Introduction
The crystallin proteins of the human eye lens need to remain folded and soluble to retain lens transparency throughout our lifetime. Thus their stability is an essential physiological property evolved during the emergence of vertebrates, or even earlier. Protein stability is typically defined in terms of the preservation of native atomic contacts within the protein's 3-dimensional conformation. The loss of these contacts and interactions – denaturation – is captured, for example, in the thermal melting transitions detected by calorimetry. Partial loss of native structure often leads to polymerization of polypeptides into high molecular weight aggregates. In this review we are particularly interested in those unfolding pathways which lead to aggregates of sufficiently high molecular weight to scatter visible light, resulting in cataract.
Though the aggregated states of proteins are often thought of us as products of non-specific reactions, this view reflects the paucity of physical methods available for characterizing high molecular weight aggregates. In almost all cases closely investigated, the aggregated states turn out to be polymers of specific partially folded or unfolded intermediates. Examples include the domain swapped polymers formed from mutants of α1-antitrypsin in liver cells (Lomas and Parfrey, 2004; Yamasaki et al., 2011); the amyloid fibers formed from mutant transthyretin molecules, probably within cells (Colon and Kelly, 1992); β2-microglobulin fibers in blood (Skora et al., 2010); the amyloid fibers formed from α-synuclein (Fink, 2006); the inclusion bodies formed from P22 tailspike and coat proteins chains (King et al., 1996); and polymers of a number of other proteins (Horwich, 2002). In fact the classic example the polymerization of sickle hemoglobin into fibers is an exception, since the precursor is a native state of the mutant protein.
The widespread industrial method of purifying misfolded human therapeutic proteins from the inclusion body state, and then refolding them in vitro, is based in part on the property of the inclusion bodies as usually relatively pure aggregates of the protein in question (Mitraki and King, 1989; Speed et al., 1996). Thus a diverse body of work indicates that the conformation of the partially folded or unfolded intermediate is critical for the efficiency of the off-pathway aggregation reaction. These aggregates are non-covalent polymers of protein folding or unfolding intermediates, and we will often use the term polymerization to communicate the notion of a specific pathway to high molecular weight complexes.
The critical measurements used to assess and quantify protein stability differ depending on the class of proteins under consideration. In the case of enzymes, stability often refers to retention of catalytic ability, which depends on a precise arrangement of local residues at the active site and sites important for allostery. An enzyme need not form aggregates to become denatured. Conversely, limited aggregation may actually rescue an enzyme's catalytic ability (Bershtein et al., 2012). Stability of structural proteins such as collagen, myosin, actin or tubulin may require maintaining intact a much larger fraction of the protein's native conformation, in part due to the requirement of proper supramolecular assembly. Thus, loss of native structure and the formation of insoluble high molecular weight aggregates are often coupled, but not necessarily proportional. Our discussion below includes examples where higher native-state stability goes hand in hand with higher aggregation propensity, and vice versa.
For the eye lens crystallins, the native folded state is required for lens transparency, while the aggregated high molecular weight complexes are the source of light scattering leading to cataract. Thus our discussion below focuses on features of the crystallins that protect from loss of native structure or from formation of high molecular weight aggregates. Within the protected environment of the eye lens, it is unlikely that fully unfolded polypeptide chain conformations are populated, in contrast to guanidinium chloride or urea denaturation. Rather, the species that form high molecular weight aggregated states are partially folded or unfolded intermediates. The partially unfolded conformations that polymerize into high molecular weight aggregates will be only a subset of the possible conformations generated upon loss of native state structure. Thus, the particular pathways of unfolding that result from specific perturbations are likely to determine whether and when light scattering aggregates will form. Generalized measures of protein stability may not reveal this information.
In some cases, aggregated states may be generated by folding intermediates formed after translation by lens cell ribosomes. This is particlularly relevant for congenital cataract-causing mutations (Zhai et al., 2014). However, the properties of folding intermediates often have very limited relationships with the stability of the native state, and cannot in general be deduced by studies of the native state. In fact for truncated chains, for example, no native state can be formed.
A comprehensive and valuable survery of the literature on crystallin biochemistry and its relation to cataract was published by Bloemendal et al. in 2004. Subsequent reviews include those by Moreau and King (Moreau and King, 2012b) and Michael and Bron (Michael and Bron, 2011). Below we concentrate on recent findings that elucidate the molecular basis of crystallin unfolding and aggregation leading to cataract.
Structure of βγ-crystallins
Details of the structure are reviewed elsewhere in this issue. Here we will only briefly recapitulate the main structural features of the βγ-crystallin family. These proteins share a common bilobed structure, composed of four Greek Key motifs, as shown in Figure 1. The Greek key motifs are intercalated within each domain, such that each domain is a double Greek key. The core of each domain is highly hydrophobic, and the sequence is unusually rich in aromatic and sulfur-containing residues. The surface is highly charged, but pI is near neutral for the γ-crystallins; the β-crystallins are subdivided into the acidic (βA) and basic (βB) classes. γ-crystallins are natively monomeric. β-crystallins form homo- or heterodimers, as well as some higher-order assemblies. Although βB1-crystallin exists as a monomer in vitro (Annunziata et al., 2005), it is a marker component of higher-order assemblies (reviewed elsewhere in this issue).
Figure 1.
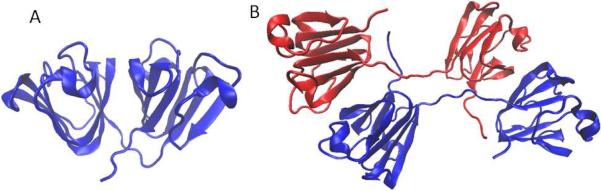
Crystal structures of human γD crystallin, PDB ID 1hk0 (Basak et al., 2003) (A) and human βB2 crystallin, PDB ID 1ytq (Smith et al., 2007) (B). Drawn using VMD (Humphrey et al., 1996).
Contributions to native state stability
Crystallin stability has been studied by a variety of methods, which vary both in the type of insult used to destabilize the protein as well as the signals monitored. Measurements have been made under both equilibrium conditions, assessing thermodynamic stability, and kinetic stability under less reversible, more physiological, conditions. The reversibility requirement for measuring thermodynamic stability generally requires suppressing the aggregation reactions which are so important in cataract formation. In measurements of kinetic stability, the aggregation reactions are usually present, and often account for the absence of reversibility.
Particularly valuable for the crystallins have been experiments that directly monitor the formation of the high molecular weight aggregates responsible for light scattering. Heat, chemical denaturants, pH, and specific mutations have all been used as perturbations to the native βγ-crystallin fold. Likewise, aggregation has been studied using each of these destabilizing perturbations. However, with rare exceptions, the proteins may not encounter these denaturing conditions in vivo. Some of these treatments may result in non-physiological conformations or conformational transitions. Aggregation has also been studied in response to UV irradiation, refolding from a denatured state, or cold-precipitation of the native state. Selecting those results which approach physiological conditions may be important for assessing the key contributions to stability in vivo.
The γ-crystallins are extremely stable, with melting temperatures up to ~80 °C as well as resistance to urea and 2-3 M guanidinium chloride (Kosinski-Collins and King, 2003). The precise origin of this high thermodynamic stability remains a subject of intensive research. Although it has often been proposed that the intercalated, tightly-packed nature of the double Greek key yields highly stable proteins, this argument applies only up to a point. The β-crystallins, which share the same double Greek key fold, are significantly less thermodynamically stable, both to thermal and chemical denaturation (Mayr et al., 1997; Wieligmann et al., 1999). It seems likely that conclusions that have been drawn by a great number of efforts to understand protein stability hold for crystallins: many different classes of interactions – H-bonds, van der waals packing, hydrophobic effect, aromatic stacking, ion pairs and salt bridges – all contribute to the overall stability.
The stability of γB-crystallin has been carefully characterized at pH 2 using urea denaturation. Under these conditions, both domains unfolded independently, and the stability of the C-terminal domain was found to be lower than that of the N-terminal domain (Rudolph et al., 1990). Mutational and crystallographic studies revealed that the C-terminal domain was stabilized by interaction with the N-terminal domain under these conditions (Mayr et al., 1997; Palme et al., 1998). This observation supports the critical role of the domain interface.
Efforts to assess the contribution of particular residues and classes of residues to native state stability of γD crystallin have been made in our lab using site-directed mutagenesis. These studies revealed the significant stabilizing effect of domain interface residues, including hydrophobic residues and glutamines (Flaugh et al., 2005b; Flaugh et al., 2006; Mills-Henry et al., 2007). Substitutions of tyrosine residues have elucidated the contributions of both conserved and variable tyrosine pairs (Kong and King, 2011). Tryptophan residues have been investigated both for their contribution to native-state stability (Kosinski-Collins et al., 2004; Xia et al., 2013); E.S., T. Takata, and J. A. K., unpublished) and for their effect on UV protection (Chen et al., 2009; Chen et al., 2006; Chen et al., 2008; Schafheimer and King, 2013). Notably, the absence of C-terminal domain tryptophans or tyrosines results in a highly cooperative unfolding transition, whereas the absence of N-terminal domain tryptophans or tyrosines leads to increased population of a partially folded intermediate. Mutations of specific core residues associated with congenital hereditary cataract have revealed a similar pattern (Moreau and King, 2009). These studies are discussed further in the following sections.
An important feature of the monomeric γ-crystallins is that they can be fully refolded from the guanidinium denatured state in vitro, in the absence of chaperones or chaperonins, at pH 7 and 37 °C (Figure 2). As a result, it has been possible to carry out a careful characterization of side chain contributions to thermodynamic stability for the human γD-crystallin. A highly efficient energy transfer mechanism that quenches tryptophan fluorescence in the native state provides a sensitive reporter of folding (Chen et al., 2006; Kosinski-Collins et al., 2004). The unfolding of this protein both in experimental studies and molecular dynamics simulations proceeds through a three-state transition; the N-terminal domain loses its structure before the C-terminal domain does (Das et al., 2010; Flaugh et al., 2005a, b; Flaugh et al., 2006). A similar situation is observed in γB crystallin (Rudolph et al., 1990). The isolated N-terminal domain and C-terminal domain of γD are able to fold in solution; however, in the full-length protein the structural integrity of the N-terminal domain is substantially augmented by the domain interface. The free energy of the domain interface is estimated to be worth ~4 kcal/mol in both γB and γD crystallins (Mayr et al., 1997; Mills-Henry et al., 2007). Mutational analysis revealed that perturbing that interface leads to much greater destabilization of the N-terminal domain than the C-terminal domain (Flaugh et al., 2005a, b; Flaugh et al., 2006). In these studies, refolding experiments with chains carrying replacements of interface residues indicate that the C-terminal face of the domain interface acts as a template for the folding of the N-terminal domain.
Figure 2.
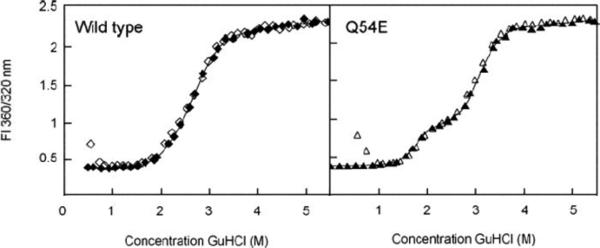
Equilibruim unfolding (closed) and refolding (open) of human γD crystallin and its deamidation-mimicking Q54E mutant monitored by changes in intrinsic fluorescence quenching. Refolding is carried out by dilution of a fully unfolded protein from 5 M GdnHCl into buffer to reach the desired denaturant concentration. The rise in the refolding curve at the lowest denaturant concentration reflects aggregation competing with productive refolding during the rapid dilution. Reprinted from Flaugh et al., 2006.
Stability and aggregation
When unfolded by chemical means, γD shows significant hysteresis, i.e., the native state is stable to higher concentrations of denaturant than the concentrations at which the unfolded state is no longer able to fold (Kosinski-Collins and King, 2003). This represents a kinetic barrier to the initiation of unfolding. Hysteresis has not been observed for the individual domains (Mills-Henry, PhD thesis), and may thus be attributable to domain-domain interactions, as has been noted for γB-crystallin (Palme et al., 1998) and for the structurally related Protein S (Wenk et al., 1998). Further evidence in support of this notion comes from the observation that stabilization of the domain interface of γD crystallin leads to an increase in the hysteresis, whereas destabilization of the C-terminal domain diminishes hysteresis (Sahin et al., 2011). Thus, hysteresis may result from the ability of the N-terminal domain to “hitch a ride” on the more structurally stable C-terminal domain by using the C-terminal side of the domain interface as a template for folding. Thus, the domain-domain interaction appears to act as a clamp on early unfolding. However, it is also possible that oligomerization of early unfolding intermediates enhances this hysteresis. Whatever its origins, kinetic stabilization is a useful strategy for a protein to preserve its native structure for a longer time than mere thermodynamic stability would allow. Nearly all known crystallins have duplicated domains, indicating significant selection for this feature of the structure.
Data for γD and γB crystallins may be considered representative of the γA-F subfamily. By contrast, the slightly more distantly related γS-crystallin does not appear to exhibit strong domain-domain interaction or extensive dependence of N-terminal domain on C-terminal domain. The unfolding of this protein is highly cooperative, and hysteresis has not been found (Mills-Henry, PhD thesis). It is notable that the interdomain linker of γS is five residues long, compared to four in γB and only three in the rest of the γ-crystallins (Bloemendal et al., 2004). Shorter linkers and tighter domain interfaces may have evolved within the γ-crystallin family to facilitate kinetic stabilization and confer longer-term resistance to formation of light-scattering aggregates.
The β-crystallins are more labile than γ-crystallins. In most cases studies of their stability are complicated by the fact that they tend to exist within heteromeric assemblies, and thus data on individual purified proteins may be misleading (see below). The notable exception is βB2-crystallin, which forms stable domain-swapped homodimers (Bax et al., 1990). The structural stability of βB2 is nonetheless greatly reduced relative to the γ-crystallins (Wieligmann et al., 1999). The N-terminal domain of human βB2-crystallin is less stable than its C-terminal domain; however, this is not the case for the calf βB2-crystallin (Evans et al., 2008).
Similarly, Evans et al. compared thermodynamic stability and thermal aggregation in human vs. calf βB2-crystallin (Evans et al., 2008). Although the human protein is more stable to both thermal and chemical denaturation, the calf protein is much more resistant to heat-induced aggregation. This striking difference may be due to aggregation from a partially folded intermediate. Since the human protein exhibits differential stability of its two double Greek key domains, it may populate a partially folded intermediate, in which the C-terminal domain is folded, and the N-terminal domain has begun to unfold. The calf protein, whose unfolding is more cooperative, appears to be protected from this aggregation pathway.
Researchers studying γ-crystallins have proposed a similar mechanism of aggregation via full or partial unfolding of the N-terminal domain, in the context of intact C-terminal domain. When fully denatured crystallin chains are diluted rapidly from denaturant into buffer at sufficiently high protein concentrations, off-pathway aggregation competes with productive refolding (Kosinski-Collins and King, 2003). These aggregates are not reversible under the conditions of the experiment. They provide, however, a model for the aggregation leading to cataract in that they derive from a wild type polypeptide chain, probably in a conformation close to that needed for productive refolding. Like folding itself, aggregation competing with refolding is enhanced by the domain interface in γD crystallin, but much less so in γS crystallin (Mills-Henry, PhD thesis). Since the former exhibits differential stability of its two domains, while the latter exhibits more cooperative folding (Mills-Henry et al., 2007), this behavior is consistent with aggregation of partially folded intermediates.
Mutational studies confirm the importance of N-terminal domain unfolding for the aggregaion pathway. Here and in what follows, we will use the PDB (1HK0) numbering convention to refer to the sites of mutations. The V75D mutation in γD-crystallin, which causes congenital cataract in mice, has been shown to destabilize the N-terminal domain, increase the population of the proposed aggregation-prone unfolding intermediate, and lead to aggregation even at physiological pH and temperature and in the absence of chemical denaturation (Moreau and King, 2009). The W42R mutation likewise induces N-terminal domain destabilization and aggregation and leads to congenital cataract in humans (Ji et al., 2013). The Y45A/Y50A double mutant of γD-crystallin also showed a folding intermediate with the N-terminal domain unfolded, in contrast to the corresponding C-terminal double mutant (Y134A/Y139A), which exhibited cooperative unfolding, both by guanidine chloride denaturation (Kong and King, 2011) and in molecular dynamics simulations (Yang et al., 2014). Although folding of the wild-type γS is cooperative, the cataract-linked F9S mutant exhibits destabilization of the N-terminal domain, and aggregation from this partially folded intermediate has been proposed (Lee et al., 2010; Mahler et al., 2011).
The common theme in these studies is that the relative thermodynamic or kinetic stability of the two domains may be more relevant as a predictor of aggregation propensity than the absolute structural stability. Mutations that reduce cooperativity of folding may put a protein at risk for aggregation regardless of its thermodynamic stability. However, as discussed below, incomplete cooperativity may also enable chaperone-like action by a folding intermediate in a mixture of crystallins.
Finally, it should be noted that structural stability is typically not informative of a protein's solubility, which is a particularly important challenge for crystallins, which exist at very high concentrations in the eye lens. Several congenital cataract mutations, such as R36S and R58H in γD, result not in significant thermodynamic destabilization or non-native aggregation, but rather in crystallization (Pande et al., 2001). In the case of the cataract-linked P23S mutation, evidence from multiple sequence alignment indicates that the reverse mutation at or near the equivalent site on the C-terminal domain (e.g., S137P) rescues the defect (Plotnikova et al., 2007). It is thus an intriguing possibility that these equivalent sites may be involved in packing interactions among γD-crystallin molecules; if the packing is too strong or disrupted, insolubilization may occur. The cataract-linked A2V mutation in the βB2 crystallin represents another interesting case: while virtually identical to the wild-type protein in thermodynamic stability and aggregation propensity, this mutant was found to be impaired in higher-order oligomeric assembly (Xu et al., 2012). Recent work on the fish γM-crystallins (Mahler et al., 2013) provides a particularly striking example. The fish crystallins exist in conditions of extreme crowding, reaching concentrations of up to 1 g/ml. However, the γM-crystallins studied to date are both less thermodynamically stable and less water-soluble than any of the mammalian γ-crystallins. A possible explanation may lie in the γM-crystallins’ scant hydration shell: water may simply be a bad approximation for their native solvent environment (Zhao et al., 2014).
Thermolabile or easily denaturable proteins may nonetheless escape aggregation. Numerous natively unstructured proteins have this character. An additional example among the lens proteins is αA-crystallin. Although its secondary structure is rapidly lost at temperatures exceeding 60 °C, which is significantly below the thermodynamic stability of the γ-crystallin monomers, αA-crystallin does not form light scattering aggregates even above the melting temperature. This property is due to the protein's ability to act as a chaperone, including for itself, by forming large supramolecular assemblies. The size of these assemblies remains too small to scatter visible light; in essence, formation of smaller assemblies diverts the polypeptide from the pathway toward larger ones.
As the chaperones of the lens core, α-crystallins are able to recognize partially folded conformations of γ-crystallins and prevent their aggregation (Acosta-Sampson and King, 2010). Certain congenital mutants of γD-crystallin have been shown to escape from this aggregation suppression, resulting in cataract that bypasses the protective chaperone system of the lens (Moreau and King, 2012a). Conversely, too-strong interactions of the E107A congenital cataract γD-crystallin mutant with the α-crystallin chaperones have been proposed as the basis of cataract in that subset of patients (Banerjee et al., 2011). The functions of α-crystallins are reviewed elsewhere in this issue.
Although few studies on βγ-crystallins have explored their potential to act as self-chaperones, they may exhibit such activity under some conditions. An interesting case study involves the attempt to engineer aggregation resistance in γD crystallin by computation-driven design of point mutants (Sahin et al., 2011). As shown in Figures 3 and 4, the M69Q mutant stabilized the domain-domain interface, increased overall thermodynamic stability as well as kinetic stability (as measured at pH 3 both by urea denaturation and by calorimetry), yet it did not substantially affect the protein's heat-induced aggregation propensity relative to the wild-type protein. It is interesting to note that M69 does not make direct contacts across the interface, but appears to affect them indirectly. By contrast, the S130P mutation resulted in a reduction of both thermodynamic and kinetic stability; however, it virtually abolished the heat-induced aggregation. Instead of aggregating, γD S130P may have formed dimeric species that were protected against aggregation.
Figure 3.
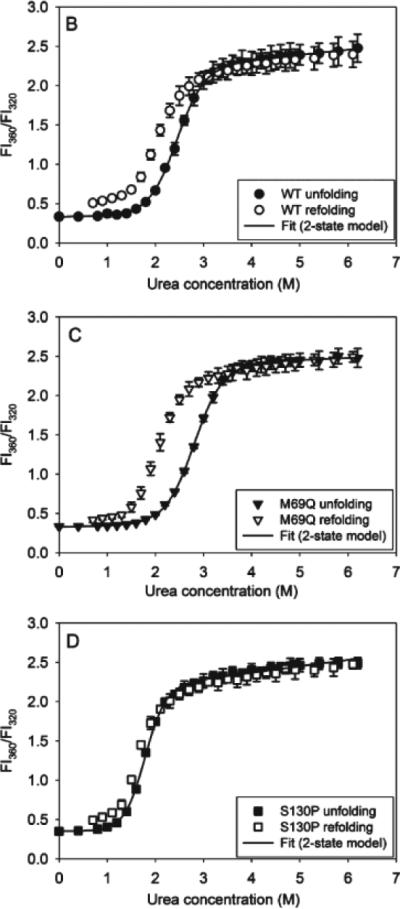
Differential stability and hysteresis in acid/urea denaturation of wild-type, M69Q, and S130P γD crystallin. Reprinted from Sahin et al., 2011
Figure 4.
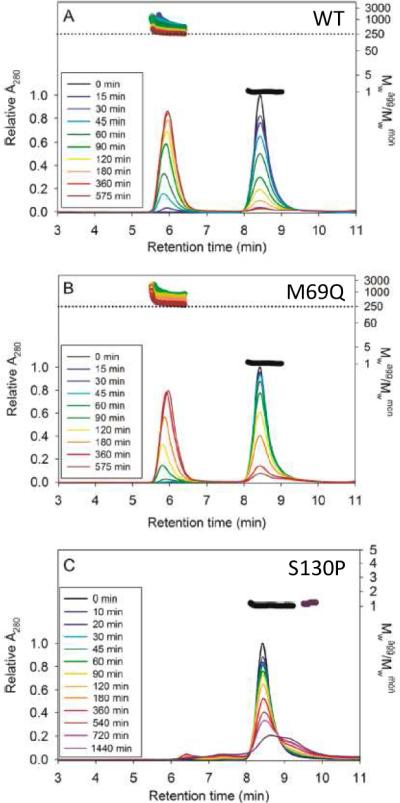
The S130P mutation suppresses aggregation relative to M69Q or WT, as seen by size exclusion and multiangle light scattering. Reprinted from Sahin et al., 2011
Effect of α-crystallin chaperones
Many of the partially folded and unfolded conformers of crystallins described above are substrates for the lens chaperones αA/B-crystallins, which suppress their in vitro aggregation (Acosta-Sampson and King, 2010; Michiel et al., 2010; Sathish et al., 2004). However some cataractogenic mutants likely escape suppression, as has been demonstrated for V75D γD-crystallin (Moreau and King, 2012a).
The structure, expression patterns and chaperone function of α-crystallins are described in detail in the companion reviews in this volume (Boelens, 2014; Hochberg and Benesch, 2014; Slingsby and Wistow, 2014). High levels of α-crystallins in the lens may account for the very low frequency of cataracts among children and young adults. However, since there is limited or no new translation in the adult lens nucleus and inner cortex, the available pool of active α-crystallin may well decline with age, contributing to the sharp increase in cataract incidence with advancing age (Heys et al., 2007).
The lens epithelia and outer cortical fibers also express the ubiquitous group II chaperonin TRiC, needed to fold tubulin, actin and many other cytosolic proteins. TriC not only suppresses the aggregation of γD-crystallin in vitro, but also can actively refold it in the presence of ATP (Knee et al., 2013; Sergeeva et al., 2013). This chaperonin may also contribute to protecting from cortical cataract.
Responses to covalent damage
For extremely long-lived proteins such as the eye lens crystallins, native structural or functional stability may ultimately be of lesser importance than resilience under conditions that induce covalent damage. Several classes of insults can result in covalent modification of βγ-crystallins in the lens, and proteomic analysis has helped reveal a variety of modifications (Truscott, 2005). These modifications often include deamidation, oxidation, or glycation of specific side chains (Hains and Truscott, 2007, 2010). The protein's behavior following such modification may change markedly.
Amino acid substitutions can be used to mimic deamidation (reviewed elsewhere in this issue) or oxidative damage at specific sites. Mimics of deamidation at the domain-domain interface thermodynamically destabilize the N-terminal domain in both γD-crystallin (Flaugh et al., 2006) and βA3-crystallin (Michiel et al., 2010; Takata et al., 2007; Takata et al., 2008; Takata et al., 2009). Mimics of cysteine oxidation (Schafheimer et al., 2014) and Trp oxidation (E.S., T. Takata, and J. A. K., unpublished) can significantly reduce structural stability, and, depending on the site of the substitution, may induce aggregation. Overall, perturbing stability via site-directed mutagenesis alleviates the need to study unfolding or aggregation under harsh denaturing conditions ordinarily never encountered in the real lens.
Exposure to UV-B light is an important contributing factor to cataract (West et al., 1998). Decades-long exposure to ultraviolet radiation from the sun may result in breakage of the indole ring of tryptophan side chains, triggering their oxidation to kynurenine (Hains and Truscott, 2007). Zhou and coworkers used molecular dynamics simulations to examine the consequences of such damage by replacing the four tryptophan residues in γD-crystallin with kynurenine residues (Xia et al., 2013). That work revealed significantly accelerated melting of the double Greek key domains, likely due to the leakage of water molecules into the tightly packed hydrophobic domain cores. A similar effect has been observed by replacing tyrosine residues with alanine (Kong and King, 2011; Yang et al., 2014).
βγ-crystallins have evolved resistance to certain types of common and damaging covalent modification. For example, the four-tryptophan cluster in the γD-crystallin core has adapted to provide fast and efficient quenching, thus minimizing the risk of loss of aromaticity as a result of UV irradiation (Chen et al., 2009; Schafheimer and King, 2013). Schafheimer et al. (2014) examined the effect of UV light on cysteine oxidation and aggregation in γD-crystallin both using specific Cys->Ser point mutations by mass spectrometry of irradiated wild-type protein. The results further demonstrate that structural stability may not be predictive of aggregation. Thus, an early effect of UV irradiation in the presence of oxygen is oxidation of Cys18 in γD-crystallin. The oxidation-mimicing C18S point mutant exhibited thermodynamic destabilization of the N-terminal domain, yet it was protected against UV-induced aggregation relative to the wild-type protein. The pathway toward aggregation may therefore be controlled by diverting potentially deleterious damage to those sites on the protein where it is less deleterious or even protective.
Resistance to damage is constrained by evolution, since it may come at the expense of other functional adaptations. Thus, the fish γM-crystallins lack the protective four-tryptophan cluster of their mammalian counterparts, presumably due to their reduced need for protection from UV exposure (Mahler et al., 2013). Some types of damage – such as from occupational exposure to heavy metals – may not have been major factors during the evolution of the human lens, but are more significant today.
Stability in mixtures
The diversity of the crystallins and polydispersity of their assemblies is thought to reflect one mechanism of inhibiting crystallization within the very concentrated lens protein solutions (Laganowsky et al., 2010). An additional factor may be that heterodimerization allows βA-crystallins to avoid aggregation in the lens; in this case, the partner protein may be thought of as a chaperone. Wang et al. (2011) examined the effect of βB1-crystallin on aggregation of βA3. As shown in Figure 5, βA3-crystallin exhibits substantial aggregation during refolding from denaturant. This aggregation persists even if natively folded βB1 is added to the refolding buffer. However, when βA3 and βB1 were refolded from denaturant as a mixture, the aggregation of βA3 was suppressed. The likely explanation is that an interaction between a folding intermediate of βA3 and a folding intermediate of βB1 allows formation of heteromers and thereby suppresses light-scattering aggregation.
Figure 5.
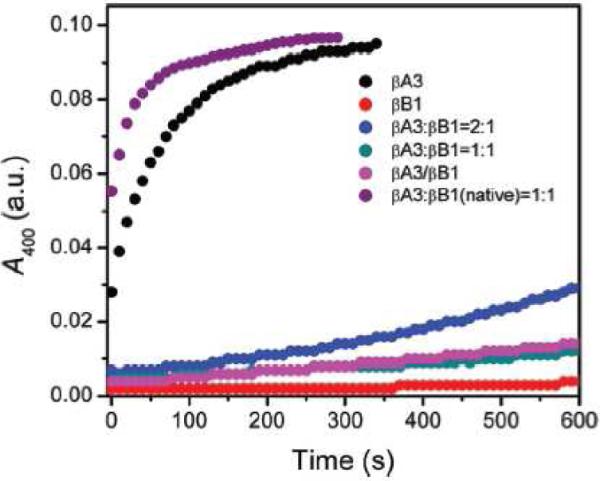
Aggregation competing with refolding of βA3 from denaturant (black) is suppressed when carried out concomitantly with refolding of βB1 (blue, pink, cyan), but not when natively folded βB1 is present in the refolding buffer (purple). Reprinted from (Wang et al., 2011).
Non-native aggregation behavior of γ-crystallins in multicomponent mixtures has not been biochemically characterized. However, the existence of interprotein interactions in these natively monomeric crystallins has been deduced from biophysical measurements. Some βγ-crystallins in aqueous solvent exhibit the property of liquid:liquid phase separation upon cooling. This phenomenon allows calculation of interaction free energies among particles in solution: the stronger the interaction, the more easily the protein partitions into a concentrated phase. Remarkably, phase separation has been observed for γA-F, but not for γS-crystallin, indicating that the former show attractive interactions, while the latter does not (Liu et al., 1996; Liu et al., 1995). It is tempting to speculate that the unusual attractive interaction energy is due to transient oligomerization, which may be present natively and facilitate crystallin packing in concentrated solutions.
The temperature of the phase transition can be suppressed in mixtures of relatively dissimilar βγ-crystallins. For example, Liu et al. (1996) demonstrated that admixture of γS-crystallin suppresses demixing of γB or γE. This suggests an interaction between γS and the other γ-crystallins that is able to reduce the magnitude of the attractive interaction among the γA-F-crystallin molecules. More recent work (Wang et al., 2010a) revealed that admixture of βB1-crystallin can suppress demixing of γD-crystallin. Moreover, as shown in Figure 6, data from quasi-elastic light scattering are consistent with formation of complexes of γD and βB1 in the mixtures. Thus, protein-protein interactions in multicomponent βγ-crystallin solutions may directly modify these proteins’ tendency toward aggregation and serve as an important protection against opacification of the lens.
Figure 6.
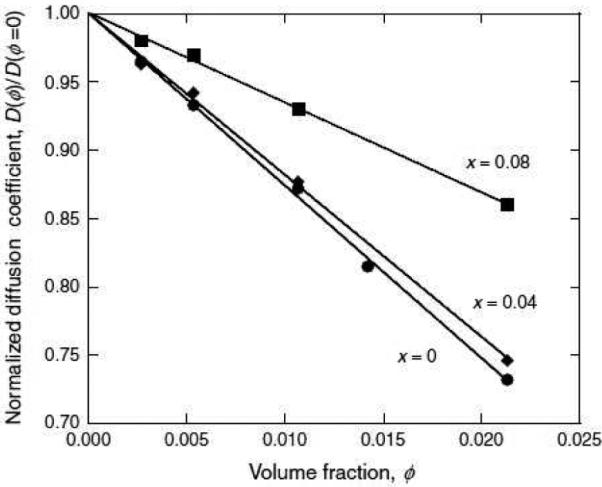
Diffusion coefficient decreases with increasing protein concentration in a pure γD crystallin solution (x=0), and this increase is attenuated by admixture of βB1 (×=0.04 and ×=0.08 are mole fractions of βB1 crystallin). Reprinted from Wang et al., 2010.
Stability over evolutionary time
Although βγ-crystallins are sometimes considered as having no function in the lens aside from their role as structural proteins, at least two other functions have been studied. Evolutionary and biophysical measurements can quantify the ability of γ-crystallins to enhance the refractive index of the lens (Zhao et al., 2011). In fact, the requirement of an unusually high index of refraction likely contributed to the aromatic- and sulfur-rich primary structure of these proteins. In addition, crystallins are likely to play a role in absorbing ultraviolet light to protect the retina from photodamage (Chen et al., 2008). The conserved tryptophan cluster in βγ-crystallins is needed for efficient dissipation of the absorbed energy (Chen et al., 2009; Schafheimer and King, 2013). Neighboring residues, such as Tyr16 and Cys18, participate in this energy transfer process (Schafheimer et al., 2014).
Balancing the functional demands of high refractive index, high UV absorptivity, and extremely high resistance to aggregation likely drove the selection of the double Greek key scaffold as the eye lens crystallin of choice in the vertebrate lineage. The extremely long life of βγ-crystallins likely provided a further constraint: the requirement for resistance to covalent modification due to environmental damage.
The structural versatility of the βγ-crystallin fold may allow it to tolerate genetic drift and allow for easy divergence among crystallin genes (Bershtein et al., 2006). However, the existence of many cases of congenital cataract due to point mutations, as discussed above, indicates that there are limitations to this versatility. Deleterious mutations may be rescuable by mutations in distal domains (Plotnikova et al., 2007) via higher-order interactions or buffered by the high concentration of α-crystallin in the mammalian lens. Exchange of sequence with the γ-crystallin pseudogenes (Dendunnen et al., 1986) may have may have allowed speedier replacement of deleterious alleles or introduction of rescuing mutations in case a mutation cannot be tolerated.
Nature of aggregates and mechanisms of aggregation
The nature of crystallin aggregates in the lens remains one of the great mysteries in the field of cataract research. Electron microscopy studies have revealed only an increased texturing of the lens fiber cell cytosol, but no evident aggregate structure (Metlapally et al., 2008). It may be that increased protective oligomerization of βγ-crystallins and α-crystallin assembly with increasing environmental damage, or even sequestration of damaged proteins into dense vesicles underlies this increased texturing (Gilliland et al., 2004). It is also possible, however, that propagating aggregates of βγ-crystallins exist in the lens, but lack the regularly repeating supramolecular structure that ordinarily aids detection by microscopy techniques.
As mentioned above, crystallins can be induced to form amyloid fibers at reduced pH (typically pH 3). Although no amyloid fibers have been observed in aged or cataractous lenses, they may be causative agents in certain childhood-onset cataract, since the developing lens does contain lysosomes, which are low-pH organelles (Wride, 2011). In addition, at least one study found amyloid fibers in γD-crystallin samples subjected to UV irradiation (Moran et al., 2013). Besides, amyloids remain the only class of non-native protein aggregates for which structural study is accessible. Thus, significant structural efforts have focused on characterizing the amyloid aggregates of γ-crystallins. Either of the isolated domains of γD- or γC-crystallin is able to form amyloid fibers when exposed to pH 3 (Papanikolopoulou et al., 2008), as does γC crystallin (Wang et al., 2010b). However, Moran et al. showed using 2-dimenstional infrared spectroscopy and mass spectrometry of pepsin fragments that the core of the full-length γD-crystallin amyloids is likely composed of C-terminal domain residues (Moran et al., 2012a; Moran et al., 2012b). Since the known amyloid cores consist of contiguous residues, sequence-based prediction can in principle be applied to help delineate a likely amyloid core. The most likely C-terminal domain amyloid core of γD-crystallin reported in these studies (residues ~118-140) overlaps with the likely core derived from sequence-based prediction algorithms (Sahin et al., 2011).
Non-native aggregation from protein folding intermediates remains challenging to study or even detect using currently available structural techniques. Analysis of disulfide bonding patterns in the congenital cataract R14C mutant of γD-crystallin suggested extensive non-native interactions (Pande et al., 2009; Pande et al., 2000). However, in this and other studies of posttranslational modification it is difficult to determine whether the modification occurs before or after the aggregation process.
Multiple studies (discussed above) link the relative stability of the N- and C-terminal domains with the proclivity for aggregation. Aggregation may proceed from a partially unfolded intermediate, such as the one with N-terminal domain unfolded and C-terminal domain intact, or it may begin with a more localized loss of structure. Mahler et al. (2013) suggest that unfolding of γM-crystallins may originate from the helical loop at the top of the N-terminal domain. Accordingly, a study of the importance of aromatic stacking for the thermodynamic and kinetic stability of γD-crystallin also found the largest effect in the N-terminal domain from the Y45A/Y50A pair (Kong and King, 2011). It is worth noting again that the site at which unfolding begins need not be the site responsible for aggregation, but may represent the initial step on a conformational change pathway that reveals such a site.
The observation of differential domain stability, coupled with the domain-swapped crystal structure of βB2-crystallin, have drawn attention to domain swapping as a potential mechanism of non-native aggregation. Computational studies using long time scale molecular dynamics simulation of unfolding followed by simulated annealing have demonstrated protein-protein contacts consistent with exchange of N-terminal domains among γD-crystallin molecules (Das et al., 2011). If such domain exchange occurs, the interdomain linker is likely to play an important role, as it does in other cases of domain swapping (Chen et al., 1999; Green et al., 1995; Rousseau et al., 2001; Rousseau et al., 2006; Schymkowitz et al., 2001). The role of the linker is further emphasized by the observation that mixtures of isolated single domains of either γB or γS crystallin do not form dimers in solution (Mayr et al., 1997). Linker engineering in βγ-crystallins has not been shown to induce swapping (Mayr et al., 1994; Trinkl et al., 1994), although altering the linker in a βA3-crystallin construct already prone to dimerization permitted researchers to enhance this propensity (Sergeev et al., 2000). The origin of this dimerization was proposed to be domain swapping. It is important to note that swapped aggregates may not be the end-product of βγ-crystallin aggregation, but instead may represent one step along an aggregation pathway completed by further levels of supramolecular packing.
An alternative swapping mechanism based on exchange of an N-terminal β-strand has been found in the structurally related bacterial protein, nitrollin, as shown in Figure 7B (Aravind et al., 2009). It is intriguing that most of the congenital cataract point mutations in the γ-crystallins (T4P, L5/F9S, R14C, P23S/T, W42R, V75D, R76S), as well as the most easily observed modifications upon UV irradiation (oxidation of Cys18 and Cys78) cluster near the N-terminal β-strand (Figure 7A). To date, no forward genetic screen for aggregation-prone β- or γ-crystallin mutants has been carried out, though its results would likely inform the biochemical and biophysical models of βγ-crystallin aggregation in the lens.
Figure 7.
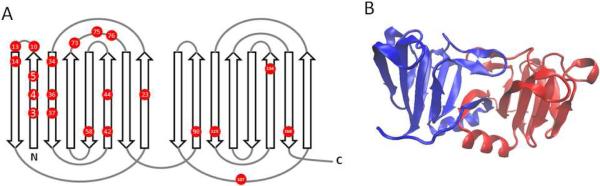
(A) A connectivity diagram of the γ-crystallin fold showing the positions of all conserved residues where known point mutations are linked to hereditary cataract. Data from (Graw, 2009); the more recently found W42 mutation is also included. (B) Crystal structure of the domain-swapped dimer of nitrollin, a prokaryotic crystallin homolog. PDB ID 3ENT (Aravind et al., 2009). Drawn using VMD (Humphrey et al., 1996).
The βA and βB crystallins have been shown to exchange subunits in vitro on the time scale of hours under physiological conditions in vitro (Hejtmancik et al., 1997; Hejtmancik et al., 2004). The binding free energy of βA3 and βB2 domain interface has been calculated as worth only 1-2 kcal/mol (Sergeev et al., 2004), in contrast to the ~4 kcal/mol for the γB and γD crystallins. Does a weakened domain interface induce domain swapping? The structure of βγ-crystallins is highly symmetric, comprising two globular domains, each consisting of two Greek Key motifs, each of which in turn is composed of two β-hairpins. The highly duplicated structure offers a number of possible interfaces that, if disrupted by mutation or post-translational damage, could open the pathway toward swapped polymerization. A pioneering study in this field (Mahler et al., 2011) used NMR relaxation dispersion to quantify the mobility of individual side chains in γS-crystallin arising either from partial urea denaturation or from the cataract-linked F9S mutation. Whereas the chemical denaturant induced relatively broad denaturation of the N-terminal domain, as observed previously (Lee et al., 2010), the mutation's effect under physiological conditions was much more precise. Increased mobility at the N-terminal β-strands indicated the formation of a transiently populated unfolding intermediate with most of its structure intact, but a distinct part of the N-terminal domain destabilized or opened. Future studies should seek to identify comparable unfolding intermediates that may reside on the pathway toward aggregate formation.
Implications for lens transparency and cataract
As the most common cause of blindness, cataract has large societal and economic costs. The World Health Organization projects that over 30 million cataract surgeries per year will be needed worldwide by the year 2020. In the United States alone, over 22 million people are affected by cataract, and the direct medical costs of treatment alone exceed $6 billion a year (Frick et al., 2007; Rein et al., 2006; Tielsch et al., 2008).
Accordingly, understanding the molecular origin of crystallin aggregation in cataract disease would be of great benefit. We have highlighted a number of studies that illustrate the distinction between structural and functional stability of βγ-crystallins. Although many advances have been made in the search for the origin of βγ- crystallins’ unusually high structural stability, revealing the nature of its physiological aggregates and the mechanisms of their formation remains a major challenge. A variety of hypotheses have been discussed here, including phase separation, amyloid aggregation, and aggregation from unfolding intermediates. Each of these possibilities offers distinct strategies for cataract prevention. For instance, the amyloid hypothesis emphasizes the role of the C-terminal domain core, so stabilizing the C-terminal domain has been proposed as the therapeutic strategy (Moran et al., 2012a). By contrast, the unfolding intermediate hypothesis appears to offer the opposite indication: increasing unfolding cooperativity by stabilizing the N-terminal domain, destabilizing the C-terminal domain, or increasing the strength of the native domain interface may reduce the population of partially unfolded intermediates. Meanwhile, the phase separation hypothesis has drawn attention to inhibitors of demixing of crystallins in their native state, thus focusing on the proteins’ surface residues. Drug search strategies are directly dependent on understanding the underlying biochemical mechanisms of aggregation.
Cataracts induced by missense mutations, radiation or oxidative damage, exposure to heavy metals, heat, electric shock, specific drugs or metabolites, or other influences may develop following distinct pathways. Thus, it is likely that each of the models discussed above is applicable to some subset of the disease. It is important to continue researching the etiology of cataract in humans in order to correlate specific insults to specific biochemical changes and thus therapeutic interventions. Conversely, further work should continue to address the mechanisms of protection that crystallins have evolved. The mechanisms of α-crystallin chaperone capacity, βγ-crystallin homo- and heteromerization, and the importance of specific residue clusters for protection against ultraviolet light or other factors, continue to be subjects of considerable interest. By understanding the protection offered by these innate mechanisms, as well as their failure, may offer new insights into the causes of lens opacification and intervention strategies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Sampson L, King J. Partially Folded Aggregation Intermediates of Human yD-, yC-, and yS-Crystallin Are Recognized and Bound by Human alpha B-Crystallin Chaperone. Journal of Molecular Biology. 2010;401:134–152. doi: 10.1016/j.jmb.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata O, Pande A, Pande J, Ogun O, Lubsen NH, Benedek GB. Oligomerization and phase transitions in aqueous solutions of native and truncated human beta B1-crystalline. Biochemistry. 2005;44:1316–1328. doi: 10.1021/bi048419f. [DOI] [PubMed] [Google Scholar]
- Aravind P, Suman SK, Mishra A, Sharma Y, Sankaranarayanan R. Three-Dimensional Domain Swapping in Nitrollin, a Single-Domain beta gamma-Crystallin from Nitrosospira multiformis, Controls Protein Conformation and Stability but Not Dimerization. Journal of Molecular Biology. 2009;385:163–177. doi: 10.1016/j.jmb.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Banerjee PR, Pande A, Patrosz J, Thurston GM, Pande J. Cataract-associated mutant E107A of human gamma D-crystallin shows increased attraction to alpha-crystallin and enhanced light scattering. Proc Natl Acad Sci U S A. 2011;108:574–579. doi: 10.1073/pnas.1014653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gamma D crystallin (1.25 angstrom) and the R58H mutant (1.15 angstrom) associated with aculeiform cataract. Journal of Molecular Biology. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- Bax B, Lapatto R, Nalini V, Driessen H, Lindley PF, Mahadevan D, Blundell TL, Slingsby C. X-ray Analysis of beta-B2-Crystallin and Evolution of Oligomeric Lens Proteins. Nature. 1990;347:776–780. doi: 10.1038/347776a0. [DOI] [PubMed] [Google Scholar]
- Bershtein S, Mu WM, Shakhnovich EI. Soluble oligomerization provides a beneficial fitness effect on destabilizing mutations. Proc Natl Acad Sci U S A. 2012;109:4857–4862. doi: 10.1073/pnas.1118157109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershtein S, Segal M, Bekerman R, Tokuriki N, Tawfik DS. Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature. 2006;444:929–932. doi: 10.1038/nature05385. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in Biophysics & Molecular Biology. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Boelens WC. Cell biological roles of αB-crystallin. Progress in Biophysics & Molecular Biology. 2014 doi: 10.1016/j.pbiomolbio.2014.02.005. http://dx.doi.org/10.1016/j.pbiomolbio.2014.02.005. [DOI] [PubMed]
- Chen J, Callis PR, King J. Mechanism of the Very Efficient Quenching of Tryptophan Fluorescence in Human gamma D- and gamma S-Crystallins: The gamma-Crystallin Fold May Have Evolved To Protect Tryptophan Residues from Ultraviolet Photodamage. Biochemistry. 2009;48:3708–3716. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Flaugh SL, Callis PR, King J. Mechanism of the highly efficient quenching of tryptophan fluorescence in human gamma D-crystallin. Biochemistry. 2006;45:11552–11563. doi: 10.1021/bi060988v. [DOI] [PubMed] [Google Scholar]
- Chen J, Toptygin D, Brand L, King J. Mechanism of the efficient tryptophan fluorescence quenching in human gamma D-crystallin studied by time-resolved fluorescence. Biochemistry. 2008;47:10705–10721. doi: 10.1021/bi800499k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Stott K, Perutz MF. Crystal structure of a dimeric chymotrypsin inhibitor 2 mutant containing an inserted glutamine repeat. Proc Natl Acad Sci U S A. 1999;96:1257–1261. doi: 10.1073/pnas.96.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon W, Kelly JW. Partial Denaturation of Transthyretin is Sufficient for Amyloid Fibril Formation in Vitro. Biochemistry. 1992;31:8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- Das P, King JA, Zhou R. beta-strand interactions at the domain interface critical for the stability of human lens gamma D-crystallin. Protein Science. 2010;19:131–140. doi: 10.1002/pro.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, King JA, Zhou R. Aggregation of gamma-crystallins associated with human cataracts via domain swapping at the C-terminal beta-strands. Proc Natl Acad Sci U S A. 2011;108:10514–10519. doi: 10.1073/pnas.1019152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dendunnen JT, Moormann RJM, Lubsen NH, Schoenmakers JGG. Concerted and Divergent Evolution within the Rat gamma-Crystallin Gene Family. Journal of Molecular Biology. 1986;189:37–46. doi: 10.1016/0022-2836(86)90379-7. [DOI] [PubMed] [Google Scholar]
- Evans P, Slingsby C, Wallace BA. Association of partially folded lens beta B2-crystallins with the alpha-crystallin molecular chaperone. Biochemical Journal. 2008;409:691–699. doi: 10.1042/BJ20070993. [DOI] [PubMed] [Google Scholar]
- Fink AL. The aggregation and fibrillation of alpha-synuclein. Accounts Chem Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- Flaugh SL, Kosinski-Collins MS, King J. Contributions of hydrophobic domain interface interactions to the folding and stability of human gamma D-crystallin. Protein Science. 2005a;14:571–581. doi: 10.1110/ps.041111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugh SL, Kosinski-Collins MS, King J. Interdomain side-chain interactions in human gamma D crystallin influencing folding and stability. Protein Science. 2005b;14:2030–2043. doi: 10.1110/ps.051460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gamma D-crystallin and lowers the kinetic barrier to unfolding. Journal of Biological Chemistry. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Archives of Ophthalmology. 2007;125:544–550. doi: 10.1001/archopht.125.4.544. [DOI] [PubMed] [Google Scholar]
- Gilliland KO, Freel CD, Johnsen S, Fowler WC, Costello MJ. Distribution, spherical structure and predicted Mie scattering of multilamellar bodies in human age-related nuclear cataracts. Exp Eye Res. 2004;79:563–576. doi: 10.1016/j.exer.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Graw J. Genetics of crystallins: Cataract and beyond. Exp Eye Res. 2009;88:173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Green SM, Gittis AG, Meeker AK, Lattman EE. One-step Evolution of a Dimer from a Monomeric Protein. Nature Structural Biology. 1995;2:746–751. doi: 10.1038/nsb0995-746. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. Journal of Proteome Research. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Age-Dependent Deamidation of Lifelong Proteins in the Human Lens. Investigative Ophthalmology & Visual Science. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik JF, Wingfield PT, Chambers C, Russell P, Chen HC, Sergeev YV, Hope JN. Association properties of beta B2- and beta A3-crystallin: ability to form dimers. Protein Engineering. 1997;10:1347–1352. doi: 10.1093/protein/10.11.1347. [DOI] [PubMed] [Google Scholar]
- Hejtmancik JF, Wingfield PT, Sergeev YV. beta-Crystallin association. Exp Eye Res. 2004;79:377–383. doi: 10.1016/j.exer.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Hochberg GKA, Benesch JLP. Dynamical structure of αB-crystallin. Progress in Biophysics & Molecular Biology. 2014 doi: 10.1016/j.pbiomolbio.2014.03.003. http://dx.doi.org/10.1016/j.pbiomolbio.2014.03.003. [DOI] [PubMed]
- Horwich A. Protein aggregation in disease: a role for folding intermediates forming specific multimeric interactions. Journal of Clinical Investigation. 2002;110:1221–1232. doi: 10.1172/JCI16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: Visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Ji F, Jung J, Koharudin LMI, Gronenborn AM. The Human W42R gamma D-Crystallin Mutant Structure Provides a Link between Congenital and Age-related Cataracts. Journal of Biological Chemistry. 2013;288:99–109. doi: 10.1074/jbc.M112.416354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, HaasePettingell C, Robinson AS, Speed M, Mitraki A. Thermolabile folding intermediates: Inclusion body precursors and chaperonin substrates. Faseb J. 1996;10:57–66. doi: 10.1096/fasebj.10.1.8566549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knee KM, Sergeeva OA, King JA. Human TRiC complex purified from HeLa cells contains all eight CCT subunits and is active in vitro. Cell Stress Chaperones. 2013;18:137–144. doi: 10.1007/s12192-012-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FR, King J. Contributions of aromatic pairs to the folding and stability of long-lived human gamma D-crystallin. Protein Science. 2011;20:513–528. doi: 10.1002/pro.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski-Collins MS, Flaugh SL, King J. Probing folding and fluorescence quenching in human gamma D crystallin Greek key domains using triple tryptophan mutant proteins. Protein Science. 2004;13:2223–2235. doi: 10.1110/ps.04627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski-Collins MS, King J. In vitro unfolding, refolding, and polymerization of human gamma D crystallin, a protein involved in cataract formation. Protein Science. 2003;12:480–490. doi: 10.1110/ps.0225503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganowsky A, Benesch JLP, Landau M, Ding L, Sawaya MR, Cascio D, Huang Q, Robinson CV, Horwitz J, Eisenberg D. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Science. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Mahler B, Toward J, Jones B, Wyatt K, Dong L, Wistow G, Wu Z. A Single Destabilizing Mutation (F9S) Promotes Concerted Unfolding of an Entire Globular Domain in gamma S-Crystallin. Journal of Molecular Biology. 2010;399:320–330. doi: 10.1016/j.jmb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Asherie N, Lomakin A, Pande J, Ogun O, Benedek GB. Phase separation in aqueous solutions of lens gamma-crystallins: Special role of gamma(s). Proc Natl Acad Sci U S A. 1996;93:377–382. doi: 10.1073/pnas.93.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CW, Lomakin A, Thurston GM, Hayden D, Pande A, Pande J, Ogun O, Asherie N, Benedek GB. Phase-Separation in Multicomponent Aqueous-Protein Solutions. Journal of Physical Chemistry. 1995;99:454–461. [Google Scholar]
- Lomas DA, Parfrey H. alpha(1)-antitrypsin deficiency 4: Molecular pathophysiology. Thorax. 2004;59:529–535. doi: 10.1136/thx.2003.006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler B, Chen Y, Ford J, Thiel C, Wistow G, Wu Z. Structure and Dynamics of the Fish Eye Lens Protein, gamma M7-Crystallin. Biochemistry. 2013;52:3579–3587. doi: 10.1021/bi400151c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler B, Doddapaneni K, Kleckner I, Yuan C, Wistow G, Wu Z. Characterization of a Transient Unfolding Intermediate in a Core Mutant of gamma S-Crystallin. Journal of Molecular Biology. 2011;405:840–850. doi: 10.1016/j.jmb.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr EM, Jaenicke R, Glockshuber R. Domain Interactions and Connecting Peptides in Lens Crystallins. Journal of Molecular Biology. 1994;235:84–88. doi: 10.1016/s0022-2836(05)80017-8. [DOI] [PubMed] [Google Scholar]
- Mayr EM, Jaenicke R, Glockshuber R. The domains in gamma B-crystallin: Identical fold-different stabilities. Journal of Molecular Biology. 1997;269:260–269. doi: 10.1006/jmbi.1997.1033. [DOI] [PubMed] [Google Scholar]
- Metlapally S, Costello MJ, Gilliland KO, Ramamurthy B, Krishna PV, Balasubramanian D, Johnsen S. Analysis of nuclear fiber cell cytoplasmic texture in advanced cataractous lenses from Indian subjects using Debye-Bueche theory. Exp Eye Res. 2008;86:434–444. doi: 10.1016/j.exer.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael R, Bron AJ. The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc B-Biol Sci. 2011;366:1278–1292. doi: 10.1098/rstb.2010.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiel M, Duprat E, Skouri-Panet F, Lampi JA, Tardieu A, Lampi KJ, Finet S. Aggregation of deamidated human beta B2-crystallin and incomplete rescue by alpha-crystallin chaperone. Exp Eye Res. 2010;90:688–698. doi: 10.1016/j.exer.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Henry IA, Flaugh SL, Kosinski-Collins MS, King JA. Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gamma D-crystallin and gamma S-crystallin. Protein Science. 2007;16:2427–2444. doi: 10.1110/ps.072970207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitraki A, King J. Protein Folding Intermediates and Inclusion Body Formation. Bio-Technology. 1989;7:690–697. [Google Scholar]
- Moran SD, Decatur SM, Zanni MT. Structural and Sequence Analysis of the Human gamma D-Crystallin Amyloid Fibril Core Using 2D IR Spectroscopy, Segmental C-13 Labeling, and Mass Spectrometry. Journal of the American Chemical Society. 2012a;134:18410–18416. doi: 10.1021/ja307898g. [DOI] [PubMed] [Google Scholar]
- Moran SD, Woys AM, Buchanan LE, Bixby E, Decatur SM, Zanni MT. Two-dimensional IR spectroscopy and segmental C-13 labeling reveals the domain structure of human gamma D-crystallin amyloid fibrils. Proc Natl Acad Sci U S A. 2012b;109:3329–3334. doi: 10.1073/pnas.1117704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran SD, Zhang TO, Decatur SM, Zanni MT. Amyloid Fiber Formation in Human gamma D-Crystallin Induced by UV-B Photodamage. Biochemistry. 2013;52:6169–6181. doi: 10.1021/bi4008353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King J. Hydrophobic Core Mutations Associated with Cataract Development in Mice Destabilize Human gamma D-Crystallin. Journal of Biological Chemistry. 2009;284:33285–33295. doi: 10.1074/jbc.M109.031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King JA. Cataract-Causing Defect of a Mutant gamma-Crystallin Proceeds through an Aggregation Pathway Which Bypasses Recognition by the alpha-Crystallin Chaperone. Plos One. 2012a;7 doi: 10.1371/journal.pone.0037256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends in Molecular Medicine. 2012b;18:273–282. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme S, Jaenicke R, Slingsby C. X-ray structures of three interface mutants of gamma B-crystallin from bovine eye lens. Protein Science. 1998;7:611–618. doi: 10.1002/pro.5560070310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Gillot D, Pande J. The Cataract-Associated R14C Mutant of Human gamma D-Crystallin Shows a Variety of Intermolecular Disulfide Cross-Links: A Raman Spectroscopic Study. Biochemistry. 2009;48:4937–4945. doi: 10.1021/bi9004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King J, Benedek GB. Crystal cataracts: Human genetic cataract caused by protein crystallization. Proc Natl Acad Sci U S A. 2001;98:6116–6120. doi: 10.1073/pnas.101124798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King JA, Lubsen NH, Walton D, Benedek GB. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc Natl Acad Sci U S A. 2000;97:1993–1998. doi: 10.1073/pnas.040554397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolopoulou K, Mills-Henry I, Tho SL, Wang Y, Gross AAR, Kirschner DA, Decatur SM, King J. Formation of amyloid fibrils in vitro by human gamma D-crystallin and its isolated domains. Molecular Vision. 2008;14:81–89. [PMC free article] [PubMed] [Google Scholar]
- Plotnikova OV, Kondrashov FA, Vlasov PK, Grigorenko AP, Ginter EK, Rogaev EI. Conversion and compensatory evolution of the gamma-crystallin genes and identification of a cataractogenic mutation that reverses the sequence of the human CRYGD gene to an ancestral state. American Journal of Human Genetics. 2007;81:32–43. doi: 10.1086/518616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J. The economic burden of major adult visual disorders in the United States. Archives of Ophthalmology. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Schymkowitz JWH, Wilkinson HR, Itzhaki LS. Three-dimensional domain swapping in p13suc1 occurs in the unfolded state and is controlled by conserved proline residues. Proc Natl Acad Sci U S A. 2001;98:5596–5601. doi: 10.1073/pnas.101542098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F, Wilkinson H, Villanueva J, Serrano L, Schymkowitz JWH, Itzhaki LS. Domain swapping in p13suc1 results in formation of native-like, cytotoxic aggregates. Journal of Molecular Biology. 2006;363:496–505. doi: 10.1016/j.jmb.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Rudolph R, Siebendritt R, Nesslauer G, Sharma AK, Jaenicke R. Folding of a All-Beta-Protein -- Independent Domain Folding in gamma-II-Crystallin from Calf Lens. Proc Natl Acad Sci U S A. 1990;87:4625–4629. doi: 10.1073/pnas.87.12.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Jordan JL, Spatara ML, Naranjo A, Costanzo JA, Weiss WF, Robinson AS, Fernandez EJ, Roberts CJ. Computational Design and Biophysical Characterization of Aggregation-Resistant Point Mutations for gamma D Crystallin Illustrate a Balance of Conformational Stability and Intrinsic Aggregation Propensity. Biochemistry. 2011;50:628–639. doi: 10.1021/bi100978r. [DOI] [PubMed] [Google Scholar]
- Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized beta B2-crystallin mutants to alpha-crystallin - The role of a folding intermediate. Journal of Biological Chemistry. 2004;279:16425–16432. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- Schafheimer N, King J. Tryptophan Cluster Protects Human D-Crystallin from Ultraviolet Radiation-Induced Photoaggregation In Vitro. Photochemistry and Photobiology. 2013;89:1106–1115. doi: 10.1111/php.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafheimer N, Wang Z, Schey KL, King JA. Tyrosine/Cysteine Cluster Sensitizing Human γD-Crystallin to Ultraviolet Radiation-Induced Photo-Aggregation In vitro. Biochemistry. 2014;53:979–990. doi: 10.1021/bi401397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymkowitz JWH, Rousseau F, Wilkinson HR, Friedler A, Itzhaki LS. Observation of signal transduction in three-dimensional domain swapping. Nature Structural Biology. 2001;8:888–892. doi: 10.1038/nsb1001-888. [DOI] [PubMed] [Google Scholar]
- Sergeev YV, Hejtmancik JF, Wingfield PT. Energetics of domain-domain interactions and entropy driven association of beta-crystallins. Biochemistry. 2004;43:415–424. doi: 10.1021/bi034617f. [DOI] [PubMed] [Google Scholar]
- Sergeev YV, Wingfield PT, Hejtmancik JF. Monomer-dimer equilibrium of normal and modified beta A3-crystallins: Experimental determination and molecular modeling. Biochemistry. 2000;39:15799–15806. doi: 10.1021/bi001882h. [DOI] [PubMed] [Google Scholar]
- Sergeeva OA, Chen B, Haase-Pettingell C, Ludtke SJ, Chiu W, King JA. Human CCT4 and CCT5 Chaperonin Subunits Expressed in Escherichia coli Form Biologically Active Homo-oligomers. Journal of Biological Chemistry. 2013;288:17734–17744. doi: 10.1074/jbc.M112.443929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skora L, Becker S, Zweckstetter M. Molten Globule Precursor States Are Conformationally Correlated to Amyloid Fibrils of Human beta-2-Microglobulin. Journal of the American Chemical Society. 2010;132:9223–9225. doi: 10.1021/ja100453e. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Wistow G. Functions of crystallins in and out of lens: Roles in elongated and post-mitotic cells. Progress in Biophysics & Molecular Biology. 2014 doi: 10.1016/j.pbiomolbio.2014.02.006. http://dx.doi.org/10.1016/j.pbiomolbio.2014.02.006. [DOI] [PMC free article] [PubMed]
- Smith MA, Bateman OA, Jaenicke R, Slingsby C. Mutation of interfaces in domain-swapped human beta B2-crystallin. Protein Science. 2007;16:615–625. doi: 10.1110/ps.062659107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed MA, Wang DIC, King J. Specific aggregation of partially folded polypeptide chains: The molecular basis of inclusion body composition. Nat Biotechnol. 1996;14:1283–1287. doi: 10.1038/nbt1096-1283. [DOI] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Brandon TR, Lampi KJ. Deamidation alters the structure and decreases the stability of human lens beta Alpha 3-crystallin. Biochemistry. 2007;46:8861–8871. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens protein, beta A3-crystallin. Protein Science. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Woodbury LG, Lampi KJ. Deamidation alters interactions of beta-crystallins in hetero-oligomers. Molecular Vision. 2009;15:241–249. [PMC free article] [PubMed] [Google Scholar]
- Tielsch JM, Kempen JH, Congdon N, Friedman DS. Vision probelms in the U.S.: Prevalence of adult visual impairment and age-related eye disease in America. Prevent Blindness America. 2008 [Google Scholar]
- Trinkl S, Glockshuber R, Jaenicke R. Dimerization fo beta-B2-Crystallin -- The Role of the Linker Peptide and the N-terminal and C-terminal Extensions. Protein Science. 1994;3:1392–1400. doi: 10.1002/pro.5560030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract - oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wang S, Leng X-Y, Yan Y-B. The Benefits of Being beta-Crystallin Heteromers: beta B1-Crystallin Protects beta A3-Crystallin against Aggregation during Co-refolding. Biochemistry. 2011;50:10451–10461. doi: 10.1021/bi201375p. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lomakin A, McManus JJ, Ogun O, Benedek GB. Phase behavior of mixtures of human lens proteins Gamma D and Beta B1. Proc Natl Acad Sci U S A. 2010a;107:13282–13287. doi: 10.1073/pnas.1008353107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Petty S, Trojanowski A, Knee K, Goulet D, Mukerji I, King J. Formation of Amyloid Fibrils In Vitro from Partially Unfolded Intermediates of Human gamma C-Crystallin. Investigative Ophthalmology & Visual Science. 2010b;51:672–678. doi: 10.1167/iovs.09-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk M, Jaenicke R, Mayr EM. Kinetic stabilisation of a modular protein by domain interactions. Febs Letters. 1998;438:127–130. doi: 10.1016/s0014-5793(98)01287-3. [DOI] [PubMed] [Google Scholar]
- West SK, Duncan DD, Munoz B, Rubin GS, Fried LP, Bandeen-Roche K, Schein OD. Sunlight exposure and risk of lens opacities in a population-based study - The Salisbury Eye Evaluation Project. JAMA-J Am Med Assoc. 1998;280:714–718. doi: 10.1001/jama.280.8.714. [DOI] [PubMed] [Google Scholar]
- Wieligmann K, Mayr EM, Jaenicke R. Folding and self-assembly of the domains of beta B2-crystallin from rat eye lens. Journal of Molecular Biology. 1999;286:989–994. doi: 10.1006/jmbi.1999.2554. [DOI] [PubMed] [Google Scholar]
- Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans R Soc B-Biol Sci. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Yang ZX, Huynh T, King JA, Zhou RH. UV-radiation Induced Disruption of Dry-Cavities in Human gamma D-crystallin Results in Decreased Stability and Faster Unfolding. Sci Rep. 2013;3 doi: 10.1038/srep01560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang S, Zhao W-J, Xi Y-B, Yan Y-B, Yao K. The Congenital Cataract-Linked A2V Mutation Impairs Tetramer Formation and Promotes Aggregation of beta B2-Crystallin. Plos One. 2012;7 doi: 10.1371/journal.pone.0051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Sendall TJ, Pearce MC, Whisstock JC, Huntington JA. Molecular basis of alpha(1)-antitrypsin deficiency revealed by the structure of a domain-swapped trimer. Embo Reports. 2011;12:1011–1017. doi: 10.1038/embor.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xia Z, Tien H, King JA, Zhou R. Dissecting the contributions of beta-hairpin tyrosine pairs to the folding and stability of long-lived human gamma-crystallins. Nanoscale. 2014;6:1797–1807. doi: 10.1039/c3nr03782g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Li J, Zhu Y, Xia Y, Wang W, Yu Y, Yao K. A Nonsense Mutation of γD-crystallin Associated with Congenital Nuclear and Posterior Polar Cataract in a Chinese Family. International Journal of Medical Science. 2014;11:158–163. doi: 10.7150/ijms.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HY, Brown PH, Magone MT, Schuck P. The Molecular Refractive Function of Lens gamma-Crystallins. Journal of Molecular Biology. 2011;411:680–699. doi: 10.1016/j.jmb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HY, Chen YW, Rezabkova L, Wu ZR, Wistow G, Schuck P. Solution properties of gamma-crystallins: Hydration of fish and mammal gamma-crystallins. Protein Science. 2014;23:88–99. doi: 10.1002/pro.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]


