Summary
The Drosophila gene pickpocket (ppk) encodes an ion channel subunit of the Degenerin/Epithelial (DEG/ENaC) family [1]. PPK is specifically expressed in nociceptive, Class IV multidendritic (md) neurons and is functionally required for mechanical nociception responses [2, 3]. In this study, in a genome-wide genetic screen for other ion channel subunits required for mechanical nociception, we identify a gene that we name balboa (a.k.a. CG8546, ppk26) [4]. Interestingly, the balboa locus encodes a DEG/ENaC ion channel subunit highly similar in amino acid sequence to PPK [5]. Moreover, laser-capture isolation of RNA from larval neurons and microarray analyses reveal that balboa is also highly enriched in nociceptive neurons. The requirement for Balboa and PPK in mechanical nociception behaviors and their specific expression in larval nociceptors led us to hypothesize that these DEG/ENaC subunits form an ion channel complex in vivo. In nociceptive neurons Balboa∷GFP proteins distribute uniformly throughout dendrites but remarkably localize to discrete foci when ectopically expressed in other neuron subtypes (where PPK is not expressed). Indeed, ectopically co-expressing ppk transforms this punctate Balboa∷GFP expression pattern to the uniform distribution observed in its native cell-type. Furthermore, ppk-RNAi in Class IV neurons alters the broad Balboa∷GFP pattern to a punctate distribution. Interestingly, this interaction is mutually codependent as balboa-RNAi eliminates Venus∷PPK from the sensory dendrites of nociceptors. Finally, using a GFP-reconstitution approach in transgenic larvae, we directly detect in vivo physical interactions among PPK and Balboa subunits. Combined, our results indicate a critical mechanical nociception function for heteromeric PPK and Balboa channels in vivo.
Results and Discussion
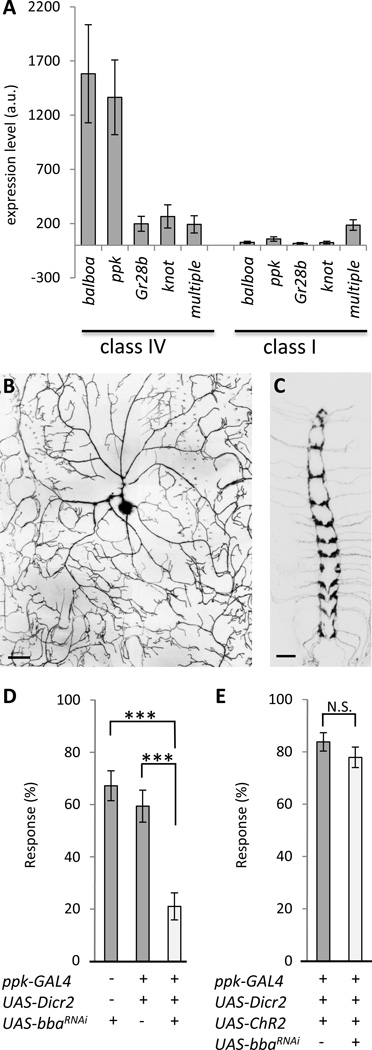
Using a set of UAS-mediated RNA interference (RNAi) lines that covers the majority of genes encoding ion channels in the genome [6], we carried out a tissue-specific RNAi screen to identify channels required for behavioral responses to noxious mechanical force [4]. Noxious force is detected by Drosophila larvae primarily through activation of the nociceptive Class IV multidendritic (md) neurons, but other classes of multidendritic neurons also contribute to noxious force detection albeit to a lesser degree [7]. Thus, to identify ion channels required for noxious force detection we first crossed our UAS-RNAi collection [4, 6] to the md-GAL4 driver [4, 8], which drives expression in all classes of multidendritic neurons, and then tested the larval progeny for normal or abnormal nocifensive rolling responses to stimulation with a 50 mN Von Frey fiber [4, 8]. This primary screen yielded defective responses in UAS-RNAi lines targeting nine channel subunits [4]. In parallel studies, we investigated the genome-wide expression levels of mRNAs isolated from Class IV and Class I multidendritic neurons by performing laser capture microdissection of the target fluorescently-labeled neurons from cryosectioned larvae. The Class IV samples showed high enrichment of ppk, Gr28b, and knot transcripts that are known to have enriched expression in these cells [1, 9, 10] (Figure 1A). As expected, the transcript for the pan-multidendritic neuron marker multiple [11] was seen in both Class IV and Class I samples. Remarkably, we found highly enriched expression in the Class IV neurons (relative to the Class I neurons) for a messenger RNA that encoded one of the ion channel subunits that was identified in our forward genetic screen (CG8546/ppk26) (Figure 1A). In addition to the microarray evidence, a ppk26-GAL4 reporter gene [5] drove expression of UAS-mCD8∷GFP exclusively in the Class IV multidendritic neurons (Figure 1B,C). Interestingly, CG8546 is highly similar in amino acid sequence to PPK [5], a DEG/ENaC previously shown to be expressed in Class IV neurons that is required for mechanical nociception [1, 2].
Figure 1. Balboa is highly expressed in nociceptive neurons and is required for mechanical nociception.
(A) Affymetrix microarrays performed on mRNA isolated Class IV and Class I neuron cell bodies show high enrichment of balboa, ppk, Gr28b and knot mRNA in nociceptors. The pan-multidendritic marker multiple is present in both Class I and Class IV neurons. (B) ppk26-GAL4 > UAS-mCD8∷GFP exclusively labels the Class IV md neurons (ddaC neuron is shown, scale bar = 20 µm). (C) Within the CNS, ppk26-GAL4 > UAS-mCD8∷ GFP exclusively labels projections of Class IV neurons and is absent from central neurons (3rd instar ventral nerve cord, dorsal view, scale bar = 20 µm). (D) Knockdown of balboa severely impairs nociceptive behavioral responses of larvae stimulated with a 30mN Von Frey fiber. The proportion of larvae exhibiting nociception responses was 67% of control animals without a driver (w/yv;; CG8546JF01843/+ [n=67]), 59% of control animals with the driver (w/yv;ppk-GAL4/+; UAS-dicer-2/attp2[n=64]), and 21% of animals with balboa-RNAi targeted in Class IV md neurons (w; ppk-GAL4/+; UAS-dicer-2/CG8546JF01843[n = 62]), (***p < 0.001, Fischer’s Exact test with Bonferroni correction). (E) Knockdown of balboa did not affect ChR2-triggered nociception behavior. Optogenetic activation of nociception behavior was seen in 84% of control animals (w;ppk-GAL4 UAS-ChR2∷eYFP Line C/+, UAS-dicer-2/+ [n = 111]). Knockdown of balboa did not significantly reduce the frequency of nociception responses to blue light (w; ppk-GAL4 UAS-ChR2∷eYFP Line C/+; UAS-dicer-2/CG8546GD2350[n = 113, mean = 78%]), (Fisher’s Exact test p=0.31). Error bars in A denote standard error of the mean, in D and E they denote standard error of the proportion.
Given the highly enriched expression in the nociceptive Class IV neurons, we tested the effects of knocking down CG8546 specifically in these cells. To do so, we crossed the UAS-RNAi lines targeting CG8546 to the Class IV-specific ppk-GAL4 driver. Consistent with the highly enriched Class IV expression pattern that was detected in our microarray, RNAi targeting of CG8546 with the Class IV driver nearly eliminated the transcript from whole animal RNA isolates (Figure S1A, B). In addition, we found that CG8546 knockdown in the Class IV neurons resulted in profound mechanical nociception behavioral defects (Figure 1D). Thus, to reflect this defective mechanical nociception phenotype, we named the gene balboa(bba) in honor of the fictional pain-resistant prizefighter hero, Rocky Balboa [4]. As previously described for ppk [2], expressing balboa-RNAi in the Class IV neurons did not cause a defect in optogenetically-triggered nociception behaviors (Figure 1E) [4]. Therefore, the effects of balboa-RNAi are unlikely to be explained by a non-specific effect on the intrinsic excitability or general health of the Class IV neurons. Indeed, balboa-RNAi did not cause a noticeable change in Class IV neuron morphology (Figure S1C, D). Given the well-established role for ion channels of this gene family in C. elegans mechanotransduction [12], an interesting possibility is that Balboa is also involved in force sensing by the Class IV cells.
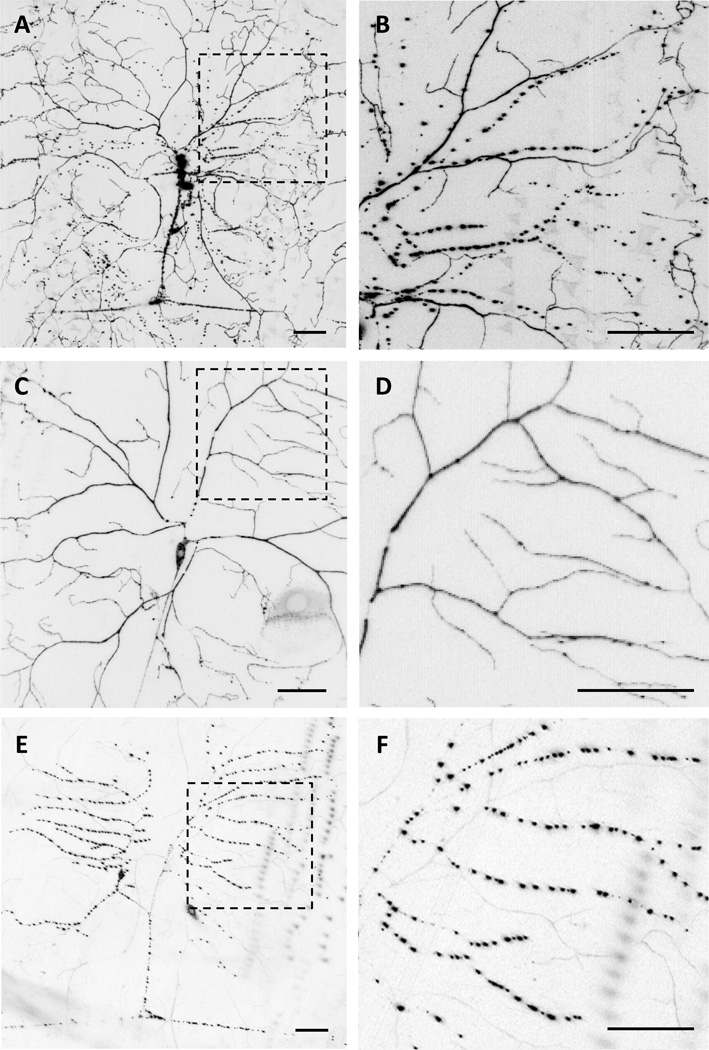
We next investigated the subcellular localization of the Balboa protein by creating transgenic flies expressing a GFP-tagged version of Balboa (UAS-balboa∷GFP). When crossed to the md-GAL4 driver, the subcellular localization of Balboa∷GFP was observed to differ between the various classes of multidendritic neurons (Figure 2A). Within the Class IV neurons, we observed uniform Balboa∷GFP fluorescence throughout the dendritic arbor, but in the Class I, II, and III neurons (where balboa may not normally be expressed) Balboa∷GFP localized to bright punctae within the dendritic arbors (Figure 2B). Although these punctae appeared similar to those previously described for expression of MEC-4 in C.elegans mechanosensory neurons [13], it is likely that these ectopic punctae are distinct and represent an intermediate stage of Balboa∷GFP protein trafficking that is not surface localized or is aggregated. Using drivers specific to different classes of neurons, we confirmed that the uniform distribution of Balboa was due to its presence in the Class IV neurons where it is endogenously expressed (Figure 2C, D) and that the highly punctate distribution was due to its ectopic expression in Class I (Figure 2E, F), II, and III neurons. These distinct patterns for Balboa∷GFP localization were unlikely to be a consequence of the C-terminal GFP tag disrupting proper protein distribution as an N-terminally tagged Balboa transgene (UAS-Venus∷balboa) showed an identical distribution (Figure S2A). In addition, the uniform pattern observed in Class IV neurons was also seen with very low expression levels of the transgene (Figure S2B) suggesting that the uniform pattern was not an abnormal consequence of a very high expression level.
Figure 2. The subcellular localization of Balboa∷GFP varies among multidendritic neuron subtypes.
(A–F) The subcellular distribution of Balboa∷GFP in the dorsal cluster of md neurons. Expression of Balboa∷GFP under the control of (A, B) md-GAL4 (C,D) ppk-GAL4 and (E,F) 2-21-GAL4 shows a broad, diffuse localization pattern throughout the arbor of Class IV ddaC neurons but a punctate expression pattern in the dendrites and axons of Class I, II, III neurons. (B, D, F) High magnification images of insets of A, C, and E, respectively. Scale bars = 50 µm. In this and all subsequent confocal micrographs, anterior is to the left, dorsal is at the top, and all images are of the dorsal, peripheral md neuron cluster of third instar larvae. Genotypes shown are (A and B) w; md-GAL4/+; UAS-balboa∷GFP/+, (C and D) w; ppk-GAL4/+; UAS-balboa∷GFP/+, and (E and F) w;; 2-21-GAL4/UAS-balboa∷GFP.
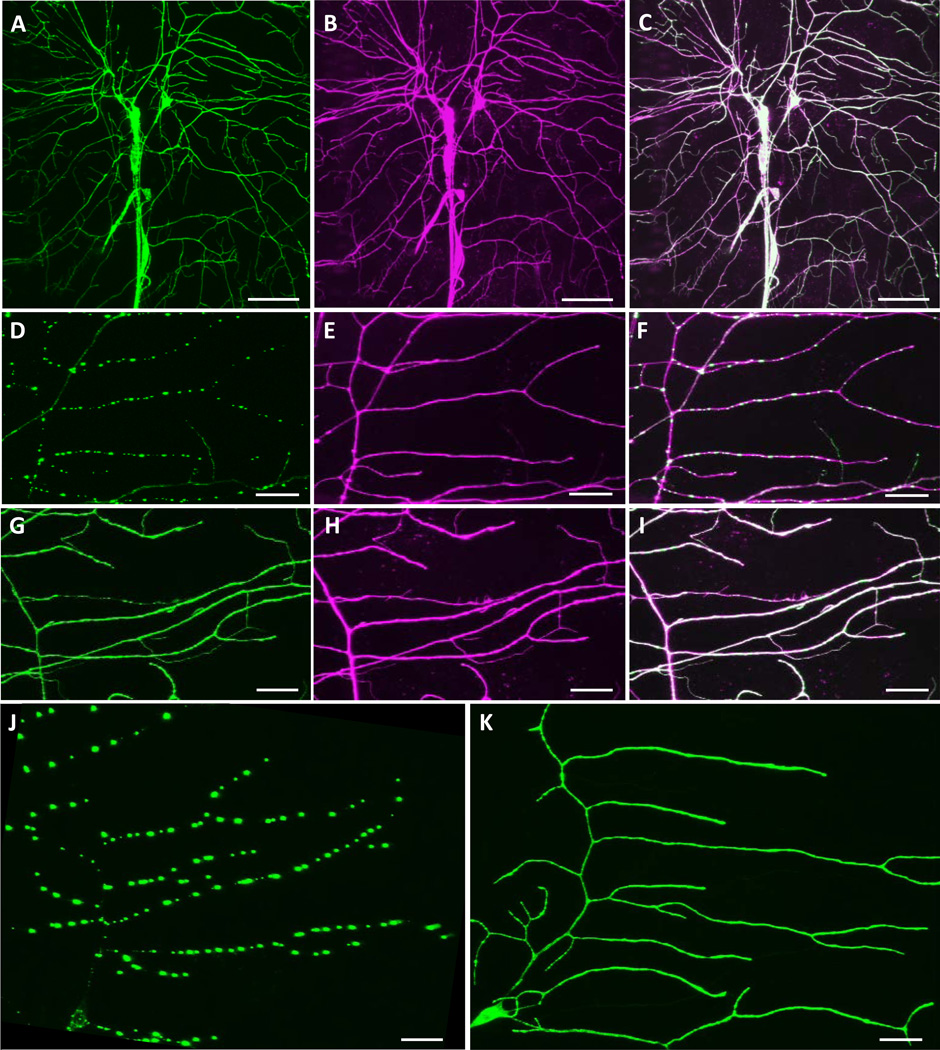
We reasoned that the distinct subcellular localization for Balboa∷GFP proteins in the different neuronal classes might be due to differential PPK expression among multidendritic neuron subtypes. ppk is expressed at high levels in the Class IV neurons but it is expressed at low or undetectable levels in the Class I (Figure 1A), II, and III neurons. Indeed, we found that simultaneous co-expression of ppk and balboa∷GFP resulted in a Balboa∷GFP distribution that was essentially uniform in all the multidendritic neurons (Figure 3A–C). These results were strikingly different from what we observed with Balboa∷GFP alone (Figure 2A, B). In the absence of additional PPK, the Balboa∷GFP foci appeared evenly spaced, like beads on a string (Figure 3D–F). This dramatic change in localization was particularly evident when we specifically examined the pattern within the Class I neurons using the 2-21-GAL4 driver (Figure 3G–K). In the presence of PPK, this Balboa∷GFP localization pattern was converted to an even and uniform one where the fluorescence evenly filled the dendrites (Figure 3G–I,K).
Figure 3. Ectopic expression of PPK alters the subcellular localization of Balboa∷GFP.
(A–C) Balboa∷GFP was smoothly distributed throughout the dendritic arbors of all four classes of multidendritic neuron when PPK was ectopically co-expressed under the control of the md-GAL4 driver (w; md-GAL4 UAS-mCD8∷DsRed /+; UAS-balboa∷ GFP/UAS-ppk). (A) Balboa∷GFP channel. (B) Counter-label of the neuronal plasma membrane (mCD8∷DsRed). (C) Merge of A and B. (D–F) Expression of Balboa∷GFP alone is punctate in Class I and II neurons, although dendrites show normal morphology (w; md-GAL4 UAS-mCD8∷DsRed /+; UAS-balboa∷GFP/+). (D) Balboa∷GFP, (E) mCD8∷DsRed, (F) Merge of D and E. (G–I) Co-expression of PPK in Class I and II shows uniformly localized Balboa∷GFP expression (w; md-GAL4 UAS-mCD8∷ DsRed /+; UAS-balboa∷GFP/UAS-ppk). (G) Balboa∷GFP, (H) mCD8∷DsRed, (I) Merge of G and H. (J) Ectopically expressed Balboa∷GFP in Class I neurons shows punctate expression under the control of the 2-21-GAL4 driver (w; UAS-balboa∷GFP/+; 2-21-GAL4/+. (K) Balboa∷GFP with co-expressed PPK under control of 2-21-GAL4 shows broad distribution in dendrites (w; UAS-balboa∷GFP/+; 2-21-GAL4/UAS-ppk). Scale bars for A–C = 50 µm; D–K = 20 µm.
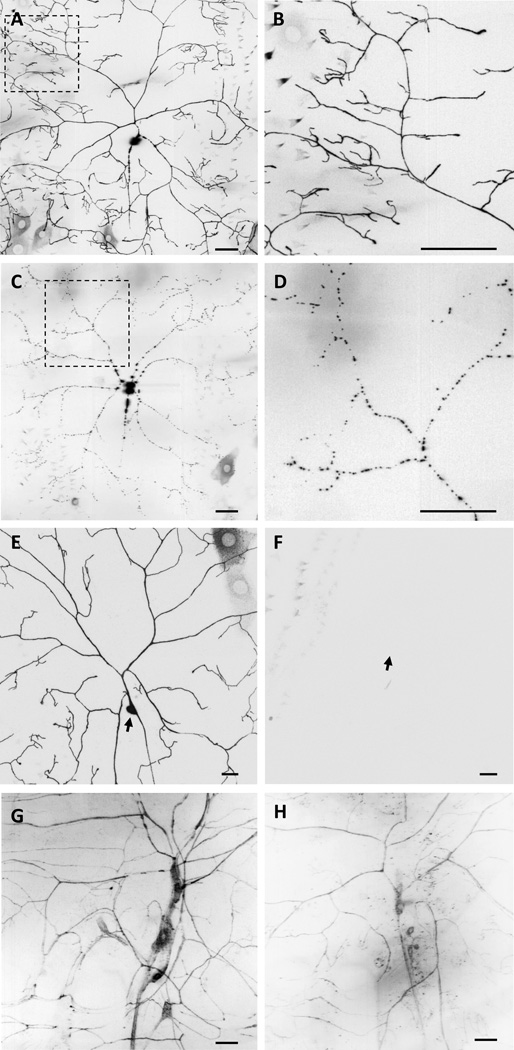
If co-expression of PPK and Balboa are indeed necessary for each other’s stability and/or localization, then removing PPK or Balboa from the Class IV neurons should alter the distribution of the cognate partner protein. Indeed, when we tested this prediction through knockdown of ppk in the Class IV neurons, the normally uniform distribution of Balboa∷GFP (Figure 4A, B) was converted to a clearly punctate distribution (Figure 4C, D). Thus, reducing ppk expression caused a mislocalization of the Balboa∷GFP protein to punctae within the Class IV neurons.
Figure 4. Balboa and PPK physically interact in vivo.
(A) Balboa∷GFP shows a broad distribution pattern in dendrites and axons of the Class IV ddaC neuron (boxed area shown in more detail in B). (B) Zoomed in view of the boxed area in A. (C) Balboa∷GFP is distributed in discrete bright foci in ppk-RNAi animals (boxed area is shown in more detail in D). (D) Zoomed in view of the boxed area in C. (E) Venus∷PPK is distributed uniformly in the dendrites of Class IV neurons (arrow indicates cell body). (F) balboa-RNAi eliminates Venus∷PPK from dendrites of Class IV neurons but the cell body remains weakly visible (arrow). (G) GFP is reconstituted in the dendrites of Class I–IV neurons when NGFP∷PPK and Balboa∷CGFP are co-expressed. (H) When Balboa∷CGFP and NGFP∷Balboa are co-expressed in Class I–IV neurons reconstitution of GFP is limited to the Class IV neurons. Scale bars are 20 µm. Genotypes were (A and B) w; ppk-GAL4/+; UAS-dicer-2/UAS-balboa∷ GFP/+, (C and D) w; ppk-GAL4/ppkKK104185; UAS-dicer-2/UAS-balboa∷GFP, (E) w; ppk-GAL4/+; UAS-dicer-2/UAS-Venus∷PPK (F) w; ppk-GAL4/UAS-Venus∷PPK; UAS-dicer-2/ UAS-balboa-RNAiJF01843, (G) w; md-GAL4/+;UAS-NGFP∷ppk/UAS-balboa∷ CGFP, and (H) w; md-GAL4/+; UAS-balboa∷CGFP/UAS-NGFP∷Balboa.
In converse experiments, we generated transgenic animals for expression of fluorescently tagged Venus∷PPK protein (UAS-Venus∷ppk). As with tagged Balboa proteins, Venus∷ppk distributed uniformly in the dendrites of Class IV neurons(Figure 4E). We then used this transgene to test whether PPK localization was Balboa dependent via RNAi knock-down of balboa in the Class IV neurons. This manipulation caused a dramatic reduction in the expression levels of Venus∷PPK to the point that it became undetectable in Class IV neurons (Figure 4F). Importantly, balboa-RNAi did not reduce expression of ppk mRNA (Figure S1B) or the strength of ppk-GAL4 expression (Figure S1C,D) indicating that these effects on Venus∷PPK were not at the level of transcription or mRNA stability.
The above observations provided strong suggestive evidence that PPK protein subunits and Balboa protein subunits may physically interact in vivo to form a functional DEG/ENaC ion channel. Cells in which Balboa and PPK were co-expressed showed a uniform distribution of Balboa∷GFP, whereas cells that expressed only Balboa∷GFP displayed the punctate or aggregated distribution pattern. In addition, the expression of Venus∷PPK was also dependent on the presence of Balboa. Nevertheless, it remained possible that these mutually dependent effects were indirect. For example, expression of PPK and/or Balboa within neurons might have regulated another protein (or proteins) that then in turn caused changes in the distribution of the cognate subunits.
Thus, to test for a direct physical interaction between Balboa protein subunits and PPK protein subunits we utilized the split-GFP technique [14, 15]. In this approach, GFP is split into an N-terminal fragment (NGFP) and a C-terminal fragment (CGFP). The cleavage is performed in such a way that neither GFP protein fragment is able to form a radiative fluorophore upon cellular co-expression. However, when the NGFP and the CGFP fragments are co-expressed as fusions to other proteins that physically interact, the two GFP fragments come into sufficiently close proximity to permit reconstitution of GFP fluorescence [14].
To apply this approach to Balboa and PPK, we generated flies containing UAS transgenes that encoded either a balboa∷CGFP transcript, an NGFP∷balboa transcript, or an NGFP∷ppk transcript. As expected, neither the balboa∷CGFP transcript nor the NGFP∷ppk transcripts produced detectable GFP fluorescence when individually expressed in neurons (data not shown). However, co-expression of the two transcripts together in all four classes of multidendritic neurons (Figure 4G) or specifically in Class IV neurons (data not shown) caused clear reconstitution of GFP in the dendrites, soma, and axon. These results are consistent with a model where the PPK dependent redistribution of Balboa∷GFP observed above is due to direct physical interactions between the proteins.
We next used the split-GFP protein approach to further test for homophilic in vivo interactions among Balboa subunits. Remarkably, upon co-expression of balboa∷CGFP and NGFP∷balboa in all four classes of multidendritic neurons we observed GFP reconstitution that was restricted to Class IV multidendritic neurons (Figure 4H). In surprising contrast, co-expression of balboa∷CGFP and NGFP∷balboa did not result in detectable GFP reconstitution in the Class I, II or III multidendritic neuron cell types. These findings further suggest that homophilic interactions among Balboa subunits do occur, but these interactions are confined to the Class IV neurons that also express PPK. Combined with the findings that Balboa∷GFP distribution in Class IV neurons also depends on PPK, these results suggest that homophilic Balboa interactions are likely to also depend on the presence of PPK. Interestingly, the Balboa∷GFP punctate structures seen in Class I, II, and III neurons are not observed in experiments with reconstituted GFP. This may be due to a relatively long maturation time for the split-GFP fluorophore [16] and might indicate that Balboa∷GFP present in punctae is less stable relative to the uniformly distributed Balboa:GFP. Alternatively, Balboa proteins in punctae do not form homophilic interactions.
In addition to these in vivo experiments we used the split-GFP approach to further investigate interactions among Balboa and PPK subunits when transiently expressed in S2R+ cells (Figure S3A–F). As in multidendritic neurons, in vitro reconstitution of GFP was detected at the plasma membrane with heteromeric channels (NGFP∷PPK + Balboa∷CGFP, Figure S3B). Interestingly, homophilic interactions among PPK subunits (Figure S3C) or Balboa subunits (Figure S3E) were confined to intracellular secretory structures in the absence of the heteromeric partner. In contrast, the homophilic interactions were clearly detectable at the plasma membrane upon co-transfection with the untagged heteromeric partner (Figure S3D, F). Combined, these data suggest that plasma membrane localization of both Balboa and PPK each depend on the presence of the heteromeric partner protein. Furthermore, as both homophilic Balboa and PPK interactions were detected at the plasma membrane, our results suggest that both types of heterotrimeric channel (i.e. either BBA/PPK/BBA or PPK/BBA/PPK) may be formed in S2R+ cells.
Are Balboa and PPK heteromeric channels sufficient to form a mechanosensitive channel? To test for this possibility we co-expressed the subunits heterologously both in HEK293t cells and in Drosophila S2R+ cells (Figure S4A–D) and tested for mechanosensitive currents. While the known mechanosensitive channel MPiezo1 produced robust currents either when stimulated with a blunt glass pipette (poke) or with negative pressure (stretch) (Figure S4-C), cells transfected with Balboa, PPK, or with Balboa and PPK together did not exhibit currents above background. Lack of currents from co-expression of ppk and balboa may be explained by the need for other accessory proteins for proper function. This is exemplified by observations indicating that the MEC-2 stomatin protein dramatically increased the currents generated from mutant versions of MEC-4 and MEC-10 in Xenopus oocytes [17]. Similarly, mammalian stomatin domain proteins also regulate acid sensing ion channels in the DEG/ENaC family [18].
In summary, our study again demonstrates the power of forward genetics in the identification of novel components important in nociception signaling pathways. Converging lines of evidence strongly support the hypothesis that Balboa and PPK physically interact and likely form a functional ion channel in vivo. First, both genes are functionally required for mechanical nociception. Second, both genes show very specific enrichment and expression in the Class IV nociceptor neurons. Third, ppk expression causes a dramatic redistribution of Balboa∷GFP fluorescence in the multidendritic neurons. Fourth, the Balboa∷GFP distribution is altered by PPK knockdown and Venus∷PPK is dramatically reduced by Balboa knockdown. Fifth, GFP is reconstituted by co-expression of balboa∷CGFP and NGFP∷ppk within neurons of the larval peripheral nervous system.
It is noteworthy that although many DEG/ENaCs have been shown to form homotrimeric or heterotrimeric channels [19, 20], our findings using the split-GFP approach provide the first direct measurement of such physical interactions between heteromeric DEG/ENaC subunits in vivo. Interestingly, GFP reconstitution measurements of homophilic Balboa interactions were confined to the Class IV neurons. Thus, it is highly likely that these in vivo homophilic interactions depend on the presence of PPK. Indeed, results in S2R+ cells suggest that the presence of PPK is needed for plasma membrane localization of Balboa.
A surprising aspect of our findings is that Balboa∷GFP, Venus∷Balboa and Venus∷PPK show uniform labeling throughout the dendritic arbor of the nociceptive Class IV neurons. This pattern differs from the previously described punctate localization pattern of MEC-4/MEC-10 in C. elegans mechanosensory neurons. Indeed, our results suggest that the uniform dendritic localization pattern for the Balboa∷GFP protein is the functionally relevant pattern for mechanical nociception. Perhaps, the uniform pattern might contribute to the high mechanosensory threshold of these cells. Further experimentation will be needed to identify the structures marked by the ectopic punctate pattern seen in Class I, II and III neurons. As Balboa localization to the plasma membrane depends on the presence of PPK, these structures likely represent an unknown component of the secretory pathway, or sites for protein degradation. To our knowledge, similarly labeled intracellular structures in multidendritic neurons have not been previously observed. Future analyses of these structures may thus provide important insight into the regulation of mechanosensory ion channel biosynthesis or turnover.
Experimental Procedures
Supplementary Material
Acknowledgements
Thanks to Andrew Bellemer and Melissa Christianson for their helpful suggestions on improving the manuscript. The NIH Microarray Consortium, Dr. Jill Gerber and Dr. J.H. Pate Skene contributed microarray analyses and procedures respectively. Dr. Greg Michelotti provided expertise on quantitative PCR methodology. Yehuda Ben-Shahar provided ppk26-GAL4, Wayne Johnson provided UAS-ppk and Boaz Cook provided pUAST-DmPiezo. We thank the VDRC and TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks used in this study. We also thank the Drosophila Genomic Resource Center and the Bloomington Drosophila Stock Center for generating and disseminating useful tools and stocks to the fly community. Funding was through grants to WDT from the National Institute of Health (1R01NS054899, 1R01GM086458, 1R21NS088926).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams CM, Anderson MG, Motto DG, Price MP, Johnson WA, Welsh MJ. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J Cell Biol. 1998;140:143–152. doi: 10.1083/jcb.140.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong L, Hwang RY, Tracey WD. Pickpocket is a DEG/ENaC protein required for mechanical nociception in Drosophila larvae. Curr Biol. 2010;20:429–434. doi: 10.1016/j.cub.2009.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang RY. PhD Dissertation. Duke University; 2009. Circuitry and Genes of Larval Nociception in Drosophila Melanogaster. http://hdl.handle.net/10161/1348. [Google Scholar]
- 5.Zelle KM, Lu B, Pyfrom SC, Ben-Shahar Y. The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3 (Bethesda) 2013;3:441–450. doi: 10.1534/g3.112.005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsubouchi A, Caldwell JC, Tracey WD. Dendritic Filopodia, Ripped Pocket, NOMPC, and NMDARs Contribute to the Sense of Touch in Drosophila Larvae. Curr Biol. 2012;22:2124–2134. doi: 10.1016/j.cub.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang RY, Zhong L, Xu Y, Johnson T, Zhang F, Deisseroth K, Tracey WD. Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol. 2007;17:2105–2116. doi: 10.1016/j.cub.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tracey WD, Wilson RL, Laurent G, Benzer S. painless, a Drosophila Gene Essential for Nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 9.Thorne N, Amrein H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J Comp Neurol. 2008;506:548–568. doi: 10.1002/cne.21547. [DOI] [PubMed] [Google Scholar]
- 10.Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Wang CC, Lo JC, Chien CT, Huang ML. Spatially controlled expression of the Drosophila pseudouridine synthase RluA-1. Int J Dev Biol. 2011;55:223–227. doi: 10.1387/ijdb.103112cw. [DOI] [PubMed] [Google Scholar]
- 12.O'Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 13.Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, R OH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- 14.Ozawa T, Umezawa Y. Detection of protein-protein interactions in vivo based on protein splicing. Current opinion in chemical biology. 2001;5:578–583. doi: 10.1016/s1367-5931(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 15.Wilson CG, Magliery TJ, Regan L. Detecting protein-protein interactions with GFP-fragment reassembly. Nat Methods. 2004;1:255–262. doi: 10.1038/nmeth1204-255. [DOI] [PubMed] [Google Scholar]
- 16.Magliery TJ, Wilson CG, Pan W, Mishler D, Ghosh I, Hamilton AD, Regan L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J Am Chem Soc. 2005;127:146–157. doi: 10.1021/ja046699g. [DOI] [PubMed] [Google Scholar]
- 17.Goodman MB, Ernstrom GG, Chelur DS, O'Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- 18.Moshourab RA, Wetzel C, Martinez-Salgado C, Lewin GR. Stomatin-domain protein interactions with acid-sensing ion channels modulate nociceptor mechanosensitivity. J Physiol. 2013;591:5555–5574. doi: 10.1113/jphysiol.2013.261180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic Force Microscopy Reveals the Architecture of the Epithelial Sodium Channel (ENaC) Journal of Biological Chemistry. 2011;286:31944–31952. doi: 10.1074/jbc.M111.275289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.