Abstract
Intrapleural photodynamic therapy (PDT) has been used as an adjuvant treatment with lung-sparing surgical treatment for mesothelioma. In the current intrapleural PDT protocol, a moving fiber-based point source is used to deliver the light and the light dose are monitored by 7 detectors placed in the pleural cavity. To improve the delivery of light dose uniformity, an infrared (IR) camera system is used to track the motion of the light sources. A treatment planning system uses feedback from the detectors as well as the IR camera to update light fluence distribution in real-time, which is used to guide the light source motion for uniform light dose distribution. We have reported previously the success of using IR camera to passively monitor the light fluence rate distribution. In this study, the real-time feedback has been implemented in the current system prototype, by transferring data from the IR camera to a computer at a rate of 20 Hz, and by calculation/displaying using Matlab. A dual-correction method is used in the feedback system, so that fluence calculation can match detector readings. Preliminary data from a phantom showed superior light uniformity using this method. Light fluence uniformity from patient treatments is also shown using the correction method dose model.
Keywords: IR Navigation, Photodynamic therapy, Pleural PDT
1. INTRODUCTION
PDT is a local treatment, suitable to treat malignant, localized tumors such as those observed in malignant pleural mesothelioma (MPM).[1, 2] MPM has no standard treatment and the median survival for diagnosed patients is 6 to 17 months, depending on the disease stage. To treat MPM, PDT is coupled with surgical debulking of the tumorous tissue, part of a trend in multi-modal regimes to increase survival rates. The photosensitizer is administered to the patient, followed by a latent period referred to as the incubation time. After incubation time, debulking surgery is performed, followed by light delivery. The Phase I pleural treatment program at Penn treats patients with MPM or pleural effusion. The photosensitizing drug, HPPH®, is administered 24–48 hours before light irradiation. The irradiation is applied using a laser of wavelength 665 nm at 15–60 J/cm2. Within the thoracic cavity, the light delivery is continuously administered by a moving point source applied by the surgeon (Fig. 1). In the current protocol, the light uniformity is monitored using 7 detectors distributed in various locations inside the cavity.
Figure 1.
An illustration of pleural PDT treatment using a moving light source.
Light, photosensitizers, and oxygen are the three most important factors for photodynamic therapy (PDT).[3] Light distribution over the treatment area is of great importance in terms of treatment efficacy. To improve the light dose uniformity, we have proposed to use an IR navigation system to track the movement of the light source during PDT in order to calculate the light fluence distribution on the treatment area.[4] In this paper, we describe the results in a clinical study of 12 patients. To improve the agreement between calculation and measurements at the 7 isotropic detector locations, we have developed a dual-correction method to correct the calculated value to match that at the detector locations.
2. MATERIALS AND METHODS
2.1 Light fluence calculation algorithm
The light from the point source is the sum of the direct and the scatter lights. The direct light can be expressed as
| (1) |
where S is the power of the point source and r is the distance from the point source to point of interest. Due to the integrating sphere effect, the scattered light is a constant due to multiple scattering, independent of the shape of the lung cavity:[5]
| (2) |
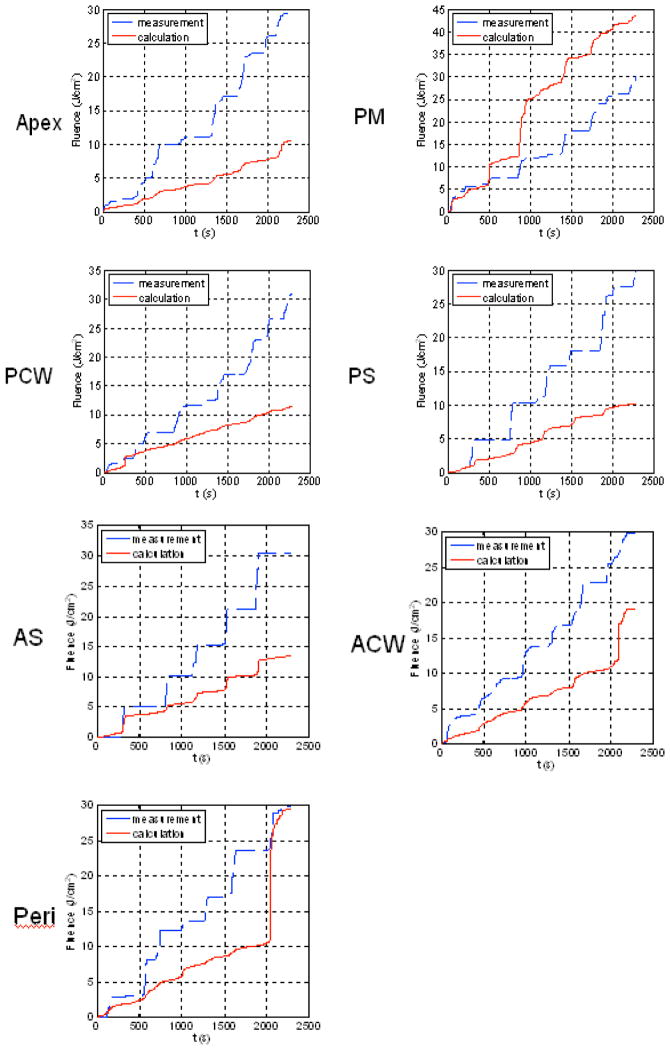
where As is the surface area of the treatment area, ρ is the diffuse reflectance, and μa is the absorption coefficient of the water medium inside the lung cavity. Several studies have been performed to characterize the patient dependence of the scatter light fluence, particularly the quantification of μa and ρ.[6, 7] In this study, we have ignored scatter light contribution for simplicity. A comparison between the measured and calculated light fluence rate is shown in Fig. 2. Clearly, the calculated light fluence rate is usually smaller than the measured light fluence rate because the scattered light is not included. But this is not always the case (see Fig. 2 for PM). The possible reasons can be partly due to the direct light being blocked by the concave surface contour but is not accounted for in the calculation and partly due to the absorption of the saline medium mixed with blood (e−μar), which is not being currently accounted for.
Figure 2.
Comparison of measurement (blue) and uncorrected calculation using Eq. 1 (red) for patient #7 for 7 detector locations: Apex, PM (posterior mediastinum), PCW (posterior chest wall), PS (posterior sulcus), AS (anterior sulcus), ACW (anterior chest wall), and Peri (Pericardium).
2.2 Dual fluence correction methodology
To improve the agreement between calculation and measurement, a dual correction method of light fluence rate is developed. The diagram of the correction formalism is shown in Fig. 3. This correction scheme include a time-dependent multiplication correction factor CF(t) applied to the entire calculated light fluence rate, i.e.,
Figure 3.

(a) Dual correction schematics to modify the calculated light fluence to match the measured light fluence. The dual correction includes (1) the light fluence for each 30 seconds interval; (2) The total cumulative light fluence at 150 seconds interval. Correction is applied only if the difference is more than 5%. (b) Schematics comparison between the corrected (red) and the measured light fluence (orange).
| (3) |
where ϕ is calculated spatially as a function of r as well as the time, t. The fluence rate is calculated as a temporal integration of ϕ. Since ϕ is calculated once every second, one can also calculate the primary dose as a summation of light fluence rate: . Every 30 seconds and 250 seconds, chosen from trial and error, a CF is applied.
For every 30 seconds, the fluence within the 30 seconds time is calculated and compared between the measurement and calculation at one of 7 detector points. This point is chosen as the point one gets the maximum light fluence, i.e., approximately the maximum measured light fluence rate at the calculation point. If the maximum difference between the two is more than 5%, then a correction factor CF is applied to reduce that difference to all data points.
For every 150 seconds, the cumulative fluence up to that time is compared between calculated and measured values (including any prior correction factors applied) at all detector points, if the difference is more 5%, then the cumulative fluence is corrected by applying an CF to the point with the mean difference among all 7 sites.
2.3 Real-time feedback of light fluence rate calculation
In order to provide real-time feedback to the measured values, we have developed a Matlab-based GUI to display the calculated light fluence on a 2D unwrapped surface. OpenIGTLink was used to provide a platform communicating between the NDI Polaris camera and a PC. The light source position data were acquired at a frequency of 20 Hz from the NDI data acquisition driver. When the PC receives the light source position data, Matlab was used to calculate the cumulative light fluence on every point of the pleural cavity contour, and to display/update the unwrapped light fluence distribution map at the frequency of 20 Hz. In the light fluence distribution map, 3D points on the pleural cavity contour were unwrapped into 2D φ-z maps, as shown in Fig. 5, given the condition that on each x-y plane of the cavity contour, there is only one point for each angle φ, where φ=atan(y/x). Calculation engine based on Eq. 1 was used so that the cumulative light fluence was displayed in real-time during treatment.results and discussions
Figure 5.
Calculated spatial distribution of light fluence rate for patient #7: (a) without (top) and (b) with (bottom) correction.
2.4 Comparison between measurement and calculation after dual-correction
Figure 4 shows the comparison between measurement and calculation for the same patient shown in Fig. 2. Clearly, the agreement between measurement and calculation is greatly improved at all detector locations except for posterior mediastinum (PM), where the calculated light fluence rate is still higher than the measured results. The reason for the large discrepancy at PM can be either blocking of the light source by the surface contour or absorption of the water medium as discussed previously. For the clinical example (Figs. 2 and 4), the standard deviation of the light fluence at all detector sites decreased from 42% to 29%. If one excludes the PM site, the standard deviation of the light fluence decreased from 26% to 12%. The later is acceptable for predicting light fluence rate for the current clinical protocol (15%). Prior studies in phantom have confirmed that the IR navigation system can determine the positions (of detectors, light source, and treatment surface) to within 2 mm.[4] The light fluence rate model (Eq. 1) can predict the measured values without scattering to within 5% based on phantom study using the same light source used for patient treatment. Thus the most likely cause of the difference is due to the scattered light in the patient.
Figure 4.
Comparison of measurement (blue) and corrected calculation using Eq. 1 (red) for patient #7 for 7 detector locations: Apex, PM (posterior mediastinum), PCW (posterior chest wall), PS (posterior sulcus), AS (anterior sulcus), ACW (anterior chest wall), and Peri (Pericardium).
Figure 5 compares the total light fluence rate before and after the application of the correction factor. As a result of applying the correction algorithm to match the results at the detector locations (shown as crosses in the diagram), the spatial distribution changes greatly and is more representative of the actual light fluence rate distribution during PDT treatment.
2.5 Fluence rate distribution characterization
Table 1 summarizes the results of the current application of the IR navigation system to 12 patients. In the column for “effective data”, “×”/ “√” means sufficient data about laser position during treatment is not/is obtained for calculation. In the column for “Reference”, “×” “/ “√” means the laser position is not/is relative to an independent reference mount that is fixed to patient table. Even if the data is not obtained relative to the reference mount, one can still analyze the calculated data. In the column for “CF”, “×” “/ “√” means the detector locations are not/are obtained during PDT, thus one cannot/can check the agreement between the calculation and measurement at the detector locations. Among the 12 patients, we obtained good data in 67% (8/12) patients. Among patients with good data, we can apply correction to 63% (5/8) patients since we didn’t get the detector positions in the other 4 patients.
Table 1.
Summary of clinical application among 12 patients treated with IR navigation system
| Index | Date | Effective data | Reference | CF |
|---|---|---|---|---|
| 1 | 9/14/10 | × | × | × |
| 2 | 2/8/11 | × | × | × |
| 3 | 2/15/11 | √ | × | √ |
| 4 | 4/19/11 | × | √ | × |
| 5 | 4/26/11 | √ | × | √ |
| 6 | 6/7/11 | √ | √ | × |
| 7 | 7/26/11 | √ | √ | √ |
| 8 | 8/30/11 | √ | × | × |
| 9 | 9/6/11 | √ | √ | √ |
| 10 | 11/1/11 | × | × | × |
| 11 | 11/8/11 | √ | √ | × |
| 12 | 11/29/11 | √ | √ | √ |
Table 2 compared the relative errors between the calculation and the measurement for the five patients before and after application of CF. The agreement always improves with correction, but not for all sites. Among patients with corrections, 20% (1/5) get agreement in most sites (at least 6 out of 7 detectors).
Table 2.
Comparison of relative deviation between calculation and measurement in each detector location before and after CF.
| Index | 3 | 5 | 7 | 9 | 12 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locations | bf CF | af CF | bf CF | af CF | bf CF | af CF | bf CF | af CF | bf CF | af CF |
| Apex | −66.7% | −37.8% | −80.0% | −37.8% | −65.0% | −8.3% | −63.3% | −23.3% | −50.0% | 13.3% |
| PM | −62.2% | 4.4% | −40.0% | 64.4% | 43.3% | 66.7% | −73.3% | −6.7% | −28.3% | 66.7% |
| PCW | −57.8% | 68.9% | −48.9% | 44.4% | −63.3% | 16.7% | −86.7% | 36.7% | −40.0% | 33.3% |
| PS | −64.4% | −2.2% | −64.4% | −2.2% | −66.7% | 0.0% | −70.0% | 20.0% | 23.3% | 0.0% |
| AS | −51.1% | 77.8% | −62.2% | 4.4% | −55.0% | −13.3% | −63.3% | 40.0% | −58.3% | −40.0% |
| ACW | −57.8% | 28.9% | −60.0% | 22.2% | −36.7% | −16.7% | −56.7% | −50.0% | −47.7% | −1.5% |
| PERI | −53.3% | −20.0% | −71.1% | −6.7% | 0.0% | 3.3% | −80.0% | 40.0% | −3.3% | 50.0% |
| St Dev | 64.0% | 47.4% | 67.2% | 36.8% | 52.0% | 27.4% | 76.8% | 36.6% | 43.1% | 40.5% |
The reason for the disagreement is two fold: First, the scatter light is not added into the current algorithm for light fluence calculation. This term is more important but is a constant term. Second, some of the direct light contribution overestimates the light fluence due to the light blockage by the surface contour. We are working on improvements in both fronts to further improve the agreement between measurement and calculation.
3. CONCLUSION
We have presented evidence that a dual-correction algorithm can be applied to calculate light fluence rate to match the measured light fluence rate at all sites in about 40% of patients, thus resulting in usable light fluence distribution map. Further study to incorporate the scatter light is needed to improve the agreement for more patients.
Acknowledgments
This work is supported by grant from National Institute of Health (NIH) P01 CA87971.
References
- 1.Pass HI, DeLaney TF, Tochner Z, Smith PE, Temeck BK, Pogrebniak HW, Kranda KC, Russo A, Friauf WS, Cole JW, et al. Intrapleural photodynamic therapy: results of a phase I trial. Ann Surg Oncol. 1994;1(1):28–37. doi: 10.1007/BF02303538. [DOI] [PubMed] [Google Scholar]
- 2.Pass HI, Tochner Z, DeLaney T, Smith P, Friauf W, Glatstein E, Travis W. Intraoperative photodynamic therapy for malignant mesothelioma. Ann Thorac Surg. 1990;50(4):687–8. doi: 10.1016/0003-4975(90)90230-4. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90(12):889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu TC, Liang X, Chang C, Sandell J, Finlay JC, Dimofte A, Rodriguez C, Cengel K, Friedberg J, Glatstein E, Hahn SM. An IR navigation system for real-time treatment guidance of pleural PDT. Proc SPIE. 2011;7886:28860L. doi: 10.1117/12.875635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandell J, Chang C, Finlay JC, Zhu TC. A treatment planning system for pleural PDT. Proc SPIE. 2010;7551:75510C. doi: 10.1117/12.843044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimofte A, Zhu TC, Finlay JC, Cullighan M, Edmonds CE, Friedberg JS, Cengel K, Hahn SM. In-vivo light dosimetry for pleural PDT. Proc SPIE. 2009;7164:71640A. doi: 10.1117/12.809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimofte A, Zhu TC, Finlay JC, Cullighan M, Edmonds CE, Friedberg JS, Cengel K, Hahn SM. In-vivo light dosimetry for HPPH-mediaed pleural PDT. Proc SPIE. 2010;7551:755115. doi: 10.1117/12.809548. [DOI] [PMC free article] [PubMed] [Google Scholar]