Abstract
This study was conducted to determine Anopheles species composition and their natural infectivity by human Plasmodium in 2 localities with the highest malaria transmission in San Jose del Guaviare, Guaviare, Colombia. A total of 1,009 Anopheles mosquitoes were collected using human landing catches during 8 months in 2010. Anopheles darlingi was the most abundant (83.2%) followed by An. albitarsis s.l. (8.6%), Anopheles braziliensis (3.8%), An. oswaldoi s.l. (1%), and An. rangeli (0.3%). Anopheles darlingi showed the highest human biting rate, and it was found naturally infected with Plasmodium vivax VK210 (0.119%) using enzyme-linked immunosorbent assays. All species were collected biting both indoors and outdoors. Anopheles darlingi showed biting activity overnight with an indoor peak between 1200–0100 h. Therefore, we recommend that malaria prevention strategies focus on 1) insecticide-treated nets to reduce human–vector contact when people are most exposed and unprotected; 2) accurate diagnoses; 3) adequate treatment for patients; 4) more timely epidemiological notification; and 5) improved entomological surveillance.
Keywords: Malaria, Anopheles, biting activity, natural infectivity, Amazonia, Colombia
INTRODUCTION
In Colombia, malaria remains an important public health problem where transmission exhibits unstable, endemic–epidemic patterns with significant variability among different areas. There are 4 malaria transmission areas: the Pacific Coast; the Orinoquia; Amazonian Region; and Urabá, Sinú and Baja Cauca located at North Region (Padilla and Peña 2002, Padilla et al. 2011).
The topography of Guaviare State is lowlands, located in the transition between Orinoquia and Amazonia regions of southeast Colombia (0°32′ to 3°09′N, 69°47′ to 73°47′W), and has an area of 42,327 km2. Guaviare consists of the capital region where the capital city San José del Guaviare is located, and 3 municipalities: El Retorno, Calamar, and Miraflores (SINCHI 2000) (Fig. 1). During the present study in 2010, Guaviare reported the highest average annual malaria parasite index (API) in Colombia, with a rate of 58.8/1,000 inhabitants, representing 39.2% of all malaria cases in Colombia. San José del Guaviare reported autochthonous malaria cases of 17.53 API attributed to Plasmodium vivax (Grassi and Feletti), 7.48 API attributed to P. falciparum (Welsh), and 1.8 cases/1,000 inhabitants attributed to a mixture of P. falciparum/P. vivax (SIVIGILA 2011). In San José del Guaviare municipality, rural and peri-urban malaria transmission is aided by immigration of infected humans from areas with high malaria transmission and enhanced by the proliferation and intensification of the cultivation of illicit crops. Settlement of illegal armed groups and intensive land use contribute to abundant Anopheles breeding sites (Padilla et al. 2011).
Fig. 1.
Map of Colombia, with the Department of Guaviare enlarged showing the municipalities.
The human population is 64.5% urban and 35.4% rural, and includes indigenous communities such as Sikuaní, Guayaberos, Tucano, Desano, Piratapuyo, and Nukak Makú (SINCHI 1999). About 10 years ago, Guaviare State, and specifically San José del Guaviare, was one of the main cocaine trafficking localities in Colombia, with high rates of forced human displacement, which resulted in a permanent floating human population moving among rural, peri-urban, and urban settlements. Because the rural area of San José de Guaviare is affected by armed conflict, samples were collected based on locality accessibility and confirmation of local security.
In general, in Colombia, Anopheles darlingi Root is considered the primary vector (SEM 1957, González and Carrejo 2009, Montoya-Lerma et al. 2011). However, other species complexes, such as the Albitarsis Complex, have been found naturally infected by human Plasmodium in the Orinoquia region in Colombia (Herrera et al. 1987, Jiménez et al. 2012). It is critical to determine if any of these species complexes play a role in local malaria transmission, and can be verified as a local or regional vector (Brochero and Quiñones 2008).
The present study was carried out in the rural area of San José del Guaviare to investigate Anopheles species composition and natural infectivity by human Plasmodium species in 2 localities with high malaria transmission, and to recommend possible prevention strategies, monitoring, and control of malaria.
MATERIALS AND METHODS
Study site
Two localities in the San José del Guaviare Municipality—Agua Bonita (AB) (02°34′43.8″N, 72°37′31.6″W) and El Progreso (EP) (02°32′17.4″N, 72°39′31.3″W)—were selected for the study. San José del Guaviare is a lowland forest ecoregion (Rubio-Palis and Zimmerman 1997) and is located at 180 m above sea level, with a mean annual precipitation of 2,800 mm (rainy season April to November; dry season December to March), mean temperature up to 26.5°C, and relative humidity >80% (IDEAM 2010).
Mosquito collection and species identification
Collections of adult mosquitoes by human-landing catches (HLC) (WHO 1975) indoors and outdoors were carried out from 1800 h to 0600 h for 3 consecutive days per month for 8 months. Collectors rotated indoors/outdoors every 2 h to avoid sampling bias. Several isofamilies were reared from females collected by HLC. These females were allowed to feed on mice in the laboratory and 3 days later were induced to oviposit by cutting off 1 wing and hind leg prior to placing them in water containers (Estrada et al. 2003). Larvae were permitted to hatch from each oviposition and reared individually. Larval and pupal exuviae of link-reared specimens were stored in 75% ethanol prior to slide-mounting, following the protocols in González and Carrejo (2009). Emerged adults were pinned and labeled with the same code as the mother for additional studies (Belkin et al. 1965). The protocol for mosquito feeding on mice was reviewed and approved by the National University of Colombia Veterinary and Animal Care Faculty, and the Institutional National Animal Care and Use Committee of the New York State Department of Health, protocol No. 11-420. Taxonomic identification was conducted using morphological features in González and Carrejo (2009). Molecular taxonomic identification, based on the barcode region (Folmer et al. 1994; Ruiz et al. 2010, 2012), confirmed species in the Albitarsis Complex. Genomic DNA was obtained from the abdomen of each specimen (Zapata et al. 2007, Cienfuegos et al. 2008) using DNAeasy tissue kits (Qiagen, Valencia, CA).
Natural infectivity status
To determine the natural infectivity by P. falciparum and P. vivax (VK210 and VK247), all mosquitoes collected in both localities were pooled (5 mosquitoes/pool) based on time, date, month, and species, and analyzed by enzyme-linked immunosorbent assays (ELISA) (Wirtz et al. 1987a, 1987b, 1991, 1992).
Data analysis
The human-biting rate (HBR) was expressed as the number of bites per person per night (b/p/n) for the total sampling period, which corresponded to 576 h (288 indoor/288 outdoor) (WHO 2008). The sporozoite rate was estimated as a percentage of CS-Plasmodium positive for mosquitoes per species divided by the total number of mosquitoes used by species. Infection rates were estimated using the mean absorbance value of negative controls plus 3 SD, with a fixed cutoff value that allowed for 20% variation in negative control absorbance values (Beier et al. 1988). The annual entomological inoculation rate (EIR) was determined as the sporozoite rate multiplied by the HBR, i.e., the mean number of female anopheline mosquitoes caught per night multiplied by 365 days (Parker et al. 2013).
Ethical considerations
Collection of wild adult mosquitoes by HLC was conducted under an informed consent agreement using a protocol and collection procedures that were reviewed and approved by the Ethics Committee of the Faculty of Medicine of the National University of Colombia and by the Institutional Review Board of the New York State Department of Health, protocol No. 02-028. No additional ethical clearance was necessary.
RESULTS
Species composition
From both localities (AB and EP) a total of 1,009 Anopheles mosquitoes were collected over an 8-month period, based on 288 h for each of the indoor and outdoor samplings. Six Anopheles (Nyssorhynchus) species were identified (Table 1). Anopheles darlingi occurred throughout the year and was the most abundant species (83.2%, n = 840) of the total identified. Anopheles albitarsis (Lynch-Arribálzaga) s.l. accounted for 8.6% (n = 87), followed by An. braziliensis (Chagas) at 3.8% (n = 39), An. oswaldoi (Peryassu) s.l. (n = 10), An. rangeli (Gabaldón, Cova García, and López) (n = 4); the latter 3 species combined accounted for <2% of the total. A subsample (n = 15) of specimens morphologically identified as An. albitarsis s.l. was confirmed as An. albitarsis F (n = 5), using molecular markers (Brochero et al. 2007, Ruiz et al. 2012).
Table 1.
Anopheles species composition in 2 localities of San José del Guaviare–Amazonia, Colombia, 2010.
| Locality | Month | Anopheles collected (n) | Anopheles species |
|---|---|---|---|
| Agua Bonita | February | 2 | An. darlingi |
| 2 | An. albitarsis s.l. | ||
| Anopheles spp.1 | |||
| May | 26 | An. darlingi2 | |
| 6 | An. albitarsis s.l. | ||
| Anopheles spp.1 | |||
| El Progreso | June | 8 | An. darlingi |
| 1 | An. albitarsis s.l. | ||
| Anopheles spp.1 | |||
| August | 560 | An. darlingi | |
| 9 | An. oswaldoi s.l. | ||
| 5 | An. albitarsis s.l. | ||
| 3 | An. rangeli | ||
| 1 | An. braziliensis | ||
| 13 | Anopheles spp.1 | ||
| September | 128 | An. darlingi | |
| 3 | An. albitarsis s.l. | ||
| 1 | An. oswaldoi s.l. | ||
| 3 | Anopheles spp.1 | ||
| October | 112 | An. darlingi | |
| 5 | An. albitarsis s.l. | ||
| 3 | An. braziliensis | ||
| 4 | Anopheles spp.1 | ||
| November | 8 | An. albitarsis s.l. | |
| 2 | An. darlingi | ||
| 4 | Anopheles spp.1 | ||
| December | 57 | An. albitarsis s.l. | |
| 35 | An. braziliensis | ||
| 2 | An. darlingi | ||
| 1 | An. rangeli | ||
| 5 | Anopheles spp.1 |
Anopheles spp. unidentifiable specimens due to loss of taxonomic characters.
One specimen of An. darlingi infected with Plasmodium vivax 210.
Biting activity and human biting rate
All species collected displayed biting activity both indoors and outdoors. Anopheles darlingi had 2 indoor peaks of 1900–2100 h and 0000–0100 h, and the greatest overall HBR (5.2 b/p/n). For this species, the average biting rate was 6 b/p/n with values ranging from 1–20.6 indoors and 1.7–72.6 outdoors. Anopheles albitarsis s.l. was caught mainly outdoors from 1800–1900 h. The HBR was 1.1 b/p/n with an average of 1 b/p/n, ranging from 1–3.2 b/p/n indoors and 1.0–6.3 b/p/n outdoors.
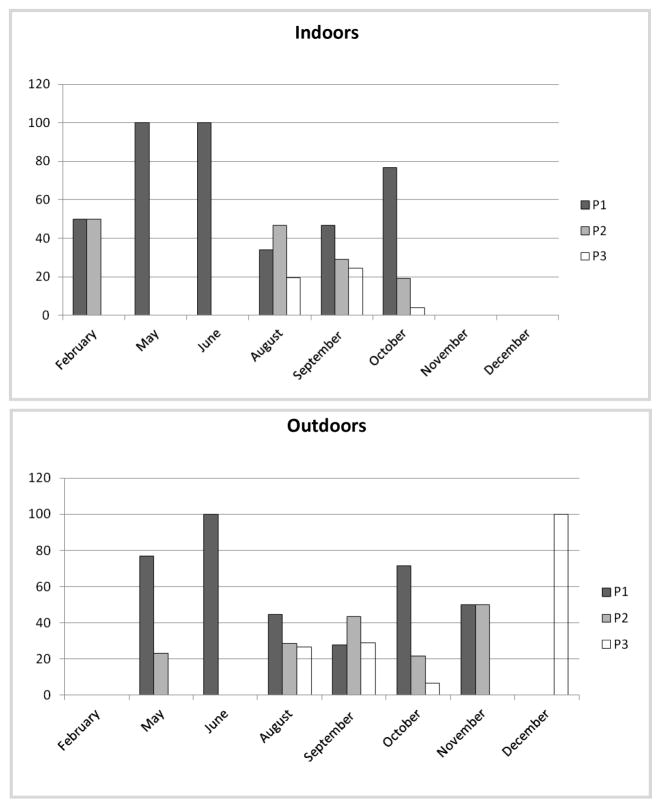
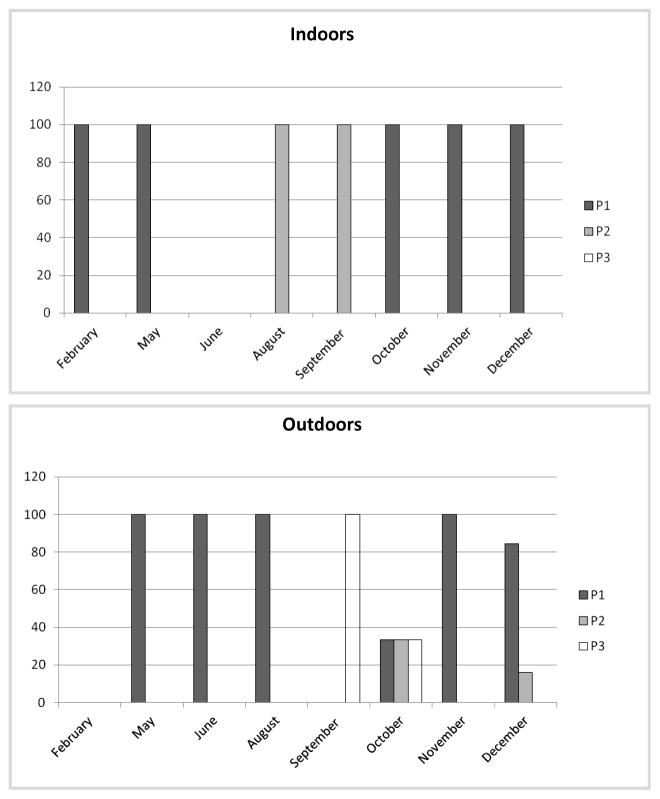
We analyzed the patterns of biting activity of An. darlingi and An. albitarsis s.l. according to human activities when people were likely exposed to biting: the 1st period (P1) between 1800–2200 h, the 2nd period (P2) between 2200–0300 h, and the 3rd period (P3) between 0300–0600 h (Figs. 2 and 3), where each concentric circle represented the month sampled, starting with February as the innermost circle and December as the outermost. For all patterns, the percentage of relative biting contribution per species was expressed as frequency of HBR per species for the total sampled.
Fig. 2.
Variation in percentage contribution of Anopheles darlingi biting activity during 3 periods of the night, 2010.
Fig. 3.
Variation in percentage contribution of Anopheles albitarsis s.l. biting activity during 3 periods of the night, 2010.
Natural infectivity status
Anopheles darling from AB was naturally infected with P. vivax VK 210, representing an infection rate of 0.119% (1/840). The infected specimen was caught indoors between 1200 and 0100 h. No specimens were ELISA-positive for P. falciparum. The annual EIR was 2.6 infective bites/person/year (b/p/y) (6 × 0.00119 × 365).
DISCUSSION
Malaria transmission in San José del Guaviare is mainly caused by P. vivax (API = 17.53 in 2010), and An. darlingi was found naturally infected by P. vivax VK-210. Similarly to other localities in Brazil and the southeastern Amazon, this species exhibited biting activity both indoors and outdoors throughout the night, with a biting peak at sunset (P1), a gradual decrease in biting activity until midnight, and a 2nd peak at 0300 h and 0400 h (P3), consistent with local human activities (Charlwood 1996, Da Silva-Vasconcelos et al. 2002, Voorham 2002). In general, in San José del Guaviare, the risk of contracting malaria is likely highest before 2200 h, when vectors are most active (Voorham 2002, Jiménez et al. 2012, Ahumada et al. 2013). However, in agreement with Charlwood’s (1996) observation that gravid females bite after midnight, in the present study, An. darlingi collected between 1200 and 0100 h was infected by P. vivax. Malaria control in San José del Guaviare should consider multiple strategies; for example, long-lasting insecticidal nets to reduce human–vector contact when people are most exposed and unprotected, and the use of repellants or other alternative controls around sunset, when the HBR is high and the human population is active.
According to the EIR, in San José del Guaviare 1 person could be exposed to 2.6 infective bites for An. darlingi per year, similar to results in the Orinoquia and Amazonian regions in Colombia, as well as in Venezuela and Brazil (Soares et al. 2003, Magris et al. 2007, Girod et al. 2008, Jiménez et al. 2012, Ahumada et al. 2013). In forested areas in Peru, the EIR for An. darling was 0.1 infective b/p/y, whereas in more deforested areas (e.g., grass, croplands), the EIR corresponded to 38 infective b/p/y (Vittor et al. 2006). A new study investigating riverine sites frequented by occupational laborers north of Iquitos, Peru, detected up to 5.3 infective b/p/n for An. darlingi (Parker et al. 2013). Economic activities in San José del Guaviare related to the establishment of fishponds and pastures for livestock generate deforestation in rural areas that is conducive to an increase in Anopheles breeding sites, reduced refuge niches for vectors, and, ultimately promote malaria outbreaks (Padilla et al. 2011).
Historically, entomological studies have reported An. darlingi and An. albitarsis s.l. in sympatry in the Orinoquia and Amazonia regions in Colombia (González and Carrejo 2009, Jiménez et al. 2012, Ruiz et al. 2012). Several members of the Albitarsis Complex have been involved in malaria transmission (Conn et al. 2002, Povoa et al. 2006, Jiménez et al. 2012, Ruiz et al. 2012). In San José del Guaviare, An. albitarsis s.l. was much less abundant than An. darlingi, exhibiting a biting activity both indoors and outdoors, with a peak at sunset (P1) between 1800–2000 h (Fig. 3) and a HBR of 2 b/p/n. None of the Albitarsis Complex specimens, nor the An. albitarsis F identified (n = 5) were infected with Plasmodium and the importance of this species in malaria epidemiology is unknown (Ruiz et al. 2012).
Anopheles braziliensis has been traditionally considered zoophilic (Faran and Linthicum 1981) and it is not often reported naturally infected with Plasmodium (but see Da Silva-Vasconcelos et al. 2002, Martins-Campo et al. 2012). In the present study, An. braziliensis was less abundant but showed anthropophilic behavior and was active outdoors, mainly between 1900–2100 h. Anopheles oswaldoi s.l. and An. rangeli were both collected outdoors between 2000–2200 h (P1). These species were not found infected and, because of low abundance in this study, it was not possible to determine their biting patterns.
Seven species reported for San José del Guaviare previously—An. apicimacula Dyar and Knab, An. forattinni Wilkeron and Sallum/An. costai Fonseca and Ramos, An. mattogrossensis Lutz and Neiva, An. neomaculipalpus Curry, An. pseudopuctipennis Theobald, An. punctimacula Dyar and Knab, and An. triannulatus Neiva and Pinto (González and Carrejo 2009)—were not detected. The reduced diversity of Anopheles species in these 2 sites compared with previous reports may have resulted from 1) differences in the sites and seasonality sampled, 2) discontinuous collections and number of collections, and 3) local changes in ecological conditions and human activities (Conn et al. 2002, Yasuoka and Levins 2007, Dantur et al. 2009).
According to the distribution of malaria cases during 2010 in San José del Guaviare, it was not possible to establish any association between the abundance of adult Anopheles species and monthly incidence of malaria; several studies have shown the infection rate for Colombian Anopheles species to be low, but still adequate to maintain malaria transmission in endemic areas (Lanelli et al. 1998, Tadei et al. 1998, Conn et al. 2002, WHO 2008). Despite the relatively low capture rates, our study incriminated An. darlingi as the most likely principal malaria vector in this municipality.
Vector control measures are conducted by Department of Health Authorities in endemic rural and urban municipalities of Guaviare. Administrative, social, and technical difficulties reduce the effectiveness of malaria programs to control malaria transmission (I. P. Jiménez, unpublished observations). These include: sociocultural characteristics of the indigenous and rural populations that contribute to floating communities moving back and forth from high malaria transmission areas to urban areas; very difficult road access; inadequate housing and limited access to public services and health care; unlimited breeding sites of An. darlingi from flooded rivers during the rainy season (April to November); and environmental degradation from slash-and-burn agriculture and livestock, resulting in drastic changes in the ecosystem.
In conclusion, malaria control strategies in San José del Guaviare should be multifaceted and consider social, environmental, technical, and administrative issues through strengthening of epidemiological, clinical, and entomological surveillance. Some possible prevention and control strategies include: 1) accurate diagnosis and adequate treatment for patients; 2) evaluation of response to treatment to detect drug resistance; 3) surveillance of asymptomatic people arriving from high transmission zones to peri-urban and urban areas; 4) strengthening of epidemiological notification; 5) improved entomological surveillance to determine, monitor, and evaluate peak anopheline biting activities for variation and plasticity of seasonal and temporal change, particularly in relation to ecosystem transformations that can dramatically increase malaria transmission (Vittor et al. 2006, Gil et al. 2007, Moreno et al. 2007).
Acknowledgments
This work was supported by National Institutes of Health, USA grant 2R01AIO54139 Malaria Vector Biology in Brazil: Genetics and Ecology to JEC, Universidad Nacional de Colombia–Bogotá, Faculty of Agronomy Quipu 201010012197 to HB, and Departamento Administrativo de Ciencia, Tecnología e Innovación–COLCIENCIAS Jóvenes Investigadores e Innovadores–“Virginia Gutiérrez de Pineda”–2010 to IPJ. The authors thank Humberto Mosquera for assistance with field collections. We thank Maria Camila Ramirez for assistance with the map (Fig. 1). We thank Laureano Mosquera, Entomological Unit Coordinator, and the technical personnel of the Guaviare Health Department, Colombia, for logistic support in San José del Guaviare.
REFERENCES CITED
- Ahumada M, Pareja P, Buitrago L, Quiñones M. Comportamiento de picadura de Anopheles darlingi Root, 1926 (Diptera: Culicidae) y su asociación con la transmisión de malaria en Villavicencio (Meta, Colombia) [accessed March 16, 2013];Biomédica [Internet] 2013 33:241–250. Available from: http://www.revistabiomedica.org/index.php/biomedica/article/view/1492/1622. [PubMed] [Google Scholar]
- Beier J, Asiago C, Onyango F, Gargan T, Wirtz R, Koech D, Roberts C. ELISA absorbance cutoff method affects malaria sporozoite rate determination in wild Afrotropical Anopheles. Med Vet Entomol. 1988;2:259–264. doi: 10.1111/j.1365-2915.1988.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Belkin JN, Hogue CS, Galindo P, Aitken THG, Schick RX, Powder WA. Mosquito studies (Diptera, Culicidae). II. Methods for the collection, rearing and preservation of mosquitoes. Contrib Am Entomol Inst. 1965;1:19–78. [Google Scholar]
- Brochero HL, Li C, Wilkerson R. A newly recognized species in the Anopheles (Nyssorhynchus) Albitarsis Complex (Diptera: Culicidae) from Puerto Carreño, Colombia. Am J Trop Med Hyg. 2007;76:1113–1117. [PubMed] [Google Scholar]
- Brochero HL, Quiñones ML. Retos de la entomología médica para la vigilancia en salud pública en Colombia: reflexión para el caso de malaria. Biomédica. 2008;28:18–24. [PubMed] [Google Scholar]
- Charlwood J. Biological variation in Anopheles darlingi Root. Mem Inst Oswaldo Cruz. 1996;91:391–398. doi: 10.1590/s0074-02761996000400001. [DOI] [PubMed] [Google Scholar]
- Cienfuegos A, Gómez F, Córdoba L, Luckhart S, Conn J, Correa M. Diseño y evaluación de metodologías basadas en PCR–RFLP de ITS2 para la identificación molecular de mosquitos Anopheles spp. (Diptera: Culicidae) de la Costa Pacífica de Colombia. Biomédica. 2008;19:35–44. [Google Scholar]
- Conn J, Wilkerson R, Segura N, De Souza R, Schlichting C, Wirtz R, Povoa M. Emergence of a new Neotropical malaria vector facilitated by human migration and changes in land use. Am J Trop Med Hyg. 2002;66:18–22. doi: 10.4269/ajtmh.2002.66.18. [DOI] [PubMed] [Google Scholar]
- Da Silva-Vasconcelos A, Kato M, Mourão E, De Souza R, Lacerda R, Sibajev A, Tsouris P, Povoa M, Momen H, Rosa-Freitas M. Biting indices, host-seeking activity and natural infection rates of anopheline species in Boa Vista, Roraima, Brazil from 1996 to 1998. Mem Inst Oswaldo Cruz. 2002;97:151–161. doi: 10.1590/s0074-02762002000200002. [DOI] [PubMed] [Google Scholar]
- Dantur M, Zaidenberg M, Claps G, Santana M, Almiron W. Malaria transmission in two localities in Northwestern Argentina. Malar J. 2009;8:18. doi: 10.1186/1475-2875-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada D, Quiñones M, Sierra D, Calle D, Ruiz F, Erazo H, Linton Y. Utilidad de la morfología de los huevos como método indirecto para identificar An. benarrochi, An oswaldoi, y An. rangeli en Putumayo, Colombia. Biomédica. 2003;23:388–395. [PubMed] [Google Scholar]
- Faran M, Linthicum K. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq Syst. 1981;13:1–81. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- Gil L, Tada M, Katsuragawa T, Ribolla P, Silva L. Urban and suburban malaria in Rondônia (Brazilian Western Amazon) II: perennial transmissions with high anopheline densities are associated with human environmental changes. Mem Inst Oswaldo Cruz. 2007;102:271–276. doi: 10.1590/s0074-02762007005000013. [DOI] [PubMed] [Google Scholar]
- Girod R, Gaborit P, Carinci R, Issaly J, Fouque F. Anopheles darlingi bionomics and transmission of Plasmodium falciparum, Plasmodium vivax and Plasmodium malariae in Amerindian villages of the Upper-Maroni Amazonian forest, French Guiana. Mem Inst Oswaldo Cruz. 2008;103:702–710. doi: 10.1590/s0074-02762008000700013. [DOI] [PubMed] [Google Scholar]
- González R, Carrejo N. Claves y notas de distribución. 2. Cali, Colombia: Universidad del Valle; 2009. Introducción al estudio taxonómico de Anopheles de Colombia. [Google Scholar]
- Herrera S, Suarez M, Sanchez G, Quiñones M, Herrera M. Uso de la técnica inmuno-radiométrica (IRMA) en Anopheles de Colombia para la identificación de esporozoitos de Plasmodium. Colombia Med. 1987;18:2–6. [Google Scholar]
- IDEAM [Instituto de Hidrología, Meteorología y Estudios Ambientales] Colombia: Primera Comunicación Nacional ante la convención Marco de las Naciones Unidas sobre el Cambio Climático. Bogotá, Colombia: Instituto de Hidrología, Meteorología, y Estudios Ambientales; 2010. [Google Scholar]
- Jiménez P, Conn J, Witz R, Brochero H. Anopheles (Díptera: Culicidae) vectores de malaria en el municipio de Puerto Carreño, Vichada, Colombia. Biomédica. 2012;1(Suppl):13–21. doi: 10.1590/S0120-41572012000500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanelli H, Honorio N, Lima D, Loureco-De-Oliveira R, Santos R, Coimbra CE., Jr Faunal composition and behavior of anopheline mosquitoes in the Xavante Indian Reservation of Pimentel Barbosa, central Brazil. Parasite. 1998;5:197–202. doi: 10.1051/parasite/1998052197. [DOI] [PubMed] [Google Scholar]
- Magris M, Rubio-Palis Y, Menares C, Villegas L. Vector bionomics and malaria transmission in the Upper Orinoco River, southern Venezuela. Mem Inst Oswaldo Cruz. 2007;102:303–311. doi: 10.1590/s0074-02762007005000049. [DOI] [PubMed] [Google Scholar]
- Martins-Campo K, Pinheiro W, Vítor-Silva S, Siqueira A, Melo G, Rodrigues I, Fé N, Barbosa M, Tadei W, Guinovart C, Bassat Q, Alonso P, Lacerda M, Monteiro W. Integrated vector management targeting Anopheles darlingi populations decreases malaria incidence in an unstable transmission area, in the rural Brazilian Amazon. Malar J. 2012;11:351. doi: 10.1186/1475-2875-11-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya-Lerma J, Solarte Y, Giraldo-Calderon G, Quiñones M, Ruiz-Lopez F, Wilkerson R, Gozalez R. Malaria vector species in Colombia—a review. Mem Inst Oswaldo Cruz. 2011;106(Suppl 1):223–238. doi: 10.1590/s0074-02762011000900028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J, Rubio-Palis Y, Páez E, Pérez E, Sánchez V. Abundance, biting behavior and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–349. doi: 10.1111/j.1365-2915.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Padilla J, Álvarez G, Montoya R, Chaparro P, Herrera S. Epidemiology and control of malaria in Colombia. Mem Inst Oswaldo Cruz. 2011;106:114–122. doi: 10.1590/s0074-02762011000900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J, Peña S. Situación epidemiológica de la malaria en Colombia. Inf Quinc Epidemiol Nac. 2002;7:333–346. [Google Scholar]
- Parker B, Paredes M, Peñataro P, Escobedo K, Florin D, Rengifo S, Roldan G, Capcha-Vega L, Rodriguez H, Pan W, Banda-Chavez C, Vinetz J, Kosek M. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar J. 2013;12:178. doi: 10.1186/1475-2875-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povoa MM, de Souza RT, Lacerda RN, Rosa ES, Galiza D, de Souza JR, Wirtz RA, Schlichting CD, Conn JE. The importance of Anopheles albitarsis E and An. darlingi in human malaria transmission in Boa Vista, state of Roraima, Brazil. Mem Inst Oswaldo Cruz. 2006;101:163–168. doi: 10.1590/s0074-02762006000200008. [DOI] [PubMed] [Google Scholar]
- Rubio-Palis Y, Zimmerman R. Ecoregional classification of malaria vectors in the Neotropics. J Med Entomol. 1997;34:499–510. doi: 10.1093/jmedent/34.5.499. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Linton Y, Ponsonby D, Conn J, Herrera M, Quinones M, Velez I, Wilkerson R. Molecular comparison of topotypic specimens confirms Anopheles (Nyssorhynchus) dunhami Causey (Diptera: Culicidae) in the Colombian Amazon. Mem Inst Oswaldo Cruz. 2010;105:899–903. doi: 10.1590/s0074-02762010000700010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F, Wilkerson R, Conn J, McKeon S, Levin D, Quinones M, Povoa M, Linton Y. DNA barcoding reveals both known and novel taxa in the Albitarsis Group (Anopheles: Nyssorhynchus) of Neotropical malaria vectors. Parasites Vectors. 2012;21:5–44. doi: 10.1186/1756-3305-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEM [Servicio de Erradicacion de la Malaria] Plan para la erradicación de la malaria en Colombia, República de Colombia. II. Bogotá, Colombia: Ministerio de Salud Pública y Oficina Sanitaria Panamericana, Oficina Regional de la Organización Mundial de la Salud; 1957. [Google Scholar]
- SINCHI [Instituto Amazónico de Investigaciones Científicas] Guaviare población y territorio. Bogotá, Colombia: Ministerio del Medio Ambiente; 1999. [Google Scholar]
- SINCHI [Instituto Amazónico de Investigaciones Científicas] Guaviare: Proyecto Investigación Científica en Ecosistemas y Recursos Naturales de la Amazonia Colombiana [Internet] Bogotá, Colombia: Instituto Amazónico de Investigaciones Científicas; 2000. [accessed June 17, 2013]. Available from: http://www.sinchi.org.co. [Google Scholar]
- SIVIGILA [Sistema de Vigilancia en Salud Pública] Instituto Nacional de Salud—Boletín Epide-miológico Semanal. Semana epidemiológica No. 52, Subdirección de Vigilancia y Control en Salud Pública [Internet]; December 26, 2010–January 1, 2011; Bogotá, Colombia: Sistema de Vigilancia en Salud Pública; 2011. [accessed June 17, 2013]. Available from: http://www.ins.gov.co/boletin-epidemiologico. [Google Scholar]
- Soares Gil L, Alves F, Zieler H, Salcedo M, Durlacher R, Cunha R. Seasonal malaria transmission and variation of anopheline density in two distinct endemic areas in Brazilian Amazonia. J Med Entomol. 2003;40:636–641. doi: 10.1603/0022-2585-40.5.636. [DOI] [PubMed] [Google Scholar]
- Tadei W, Thatcher B, Santos J, Scarpassa V, Rodrigues I, Rafael M. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Vittor A, Gilman R, Tielsch J, Glass G, Shields T, Lozano W, Pinedo-Cancino V, Patz J. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. [PubMed] [Google Scholar]
- Voorham J. Intra-population plasticity of Anopheles darlingi (Diptera: Culicidae) biting activity patterns in the state of Amapa, Brazil. Rev Saude Publica. 2002;36:75–80. doi: 10.1590/s0034-89102002000100012. [DOI] [PubMed] [Google Scholar]
- WHO [World Health Organization] Manual on practical entomology, parts I and II. Geneva, Switzerland: World Health Organization; 1975. [Google Scholar]
- WHO [World Health Organization] World malaria report. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- Wirtz R, Burkot T, Graves P, Andre R. Field evaluation of enzymelinked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987a;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- Wirtz R, Charoenvit Y, Burkot T, Esser K, Beaudoin R, Collins W, Andre R. Evaluation of monoclonal antibodies against Plasmodium vivax sporozoites for ELISA development. Med Vet Entomol. 1991;5:17–22. doi: 10.1111/j.1365-2915.1991.tb00515.x. [DOI] [PubMed] [Google Scholar]
- Wirtz R, Sattabongkot J, Hall T, Burkot T, Rosenberg R. Development and evaluation of an enzyme-linked immunosorbent assay for Plasmodium vivax-VK247 sporozoites. J Med Entomol. 1992;29:854–857. doi: 10.1093/jmedent/29.5.854. [DOI] [PubMed] [Google Scholar]
- Wirtz R, Zavala F, Charoenvit Y, Campbell G, Burkot T, Scheneider I, Esser K, Beaudoin R, Andre R. Comparative testing of Plasmodium falciparum sporozoite monoclonal antibodies for ELISA development. Bull WHO. 1987b;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–460. [PubMed] [Google Scholar]
- Zapata M, Cienfuegos A, Quiros O, Quinones M, Luckhart S, Correa M. Discrimination of seven Anopheles species from San Pedro de Uraba, Anti-oquia, Colombia, by polymerase chain reaction-restriction fragment length polymorphism analysis of ITS sequences. Am J Trop Med Hyg. 2007;77:67–72. [PubMed] [Google Scholar]