigen et al. (2006) reported a mean-level decline in MPQ -estimated IA scores from adolescence to young adulthood (17 to 24 years), but no significant change in MPQ-estimated FD scores in a large community sample of twins. Another recent investigation examining mean-level changes in MPQ-estimated FD and IA scores from adolescence to young adulthood (17 to 27 years) reported a small decline in FD scores and a substantially larger decline in IA scores (Witt et al,, 2009). Although the twin method is not, in and of itself, informative as to the etiology of mean-level change in traits, the developmental malleability of IA personality traits raises the possibility that the relative contributions of genes and environments may differ In older versus younger samples.
BEHAVIOR GENETIC STUDIES OF PSYCHOPATHIC PERSONALITY TRAITS
Several prior investigations have examined the genetic and environmental influences on variation in self-reported psychopathic personality traits, mostly using samples ranging in age from adolescence to young adulthood. Studies have reported that approximately equal proportions of the variance in these traits were accounted by heritability (i.e., genetic influences) and factors in the environment that make members of a twin pair phenotypically different (i.e., nonshared environmental influences). This was true when psychopathic traits were measured using the Socialization scale from the California Psychological Inventory (Taylor, McGue, lacono, & Lykken, 2000), callous/detached and Impulsive/antisocial dimensions of the Minnesota Temperament Inventory (Taylor, Loney, Bobadilla, lacono, & McGue, 2003), or grandiose/manipulative, callous/unemotional, and Impulsive/irresponsible dimensions of the Youth Psychopathic Inventory (Cooke, Michle, Hart, & Clark, 2004; Larsson, Andershed, & Lichten-stein, 2006). None of the above studies reported a significant contribution from those environmental factors that make co-twins more similar (shared environmental influences).
Similar behavior genetic findings have been reported using the PPI. Blonigen, Carlson, Krueger, and Patrick (2003) reported approximately equal contributions of genetic and nonshared environmental factors to PPI total scores, Notably, twins in this sample were approximately 40 years of age at the time of assessment, which, until the present study, represented the only behavior genetic investigation of middle-aged twins in relation to psychopathic personality. However, Blonigen et al. cautioned that their results should be regarded as preliminary because their sample size (136 twin pail's plus 81 single twins) is smaller than that typically called for in biometric modeling analyses (Neale & Maes, 2005) due to the modest size of their sample. Furthermore, because the two PPI dimensions are generally phenotypically uncorrelated, researchers have argued that FD and IA scores may be more informative than PPI total scores (Patrick et al,, 2006; Lilienfeld, personal communication, Feb 27, 2008).
The finding of approximately equal contributions from genes and non-shared environment with no contribution from shared genetic factors was replicated by Blonigen, Hicks, Krueger, Patrick, and lacono (2005) in an independent sample using MPQ-estimated FD and IA scores. A more recent paper by the same group examined the genetic architecture of these scores from adolescence to young adulthood. Again, results indicated that the variation in FD and IA scores at both time points was accounted by equivalent proportions of genetic and nonshared environmental factors (Blonigen et al., 2006).
In summary, research has suggested that fearless-dominant and impulsive-antisocial dimensions of psychopathic personality may be Indexed using the MPQ. Furthermore, studies examining the developmental trajectories of the two dimensions suggest a mean-level decline in impulsive-antisocial traits in adulthood. Prior research with adolescent and young adult twin samples have suggested that variation in self-reported psychopathic personality traits is influenced by approximately equal proportions of genetic and nonshared environmental factors, with no contribution from shared environment. To the best of our knowledge, no prior study has examined genetic and environmental contributions to the phenotypic expression of the two psychopathic personality dimensions in midlife. It is plausible that the FD and the IA traits, which show different developmental trajectories in adulthood, will evidence different contributions from genes and environments in middle age. To this extent, the current study extended findings from prior behavior genetic studies of psychopathic personality by explicating the genetic and environmental contributions to the two psychopathic personality dimensions indexed by the MPQ-estimated PPI scores in a cross-sectional sample of middle-aged, male twins. Based on prior findings, we expected that the variance in both PPI factors would be explained primarily by genetic and nonshared environmental influences, with little or no contribution from shared environmental influences.
METHOD
PARTICIPANTS
Participants were drawn from the Vietnam Era Twin Registry (VETR), a nation-wide register of 7,375 American male veteran twin pairs born between 1939 and 1955 who served in the United States armed forces between 1965 and 1975. The construction of the VETR and its history are detailed elsewhere (Eisen, True, Goldberg, Henderson, & Robinette, 1987; Goldberg, Curran, Vitek, Henderson, & Boyko, 2002). Data for this study were collected as pail of the larger Twin Study of Vulnerability to Alcoholism. Twins were randomly selected from among 8,169 individuals who participated in the Harvard Twin Study of Substance Abuse (Tsuang, Bar, Harley, & Lyons, 2001), and were not selected on the basis of alcohol/drug use or any other characteristics. Only twins without service in Vietnam were recruited for the alcoholism study to avoid the potential confounding Influence of combat exposure. To be included, both members of a twin pair had to agree to participate. The study sample consisted of 345 twin pairs and 3 unpaired twins whose co-twin data were missing, for a total of 693 individuals. The mean age for all participants was 47.8 years (SD= 3.3, range-41–58), 92,2% were European American, 5.5% were African-American, 1.9% were Hispanic, and 0.4% reported other ethnic origin. In addition, 96.7% were high school graduates and 33% were college graduates; 79.1% were married, 12.1% divorced, and 8.8% widowed, separated, never married, or refused response. Among participants reporting full-time (92.2%) or part-time (1.6%) employment, 33,5% held service or manual labor positions, 24.4% held clerical or semiprofessional positions, and 41,1% held professional positions. Median household income category was $60,000–$70,000 (range, <$10,000–$100,000+), Zygoslty was determined using the blood genetic marker analysis (blood group typing) supplemented with a questionnaire that elicited twin responses to questions about sibling similarity. This approach was found to be 95% accurate compared to molecular DNA analysis (Eisen, Neuman, Goldberg, Rice, & True, 1989; Eisen et al., 1987). In the current sample, 176 pairs were monozygotic (MZ, identical) and 169 pairs were dizygotic (DZ, fraternal). Analyses by demographic group revealed no significant differences at the a = .05 level between MZ and DZ twin pairs on any of the above mentioned demographic variables. One hundred and forty-eight participants were discarded from the MPQ analyses because they did not return their questionnaires or because their responses contained excessive missing items, yielding a final sample of 545 individuals (269 MZ, 276 DZ). There were no significant differences (α = .05) between the participants with missing MPQ data and the rest of the sample on any of the abovementioned demographic variables.
CRITERION MEASURE
Scores on FD and IA dimensions of the PPI were estimated using the 178-item New Zealand (NZ) version of the MPQ, This version has been used in prior community investigations of personality (e.g., Krueger et al., 1994). Because this version omits items that load on the absorption scale, we supplemented the NZ version with the 33 absorption items from the original MPQ (Tellegen, 1982). The resultant 211-item scale demonstrated good overall internal consistency for all 11 primary scales needed to estimate FD and IA scores, with αs ranging from 0.64 to 0.89. Internal consistency estimates for the 11 primary scales and higher-order factors of the MPQ are listed in Table I. The MPQ was mailed to participants as pail of their pre-test questionnaire packet.
TABLE 1.
Reliability (Internal Consistency) Analyses for the Primary Trait Scales and Higher-Order Factors of the 211-Item Version of the Multiphasic Personality Questionnaire
| MPQ Scale | Cronbach' α | N of items |
|---|---|---|
| Primary trait scales | ||
| Well-Being | .821 | 11 |
| Social Potency | .794 | 12 |
| Achievement | .774 | 17 |
| Social Closeness | .830 | 19 |
| Stress Reaction | .857 | 14 |
| Alienation | .862 | 17 |
| Aggression | .635 | 18 |
| Control | .777 | 20 |
| Harm Avoidance | .784 | 22 |
| Traditionalism | .704 | 22 |
| Absorption | .874 | 33 |
| Higher order factors | ||
| PEM Agentic | .863 | 40 |
| PEM Communal | .875 | 42 |
| NEM | .887 | 49 |
| CON | .813 | 64 |
STATISTICAL ANALYSES
The behavior genetic method is built on the premise that observable phenotypic variation results from differential contributions of genes and environment. Additive genetic influence is the extent to which genes summate to contribute to a given phenotype and is also described as heritability, or the proportion of phenotypic variance due to genetic influences. Because MZ twins share 100%, and DZ twins share, on average, approximately 50% of their genes, genetic Influences are implicated when MZ correlations are larger than DZ correlations. The total environmental influence on a phenotype can arise from shared and nonshared sources. Nonshared environmental effects are those resulting in phenotypic differences between members of the same twin pair, whereas shared environmental influences contribute to phenotypic traits that make co-twins more similar. Consistent with this method, we expressed phenotypic variation on the dependent measure (MPQ-estimated FD and IA scores) as product-moment correlations within MZ and DZ twin pairs. We then used these intraclass correlations to compare phenotypic resemblances between members of twin pairs to ascertain heritability estimates for FD and IA.
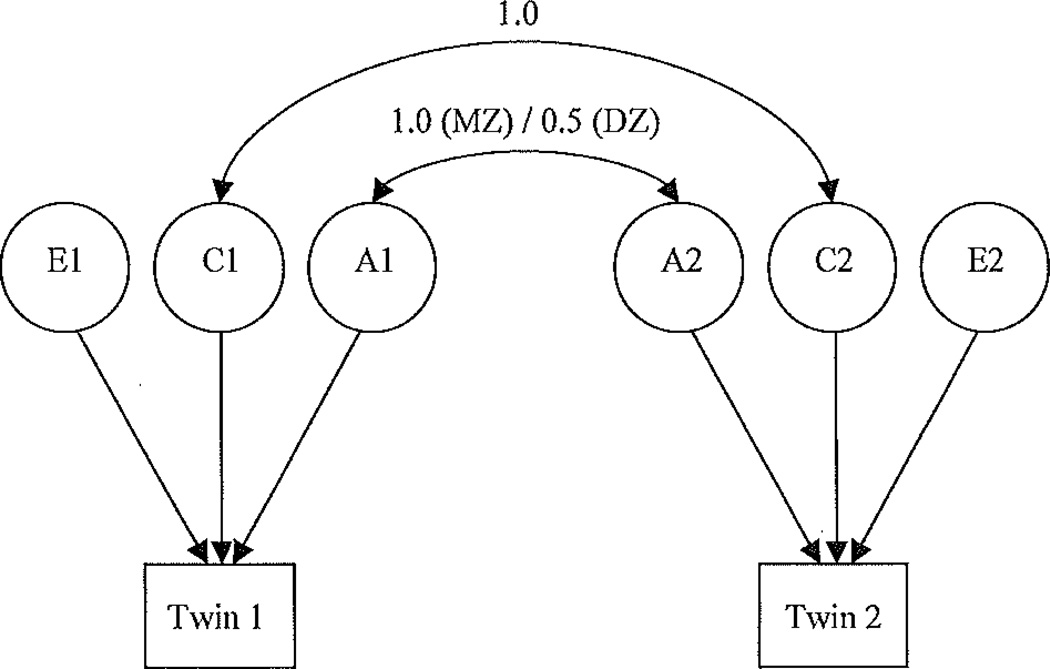
Twin (biometric modeling) analyses of FD and IA scores were carried out using Mx, a maximum-likelihood-based statistical modeling software package (Neale, Boker, Xle, & Maes, 2002). In biometric modeling, genetic and environmental variance components are expressed as latent variables whose effects are measured through their influence on the covarianee of criterion variables within MZ and DZ twin pairs. In the classic univariate twin model, referred to as the ACE model (Figure 1), variance in any single trait is decomposed into that arising from, additive genetic influences (A), shared environmental influences (C), and nonshared environmental (E) influences, Structural equation models express possible paths to and from the criterion variable using relatedness coefficients. Given that twins in a pair are reared together, the relatedness coefficient is 1.0 for shared environmental influences. For additive genetic influences, the coefficient is 1.0 for MZ and 0.5 for DZ twin pairs. The resulting models comprising different combinations of variance components (e.g., by removing C or E) are then compared to the fully saturated (or ACE) model using several fit indices. The likelihood-ratio chi-square (LRC) statistic represents the difference between the −2 log-likelihood of a fully saturated model and that of a reduced model; a nonsignificant χ2 would indicate that the reduced model has reasonable fit to the data. That is, its fit is not significantly reduced despite having fewer parameters. The Akaike Information Criterion (AIC) reflects both goodness-of-fit and parsimony, such that the lowest AIC indicates the best balance between goodness-of-fit and parsimony of the model.
FIGURE 1.
The classic ACE twin model. A= additive genetic variance; C = shared environmental variance; E = nonshared environmental variance.
In the multivariate model, the covariance between two or more traits is decomposed into genetic and environmental variance components in order to ascertain the degree to which the genetic and environmental influences are shared between the traits. As in the univariate analyses, the multivariate analyses test all possible combinations of genetic and environmental correlations against a fully saturated model. As an additional index of model fit, multivariate analyses may also Include a null model in which all covariances are fixed to zero.
RESULTS
Twin intraclass correlations and biometric modeling were carried out using standardized FD and IA scores (z-scores) indexed from primary MPQ scales. These scores were slightly but significantly correlated, Pearson r = .09, p = .027. Results of univariate behavior genetic analyses are presented in Table 2. The magnitudes of MZ correlations were FD = 0.48 (95% CI = 0.33–0.61) and IA = 0.35 (95% CI = 0.18–0.49). The magnitudes of DZ correlations were FD = 0.17, (95% CI = −0.01–0.34) and IA= 0.16 (95% CI = −0.04–0.35), suggesting additive patterns of inheritance.3 The model with the best fit included additive genetic and nonshared environmental influences, with no contribution from shared environment for both FD and IA. Each of these AE models had the lowest AIC and the largest p value of all nested models for each trait. According to this model, genetic factors explained 51% of the variation in FD traits and 32% of the variation in IA traits with the rest of the variance accounted by nonshared environmental factors. Notably, the nonshared environmental contribution to IA appears twice as great as the genetic contribution; moreover, a visual inspection of the 95% confidence intervals for the A and E variance components of IA reveals that they do not overlap. In order to obtain a statistical test of the difference in the magnitude of these two components, we computed a model in which the A and E variance components were constrained to be equal. Compared to the freely estimated model, the constrained model resulted in significantly poorer fit (Δ-2LL = 8.24; Δdf = 1; p = 0.004), suggesting that a model in which the E component of IA is greater than the A component provides a better fit to the data.
TABLE 2.
Results of the Univariate Twin Analyses (Models with Best Statistical Fit are Highlighted in Bold)**
| Model Fit Indices | Variance Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | −2LL | Df | Δ-2LL | Δdf | P | AIC | A (95% CI) | C (95% CI) | E (95% CI) | |
| FD | Saturated | 1105.59 | 535 | — | — | — | — | — | — | — |
| ACE | 1116.68 | 541 | 11.09 | 6 | .096 | 0.91 | .51 (.26–.63) | .00 (.00–. 17) | .49 (.37–.65) | |
| AE | 1116.68 | 542 | 11.09 | 7 | .135 | −2.91 | .51 (.35–.63) | — | .49 (.37–.65) | |
| CE | 1126.82 | 542 | 21.23 | 7 | .003 | 7.23 | — | .30 (.18–.42) | .70 (.58–.82) | |
| E | 1147.65 | 543 | 42.06 | 8 | <.001 | 26.06 | — | — | 1.0 | |
| IA | Saturated | 1136.09 | 535 | — | — | — | — | — | — | — |
| ACE | 1140.64 | 541 | 4.55 | 6 | .603 | −7.45 | .26 (.00–.45) | .06 (.00–.37) | .68 (.55–.83) | |
| AE | 1140.69 | 542 | 4.60 | 7 | .708 | −9.40 | .32 (.18–.45) | — | .68 (.55–.82) | |
| CE | 1141.68 | 542 | 5.59 | 7 | .589 | −8.41 | —. | 27 (.15–.39) | .73 (−61–.85) | |
| E | 1159.37 | 543 | 23.28 | 8 | .003 | 7.28 | — | — | 1.0 | |
Note. N = 545 individuals (272 twin pairs + 1 single twin). FD = fearless dominance; IA = impulsive antisociality. −2LL = likelihood-ratio chi-square statistic; df = degrees of freedom; p = p-value; AIC = Akaile Information Criterion. A = additive genetic variance; C = shared environmental variance; E = nonshared environmental variance.
The results of bivariate analyses are presented in Table 3. The magnitude of the phenotypic correlation between FD and IA was 0.11 (95% CI −0.02–0.20) in the full bivariate model, and 0.11 (95% CI = 0.04–0.18) in the best fitting model, which included the E variance component only.4 Model fitting results demonstrate that the observed phenotypic relationship was entirely due to nonshared environmental factors; the magnitude of environmental correlation between FD and IA was 0.19 (95% CI = 0.07–0,31). The correlation between genetic factors could be fixed at zero without a significant change In fit, suggesting that the two traits are influenced by different sets of genetic factors.
TABLE 3.
Results of the Bivariate Twin Analysis (The Model with Best Statistical Fit is Highlighted in Bold)
| Model Fit Indices | ||||||
|---|---|---|---|---|---|---|
| Model | −2LL | df | Δ-2LL | Δdf | P | AIC |
| Full bivariate | ||||||
| Cholesky model | 2247.952 | 1079 | — | — | — | — |
| CE | 2247.95 | 1080 | 0.001 | 1 | .981 | −1.999 |
| AE | 2247.95 | 1080 | 0.000 | 1 | .999 | −2.000 |
| AC | 2252.94 | 1080 | 4.988 | 1 | .0262 | .988 |
| E | 2247.98 | 1081 | 0.001 | 2 | >.999 | −3.999 |
| A | 2253.23 | 1081 | 5.272 | 2 | .072 | 1.272 |
| C | 2255.38 | 1081 | 7.424 | 2 | .024 | 3.424 |
| Null model | 2257.3 | 1082 | 9.362 | 3 | .025 | 3.362 |
Note. N = 545 individuals (272 twin pairs + 1 single twin). −2LL = likelihood-ratio chi-square statistic; df = degrees of freedom; p = p-value; AIC = Akaile Information Criterion; Null model = all covariances are fixed to zero.
DISCUSSION
The present study was designed to address the lack of behavior genetic findings regarding the two psychopathic personality dimensions beyond early adulthood. Results were consistent with our prediction in that genetic and nonshared environmental factors, but not common environmental factors, contributed to variation in both FD and IA. Furthermore, whereas the FD dimension was influenced approximately equally by genetic and environmental factors, the IA dimension evidenced a greater contribution from the environment than the genes. With respect to the proportion of variance in the FD dimension attributable to genetic and environmental factors, our results are similar to findings previously reported from adolescent and young adult samples (Blonigen et al,, 2005, 2006). Conversely, our finding of greater environmental than genetic contributions to IA traits in midlife appears to differ from the findings of equal contributions from genes and environment reported from younger samples (Blonigen et al., 2005, 2006).
It is important to note that our findings are strictly cross-sectional and are thus not informative regarding age-related changes in the heritability of IA over time. Longitudinal research is needed to confirm any change in the heritability of IA traits over time. However, our observation of a smaller genetic than environmental contribution in midlife is theoretically consistent with a number of large behavior genetic studies that have reported varying heritabilities in a number of self-reported personality traits as a function of age (McCartney, Harris, & Bemieri, 1990; Viken, Rose, Kaprio, & Koskenvuo, 1994). Interestingly, our cross-sectional comparisons suggest the possibility of a very different trend compared with other constructs, such as general cognitive ability, which appear to suggest increasing heritability and decreasing environmental influences from young adulthood to middle age, If a longitudinal study were to provide evidence of a change in heritabillty consistent with our cross-sectional findings, such findings would suggest a unique pattern of influence of genes and environment on personality traits related to antisocial behavior. If nonshared environmental influences on variation in IA increase from early to middle adulthood, such a change would suggest increasing malleability of impulsive and antisocial aspects of psychopathic personality in this age range.
Our analyses revealed a small but statistically significant correlation between FD and IA. This finding may appear inconsistent with some prior reports of no con-elation between these two dimensions; however, the effect size of this correlation is quite small, and the results of bivariate analyses revealed that FD and IA are genetically uncorrelated, corroborating a prior report that these two traits are influenced by different sets of genetic factors (Blonigen et al., 2005). Additionally, analyses indicated that the small observed phenotypic con-elation between these dimensions was entirely attributable to nonshared environmental factors. The observed environmental correlation between FD and IA may reflect some nonshared environmental factor(s); however, we cannot rule out the possibility that noise from measurement error underlies the small phenotypic correlation between these two dimensions.
As noted above, our findings are limited by the cross-sectional nature of our sample. Consequently, although our findings suggest differences from findings previously reported for younger samples, our design does not permit us to conduct statistical comparisons that directly address continuity or change. Our conclusions are further limited by the demographically homogeneous composition of the VETR, which is predominantly composed of European American males. Clearly, a more diverse sample that included female twin pairs and participants of more widely varied sociodemographic backgrounds would result in greater generalizability to the US population. However, although studies have found female participants to have lower scores on MPQ-estimated psychopathic personality traits (Blonigen et al, 2005, 2006), past behavior genetic investigations that included female participants found few, if any, sex differences in the magnitude of biometric components underlying these traits (Blonigen et al., 2005; Larsson et al., 2006; Taylor, Iacono, & McGue, 2000). Furthermore, VETR participants have been shown to be representative of their communities with respect to various socioeconomic variables (Eisen et al., 1987; Goldberg et al., 2002).
Lastly, there is increasing awareness about the potential influence of gene-environment interplay on the expression of maladaptive phenotypes (Rutter, Moffltt, & Caspi, 2006), For example, research has shown that genetic factors can alter individuals' vulnerability to environmental risk factors associated with antisociality or predispose them to self-select environments that increase the probability of engaging in future antisocial behavior (Moffitt, 2005). Future behavior genetic investigations may benefit from the assessment of maladaptive environments in order to explicate the possible role of gene-environment effects in the development and expression of the psychopathic personality phenotype.
Acknowledgments
This work was completed by Michael Brook in partial fulfillment of the requirements for tile degree of Master of Science at the Rosalind Franklin University of Medicine and Science under the supervision of David S. Kosson. The authors are indebted to the Vietnam Era Twin Registry and the participating twins far their support of this research. The authors are also grateful to Daniel M. Blonigen for his extensive comments on an earlier version of this manuscript.
Footnotes
Poythress and Edens (1998) reported the following correlations between PPI total scores and PCL-R total and factor scores: PCL-R Total (r = .54), PCL-R Factor 1 (r = .54), PCL-RFac-tor 2 (r = .40). Benning et al. (2005) reported the following correlations between PPI dimensions PCL-R factor scores: between FD and PCL-R Factor 1 (r = .23), between IA and PCL-R Factor 2 (r =. 36).
It must be acknowledged that self-report measures capture only a moderate proportion of variance In PCL-R factor scores. As such, we do not regard MPQ-estimated PPI scores as a tool for the clinical assessment of psychopathy. However, there are distinct advantages to using this method for psychopathy research in the community. First, both the MPQ and the PPI have been standardized on noninstltutlonalized samples. Second, the use of MPQ-estimated PPI scores makes It possible to employ large extant datasets few of which contain PCL-R or PPI data, but many of which contain MPQ data. These features enable investigators to study variation In psychopathic personality traits in the general population
Because the observed MZ correlation (.48) Is higher than the DZ correlation (17) for the FD dimension, the possibility of nonadditive (dominance) effects cannot be ruled out. A test of the model that Included dominance effects resulted In poor fit with the data and was therefore excluded from subsequent analyses.
It is prudent to explain the slight discrepancy between the Pearson correlation (0.09) between FD and IA reported above and the phenotypic correlation (0.11) reported here. The Pearson correlation is a “within-individual” statistic that reflects the relationship between FD and IA scores in the entire dataset. The phenotypic correlation resultant from biometric modeling reflects the average correlation between these two variables from groups based on two factors: twin pair membership (twin A/twin B) and zygoslty (MZ/DZ). Additional variation results from the constraints placed on analyses by the Mx program In order to account for the basic assumptions of the twin method
REFERENCES
- Benning SD, Patrick CJ, Blonigen DM, Hicks BM, lacono WG. Estimating facets of psychopathy from normal personality traits: A step toward community epidemiological Investigations. Assessment. 2005;12:3–18. doi: 10.1177/1073191104271223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the psychopathic personality Inventory: Validity and implications for clinical assessment. Psychological Assessment. 2003;15:340–350. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekln RT, Leistico AR. Convergent and discriminant validity of psychopathy factors assessed via self-report: A comparison of three instruments. Assessment. 2005;12:270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Carlson SR, Krueger RF, Patrick CJ. A twin study of self-reported psychopathic personality traits. Personality & Individual Differences. 2003;35:179–197. [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, lacono WG. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychological Medicine. 2005;35:637–648. doi: 10.1017/S0033291704004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonigen DM, Hicks BM, Krueger RF, Patrick CJ, lacono WG. Continuity and change in psychopathic personality traits as measured via normal-range personality: A longitudinal-biometric study. Journal of Abnormal Psychology. 2006;115:85–95. doi: 10.1037/0021-843X.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colligan RC, Offord KP. Age, stage, and the MMPI: Changes In response patterns over an 85-year age span. Journal of Clinical Psychology. 1992;48:476–493. doi: 10.1002/1097-4679(199207)48:4<476::aid-jclp2270480408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cooke DJ, Michie C, Hart SD, Clark DA. Reconstructing psychopathy: Clarifying the significance of antisocial and socially deviant behavior in the diagnosis of psychopathic personality disorder. Journal of Personality Disorders. 2004;18:337–357. doi: 10.1521/pedi.18.4.337.40347. [DOI] [PubMed] [Google Scholar]
- Edens JF, Poythress NG, Watkins MM. Further validation of the psychopathic personality Inventory among offenders: Personality and behavioral correlates. Journal of Personality Disorders, Id. 2001:403–415. doi: 10.1521/pedi.15.5.403.19202. [DOI] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosliy in the Vietnam era twin registry: An approach using questionnaires. Clinical Genetetics. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam era twin (VET) registry: Method of construction. Acta Genet Med Gemellol (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Curran B, Vltek ME, Henderson WG, Boyko EJ. The Vietnam era twin registry. Twin Research. 2002;5:476–481. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- Hare RD. Hare psychopathy check-list—revised (PCL-R) technical manual. 2nd ed. Toronto: Multi-Health Systems Inc.; 2003. [Google Scholar]
- Harpur TJ, Hare RD. Assessment of psychopathy as a function of age. Journal of Abnormal Psychology. 1994;103:604–609. doi: 10.1037//0021-843x.103.4.604. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Schmutte PS, Caspl A, Moffltt TE, Campbell K, Sllva PA. Personality traits are linked to crime among men and women: Evidence from a birth cohort. Journal of Abnormal Psychology. 1994;103:328–338. doi: 10.1037//0021-843x.103.2.328. [DOI] [PubMed] [Google Scholar]
- Larsson H, Andershed H, Lichtenstein P. A genetic factor explains most of the variation In the psychopathic personality. Journal of Abnormal Psychology. 2006;115:221–230. doi: 10.1037/0021-843X.115.2.221. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- McCartney K, Harris MJ, Bernieri F. Growing up and growing apart: A developmental meta-analysis of twin studies. Psychological Bulletin. 1990;107:226–237. doi: 10.1037/0033-2909.107.2.226. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psycho-pathology: Gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Neale MC, Maes HM. Methodology for genetic studies of twin and families. Dordrecht, The Netherlands: Kluwer Academic Publishers B.V.; 2005. [Google Scholar]
- Neale MC, Boker S, Xie G, Maes HM. Mx: Statistical modeling (Version 6) Richmond, VA: Medical College of Virginia; 2002. [Google Scholar]
- Neumann CS, Malterer MB, Newman JP. Factor structure of the psychopathic personality Inventory (PP1): Findings from a large incarcerated sample. Psychological Assessment. 2008;20:169–174. doi: 10.1037/1040-3590.20.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Edens JF, Poythress NG, Lilienfeld SO, Benning SD. Construct validity of the psychopathic personality inventory two-factor model with offenders. Psychological Assessment. 2006;18:204–208. doi: 10.1037/1040-3590.18.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poythress NG, Edens JF, Lilienfeld SO. Criterion-related validity of the psychopathic personality Inventory in a prison sample. Psychological Assessment. 1998;10:426–430. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of CMd Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Salekin RT, Trobst KK, Krloukova M. Construct validity of psychopathy in a community sample: A nomo-logical net approach. Journal of Personality Disorders. 2001;15:425–441. doi: 10.1521/pedi.15.5.425.19196. [DOI] [PubMed] [Google Scholar]
- Taylor J, Iacono WG, McGue M. Evidence for a genetic etiology of early-onset delinquency. Journal of Abnormal Psychology. 2000;109:634–643. doi: 10.1037//0021-843x.109.4.634. [DOI] [PubMed] [Google Scholar]
- Taylor J, Loney BR, Bobadilla L, Iacono WG, McGue M. Genetic and environmental Influences on psychopathy trait dimensions in a community sample of male twins. Journal of Abnormal Child Psychology. 2003;31:633–645. doi: 10.1023/a:1026262207449. [DOI] [PubMed] [Google Scholar]
- Taylor J, McGue M, Iacono WG, Lykken DT. A behavioral genetic analysis of the relationship between the socialization scale and self-reported delinquency. Journal of Personality. 2000;68:29–50. doi: 10.1111/j.1467-6494.2000.t01-1-.x. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Brief manual for the multidimensional personality questionnaire. Unpublished manuscript, University of Minnesota, Minneapolis. 1982 [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. The Harvard twin study of substance abuse: What we have learned. Harvard Review of Psychiatry. 2001;9:267–279. [PubMed] [Google Scholar]
- Verschuere B, Crombez G, De Clercq A, Koster EHW. Psychopathic traits and autonomic responding to concealed information in a prison sample. Psychophyslology. 2005;42:239–245. doi: 10.1111/j.1469-8986.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Rose RJ, Kaprio J, Koskenvuo M. A developmental genetic analysis of adult personality: Extraversion and neuroticism from 18 to 59 years of age. Journal of Personality and Social Psychology. 1994;66:722–730. doi: 10.1037//0022-3514.66.4.722. [DOI] [PubMed] [Google Scholar]
- Walton KE, Roberts BW, Krueger RF, Blonigen DM, Hicks BM. Capturing abnormal personality with normal personality inventories: An item response theory approach. Journal of Personality. 2008;76:1623–1648. doi: 10.1111/j.1467-6494.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DL, Frick PJ, Clements CB. Gender, somatization, and psychopathic traits In a college sample. Journal of Psychopathology and Behavioral Assessment. 1999;21:221–235. [Google Scholar]
- Witt EA, Donnellan MB. Furthering the case for the MPQ-based measures of psychopathy. Personality and Individual Differences. 2008;45:3. [Google Scholar]
- Witt EA, Donnellan MB, Blonigen DM, Krueger RF, Conger RD. Assessment of fearless dominance and impulsive antisociality via normal personality measures: Convergent validity, criterion validity, and developmental change. Journal of Personality Assessment. 2009;91:17. doi: 10.1080/00223890902794317. [DOI] [PMC free article] [PubMed] [Google Scholar]