Abstract
Dorsal root ganglion neurons (DRGs) are sensory neurons that facilitate somatosensation and have been used to study neurite outgrowth, regeneration, and degeneration and PNS and CNS myelination. Studies of DRGs have relied on cell isolation strategies that generally involve extended culture in the presence of antimitotic agents or other cytotoxic treatments that target dividing cells. The surviving cells typically are dependent on serum for growth. Other methods, involving purification of DRGs based on their large size, produce low yield. In contrast, the immunopanning-based method described here for prospective isolation of DRGs from rodents allows for rapid purification in the absence of antimitotic agents and serum. These DRG cultures take place in a defined medium. They are free of Schwann cells and other glia and thus can be used to study the role of glia in the biology of DRG neurons.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPES: Please see the end of this article for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
CD9 antibody, mouse anti-rat (BD Biosciences Pharmingen 551808)
-
Bovine serum albumin (BSA) (4%)
To prepare a stock of 4% BSA in Dulbecco’s phosphate-buffered saline (D-PBS), dissolve 8 g of BSA (Sigma-Aldrich A4161) in 150 mL of D-PBS (Thermo Scientific HyClone SH3026401) at 37°C. Adjust the pH to 7.4 with ~ 1 mL of 1 N NaOH. Bring the volume to 200 mL. Filter through a 0.22-μm filter. Store in 1-mL aliquots at −20°C. BSL-1 (Griffonia simplicifolia lectin; Vector Labs L-1100) (5 mg/mL)
Collagen I, rat tail (BD Biosciences Pharmingen 354236) (optional; see Step 1)
-
DNase (0.4%)
To prepare a 0.4% stock of DNase in Earle’s balanced salt solution (EBSS), add 1 mL of EBSS (Ca-, Mg-free; Sigma-Aldrich E6267) per 12,500 units of DNase (Worthington LS002007). Keep on ice. Filter-sterilize, and store in 200-μL aliquots at −20°C. -
Dulbecco’s phosphate-buffered saline (D-PBS) with calcium and magnesium (e.g., Thermo Scientific HyClone SH3026401)
We add phenol red to the D-PBS to help ensure that all solutions are at neutral pH before using. Ethanol (70%)
-
#x02022; Fetal calf serum (FCS)
Make 50-mL aliquots of 100% FCS (Gibco/Life Technologies 10437-028) and heat-inactivate for 30 min at 55°C.Store the aliquots at −20°C. 5-Fluoro-2′-deoxyuridine (FUDR) (Sigma-Aldrich F0503) (for long-term culture; see Step 36)
Goat anti-mouse IgG + IgM (H + L) (Jackson ImmunoResearch 115-005-044)
-
Growth factors
Forskolin stock (4.2 mg/mL) <R>
Mouse nerve growth factor (NGF) (1 mg/mL stock; AbD Serotec PMP04Z)
BDNF stock (50 μg/mL) (optional; see Step 33) <R>
NT-3 stock (1 μg/mL) (optional; see Step 33) <R>
High-ovomucoid (high-ovo) stock solution (6×) <R>
Insulin (0.5 mg/mL) <R>
Laminin, mouse (1 mg/mL; Cultrex 3400-010-01)
L-Cysteine hydrochloride monohydrate (Sigma-Aldrich C7880)
Low-ovomucoid (low-ovo) stock solution (10×) <R>
-
Media
Neurobasal medium, filtered (Gibco 21103-049)
DRG base medium <R>
-
L15 dissection medium (Life Technologies 11415-064)
Combine 450 mL of Leibovitz’s L15 medium with 50 mL of FCS. Filter-sterilize through a 0.22-μm filter and store at 4°C.
NaOH (1 M)
Papain (Worthington LS003126)
Phosphate-buffered saline (PBS) (e.g., Diamedix 1000-3)
Poly-D-lysine (PDL) stock (1 mg/mL) <R>
Rat, pregnant with E15 embryos, obtained on the second day of procedure
Tris-HCl (50 mM, pH 9.5)
Trypan blue (Life Technologies 15250-061)
Equipment
Aspirator
Bunsen burner
CO2 chamber and tank for euthanizing rats
Conical tubes (15 and 50 mL)
Coverslips, glass, ethanol-washed
Dissection scissors, curved (e.g., ROBOZ RS-5675)
Forceps (#5, #55)
Hemacytometer
Hood, sterile for tissue culture work
Incubator at 37°C, 10% CO2
Micropipettor (1 mL)
Microscopes, dissecting and phase contrast
-
Nitex mesh filter (20 μm) (Small Parts B0015H4H1A)
Cut the mesh into 4-inch squares and autoclave. Pasteur pipettes
Petri dishes (6, 10, and 15 cm; Falcon or Nunc)
Pipettes, serological (2 and 10 mL)
Pipettor, powered (e.g., Pipet-Aid)
Razor blade, sterile
Syringe filter (0.22 μm)
Tabletop centrifuge (with 15 mL/50 mL centrifuge tube adaptors) at room temperature
Tissue culture plates, plastic (e.g., 24 well, Falcon, or NUNC)
Water bath, preset to 34°C
METHOD
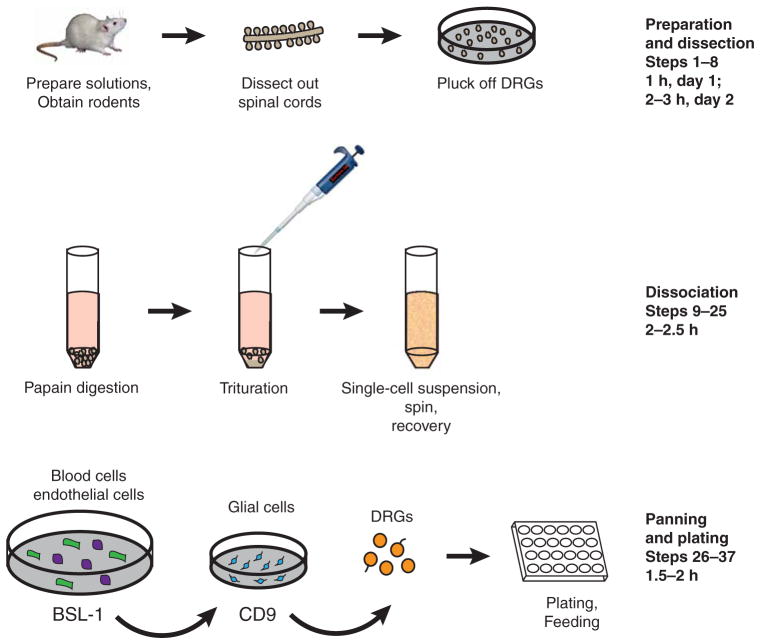
This method begins with dissection of the rat embryos and creation of a single-cell suspension of DRGs. These cells then undergo several immunopanning steps before they are plated (see Fig. 1 for an overview). The entire protocol is a 2-d procedure; Steps 1and 2 are performed on the first day. Day 1 procedures (Steps 1 and 2) require ~ 1 h, plus a 4 h–overnight incubation for laminin coating. Day 2 procedures take 6–8 h. Perform all steps in a sterile tissue culture hood, with the exception of the dissection. All solutions in contact with cells must be sterile.
FIGURE 1.
Immunopanning of DRGs by depleting glia with CD9 antibody.
Preparation of Coverslips, Solutions, and Panning Dishes
-
1
Coat coverslips with PDL and laminin.
Rinse ethanol-washed glass coverslips three times with sterile H2O and dry completely (can be done in a Petri dish).
Dilute PDL stock 1:100 in sterile H2O.
Cover each coverslip with 100 μL of diluted PDL and incubate for 30 min at room temperature.
Rinse coverslips three times with sterile H2O. During the third rinse, transfer the coverslips to tissue culture plates (e.g., 24-well plates).
Aspirate the H2O and allow the coverslips to dry completely.
Dilute the 1 mg/mL laminin 1:500 in filtered neurobasal medium.
-
Cover each coverslip with 100 μL of diluted laminin and incubate for 4 h to 1 d at 37°C. If drops of laminin are disturbed and spill off the coverslips, add more laminin to cover.
Alternatively, to plate dense spots of DRGs (see Fig. 2 and Step 34), coat coverslips with a mixture of collagen and laminin as follows: After coating with poly-D-lysine and drying as in Steps 1.i–1.v, cover with laminin diluted 1:500 in 1:3 rat collagen:neurobasal medium. Incubate for 4–6 h at 37°C, and then aspirate the gelled collagen/laminin mixture thoroughly. Air-dry with the lids off in a sterile hood for at least 24 h, up to 4 d. Complete drying is essential for plating droplets to confine the DRG cell bodies to a small area.
-
2
Prepare panning dishes and incubate them overnight at 4°C.
BSL1 dish: To a 15-cm Petri dish, add 20 mL PBS + 20 μL of 5 mg/mL BSL-1.
-
CD9 dish: To a 10-cm Petri dish, add 10 mL of 50 mM Tris-HCl (pH 9.5) + 30 μL of goat-anti-mouse IgG + IgM (H + L).
Plates are initially hydrophobic, but after coating overnight, they become visibly hydrophilic. If plates are needed immediately, a quick but less preferable way to make them is to coat with secondary antibodies for 2 h at 37°C.For preparing DRGs from two litters, prepare the CD9 dish by coating a 15-cm Petri dish with 20 mL of 50 mM Tris-HCl (pH 9.5) and 60 μL of goat-anti-mouse secondary antibody. The 15-cm BSL1 dish can be used for one or two litters.
-
3
Prepare solutions and tools.
Pour 10–20 mL of L15 dissection medium into a 15-cm dish and a 10-cm dish, and leave at room temperature for dissections.
Pour ~ 3–4 mL of D-PBS into a 6-cm dish for dissections.
In a 15-mL conical tube labelled “papain,” combine 5 mL of D-PBS + 3 μL of 1 M NaOH, and warm in a 34°C water bath.
Make 40 mL of 0.2% BSA by combining 38 mL of D-PBS + 2 mL of 4% BSA.
Warm and equilibrate DRG base medium in a 37°C, 10% CO2 incubator, loosely capped to allow gas exchange.
Sterilize the dissection area and tools by spraying with 70% ethanol.
-
4
Finish preparing the panning dishes by washing each dish from Step 2 three times with PBS and then incubating with primary antibody or blocking solution for ≥ 2 h at room temperature. Do not allow the coated dishes to dry.
BSL-1 dish: 9 mL of 0.2% BSA from Step 3.iv.
-
CD9 dish: 5 mL of 0.2% BSA + 15 μL of anti-rat CD9.
For preparing DRGs from two litters, coat a 15-cm CD9 dish (from Step 2) with 10 mL of 0.2% BSA + 30 μL of anti-rat CD9.
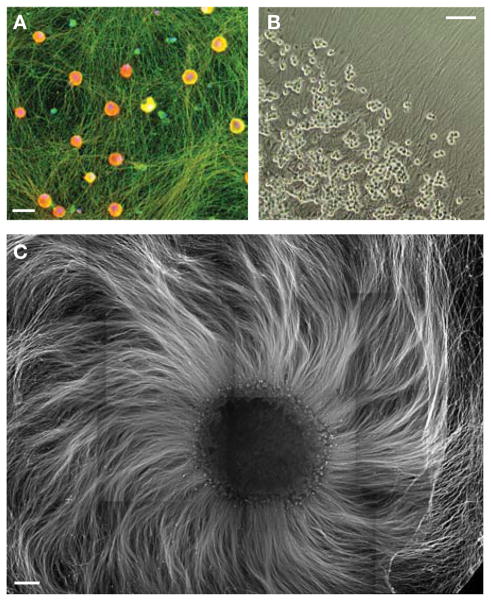
FIGURE 2.
Example images of immunopanned and cultured DRG neurons. (A) DRGs plated in dispersed culture for 12 d, fixed and stained for axonal marker Tuj1 (green), pan-sodium channel (red), and nuclei (DAPI, blue). Scale bar, 20 μm. (B) DRG spots grown for 4 d, imaged in phase. Scale bar, 100 μm. (C) 40,000 DRGs plated in a dense spot were grown for 13 d, and fixed and stained for neurofilament to show the dense bed of axons. Scale bar, 500 μm.
Dissection
-
5
Euthanize a timed pregnant rat (E15) with CO2 according to appropriate animal protocol.
-
6
Dissect out the embryos.
Spray the abdomen with 70% ethanol, lift the skin above the stomach with forceps, and use a sterile razor to cut open the skin and muscle, taking care not to cut internal organs.
Dissect out the placenta, containing the embryos, and pull into a 15-cm dish containing L15 dissection medium (prepared in Step 3).
Using forceps and curved dissection scissors, carefully cut the placenta and enclosed amni-otic sacs to pop out the embryos.
Decapitate the embryos carefully without damaging their spinal cords.
Transfer the embryos to a 10-cm dish containing L15 dissection medium (prepared in Step 3).
-
7
Using the dissecting microscope, dissect out the spinal cords, one embryo at a time.
Cut off the tail.
Cut off the ventral side (the belly) including the limbs; be careful to not cut too close to the spinal cord. Discard the ventral tissue.
Use forceps to flip the embryo so that the ventral side faces up, and gently remove tissue, including the digestive track and blood vessels, until the rib cage is completely visible.
Flip the embryo so that the dorsal side is facing up, with rostral side facing right.
Using forceps in your left hand, brace the embryo on either side of the spinal cord near the rostral side. Using forceps in your right hand, gently peel the skin above the spinal cord away from the bracing forceps.
Move the bracing forceps (in your left hand) a few millimeters caudal, and repeat the gentle peeling and tearing of skin with the other forceps to begin to reveal the spinal cord and attached DRGs. Repeat until all of the dorsal skin has been removed from above the spinal cord.
Flip the spinal cord 90° around its axis and use forceps in both hands to dissociate the spinal cord from the remaining ventral tissue (rib cage, etc.). Be careful not to strip the DRGs off of the spinal cord. This should result in a fully detached spinal cord with DRGs attached to its entire length. Set these aside in the dish until all the spinal cords are dissected.
-
8
Transfer spinal cords to a 6-cm Petri dish with D-PBS (prepared in Step 3), and again using the dissecting microscope, collect the DRGs.
Working down the length of each spinal cord, grasp the DRGs at their bases and pull off.
Discard the stripped spinal cords.
-
Bring the dish with the DRGs into the tissue culture hood and use a Pasteur pipette to transfer the DRGs into a 15-mL conical tube.
Swirling the dish to collect the DRGs in the center aids in collecting. Do not aspirate the DRGs past the flare of the Pasteur pipette, or it will be difficult to get them out.
Dissociation of DRGs
-
9
Allow the DRGs to settle, then aspirate the D-PBS. Wash the DRGs by adding 10 mL of D-PBS.
-
10
Allow the DRGs to resettle while you prepare the papain/DNase solution.
Add 50 units of papain to the “papain” tube from Step 3.
Swirl the tube and return it to the 34°C water bath to dissolve the papain.
Measure 0.002 g of L-cysteine, add it to the papain tube, and bring the tube to the sterile hood.
Sterilize the papain solution by filtering it through a 0.22-μm filter into a new 15-mL conical tube.
-
Add 50 μL of 0.4% DNase to the tube and invert to mix.
For DRGs from two litters, increase the papain to 100 U and the DNase to 100 μL in 10 mL of D-PBS.
-
11
Without disturbing the settled DRGs, aspirate the D-PBS, and replace it with the papain/DNase solution.
-
12
Swirl the conical tube, incubate for 30 min in a 34°C water bath. Swirl the tube again halfway through the incubation to mix thoroughly.
-
13
During the incubation, make the following solutions:
Low-ovo inhibitor solution: 9 mL D-PBS + 1 mL 10× low-ovo stock solution + 100 μL of 0.4% DNase
High-ovo inhibitor solution: 5 mL D-PBS + 1 mL 6× high-ovo stock solution + ~ 3 μL of 1 M NaOH (to bring the solution to neutral pH)
Panning buffer: 18 mL D-BPS + 2 mL of 0.2% BSA + 200 μL of 0.5 mg/mL insulin
-
14
After the digestion in Step 12, wash the DRGs carefully.
Bring the tube to the sterile hood and wait ~ 1 min for the tissue to settle.
Aspirate the excess liquid.
Gently add 4 mL of low-ovo inhibitor solution.
Wait for the tissue to settle; aspirate and discard the excess liquid.
-
15
Triturate the cells.
Add 2 mL of low-ovo inhibitor solution to the DRGs.
-
Use a 1-mL micropipettor to triturate the cells up and down eight to 10 times, very slowly and gently.
Be careful not to introduce bubbles, and to minimize the introduction of CO2, avoid lifting the tip of the pipettor out of the solution. The low-ovo inhibitor solution will become cloudy. Allow the tissue chunks to settle for 1–2 min.
Transfer the single cells (the cloudy solution on top of the chunks) to a new 50-mL conical tube. Avoid transferring any undissociated tissue.
Add 1 mL of low-ovo inhibitor solution and repeat the trituration steps until all the chunks of tissue are gone. Each round of trituration can be gradually more forceful.
-
Use the rest of the low-ovo inhibitor solution to wash the remaining dissociated cells out of the tube and transfer to a 50-mL collection tube.
The goals of trituration are to (1) obtain isolated single cells and (2) subject those cells to as little manipulation as possible. It is essential to let the tissue chunks settle completely before collecting the single-cell suspension, but collect as much of this suspension as possible without disturbing the chunks (to avoid subjecting the single cells to more rounds of trituration). When first using this procedure, poor cell viability is common; with practice, however, improvements in viability should be seen. We typically see ≥ 95% viability at this step, with ~ 5–10 × 106 cells total.
-
16
Count the cells and assay viability by trypan blue exclusion.
Combine 20 μL of cells with 80 μL of panning buffer or D-PBS.
-
Dilute this mixture 1:2 with trypan blue and count the cells using a hemacytometer.
Expect ~ 5–10 × 106cells per 10 to 12 embryos. This step can be done during the centrifugation in Step 19.
-
17
Use a 2-mL pipette to remove any chunks that have settled at the bottom of the 50-mL collection tube.
-
18
Carefully use a 10-mL pipette to slowly layer 6 mL of high-ovo inhibitor solution under the single-cell suspension.
This step should lead to a clear layer of liquid beneath a cloudy cell suspension. -
19
Pellet the cells through the high-ovo inhibitor solution by centrifuging for 10 min at 220g in a tabletop centrifuge at room temperature.
We use a clinical centrifuge with ramp-up and ramp-down speeds set to moderate/low to prevent damage to cells. During the centrifugation, dissociated cells will descend through the high-ovo inhibitor solution, ensuring that complete inhibition of the papain occurs. -
20
During the centrifugation in Step 19, make a cone from a Nitex filter.
Use sterilized forceps (sprayed with ethanol and flamed with a Bunsen burner) to put a Nitex filter on top of a 50-mL conical tube.
Use a 1-mL micropipettor tip to depress the center of the filter into a cone (with the bottom of the cone inside the conical tube) while wetting with 1–2 mL of panning buffer.
-
21
After the centrifugation in Step 19, aspirate and discard the liquid, being careful to not disturb the pellet of cells.
-
22
Resuspend the cell pellet.
Add 2 mL of panning buffer and gently pipette the solution and cells up and down with a 1-mL micropipettor.
Add 6 mL of panning buffer and mix gently.
-
23
Filter the solution, 1 mL at a time, through the Nitex mesh cone to separate the single cells from any remaining clumps of cells and chunks of tissue.
-
24
Wash the filter with 3 mL of panning buffer.
-
25
Incubate the tube in a 37°C, 10% CO2 incubator for 30 min. Loosely cap the tube to allow gas exchange.
This step allows antigens to return to the cell surface before immunopanning.
Panning
-
26
Rinse the BSL-1 panning dish three times with D-PBS, and pour off the final rinse.
-
27
Decant the cell suspension into the BSL-1 dish. Rinse the tube with 2–3 mL of panning buffer and add that buffer to the dish. Incubate the dish for 20 min at room temperature, gently shake, and then incubate for another 10 min (20 min total).
It is important that the cells be allowed to settle onto the panning dish during this time. Periodic shaking ensures that all cells have access to the surface of the dish. -
28
Rinse the CD9 dish nine times with D-PBS, and pour off the final rinse.
Rinse the CD9 plate thoroughly, because CD9 antibody stock contains sodium azide. -
29
Transfer the unbound cells from the BSL-1 dish to the CD9 dish.
Shake the BSL-1 dish and decant it into the CD9 dish.
Prop the BSL-1 dish up at an angle and use a 1-mL micropipettor to transfer the ~ 1 mL of remaining cell suspension to the CD9 plate.
Incubate the CD9 plate for 15 min at room temperature, gently shake, and then incubate for another 15 min (30 min total).
-
30
Gently shake the CD9 plate, and decant the suspension of unbound cells into a 50-mL conical tube. Prop the dish up at an angle, and use a 1-mL micropipettor to transfer the ~ 1 mL of remaining cell suspension into the tube.
-
31
Count the cells and assay viability by trypan blue exclusion.
Combine 20 μL of cells with 80 μL of panning buffer or D-PBS.
-
Dilute this mixture 1:2 with trypan blue, and count the cells using a hemacytometer.
Expect ~ 1–2 × 106 cells per 10 to 12 embryos. This step can be done during the centrifugation in Step 32. We typically see ≥ 95% viability at this step. DRG neurons are easily distinguished by their large cell bodies and occasional axon stumps if dissociation was performed gently. We typically count only the DRG neurons and achieve >90% purity.
Plating
-
32
Pellet the cells by centrifuging at 220g in a tabletop centrifuge for 10 min at room temperature.
-
33
Add the following growth factors to the warmed DRG base medium from Step 3:
NGF at a 1:10,000 dilution (to 100 ng/mL)
Forskolin at a 1:1000 dilution (to 10 μM)
(Optional) BDNF at a 1:1000 dilution (to 50 ng/mL)
-
(Optional) NT-3 at a 1:1000 dilution (to 1 ng/mL)
The presence of NGF immediately after the isolation procedure is critical for survival of DRGs, but it can be omitted after that time if other neurotrophins are present. For maximum survival, we typically grow DRGs in the presence of NGF, BDNF, and NT-3. If you wish to avoid growing DRGs in the presence of NGF (e.g., to establish myelinating cocultures with oligodendrocytes), replace the medium the next day with DRG base medium plus BDNF, NT-3, and forskolin (no NGF).
-
34
Aspirate the supernatant, resuspend the cell pellet in DRG base medium + growth factors, and plate the cells in a 24-well plate on the coverslips prepared in Step 1.
To plate dispersed DRGs, resuspend the cells at 25,000–50,000 per 500 μL. Add the DRGs to the wells, and allow the plate to sit in the hood at room temperature for 5–10 min so the cells can settle and attach to the coverslips. Then gently transfer the plates to a 37°C, 10% CO2 incubator.
To plate the DRGs in dense spots on dried collagen/laminin (see Fig. 2C), resuspend the cells at 20,000 DRGs per μL of medium. Plate 2–2.5 μL of cells in the center of dried coverslips, and allow the cells to settle for 20 min in the 37°C, 10% CO2 incubator. The DRGs must not be allowed to dry out during this time. To prevent drying when plating in a 24-well plate, add 5–10 mL of D-PBS to the spaces between the wells, and carefully add 20–30 μL of medium around the edges of the coverslips. After 20 min, slowly add 500 μL of medium to the wells without flooding them (to avoid washing off the DRGs). To do so, pipette the medium in a circular motion around the outside of the well, allowing it to slowly close in around the central drop of DRGs.
Feeding and Culturing DRGs
-
35
Feed DRGs by replacing half of their medium with fresh DRG base medium containing growth factors. To account for evaporation, remove 225 μL of medium from each well and replace it with 250 μL of fresh medium. Feed every 2–3 d.
-
36
For long-term culture, for the first feeding after the prep include 10 μM (final concentration) 5-fluoro-2′-deoxyuridine (FUDR) antimitotic in the medium to kill any contaminating dividing cells.
Prepare the FUDR at 20 μM in DRG medium with growth factors and feed half volume.
The FUDR does not need to be washed out of the wells after use. Subsequent feedings do not normally require FUDR.
-
37
Monitor DRG axon growth carefully. DRGs will continue to extend axons for several weeks, although good coverage of the surface is typically achieved after 4–7 d of growth.
RELATED INFORMATION
For background information on this protocol, see Purification and Culture of Dorsal Root Ganglion Neurons (Zuchero 2014).
RECIPES
BDNF Stock (50 μg/mL)
Prepare a master BDNF stock (1 mg/mL) by resuspending 1 mg of human brain-derived neurotrophic factor in powder form (BDNF; Peprotech 450-02) in 1 mL of cold, sterile 0.2% BSA (Sigma-Aldrich A-4161) that was prepared in D-PBS (Gibco 14287). Make 200-μL aliquots of the master stock, flash-freeze in liquid nitrogen, and store at −80°C.
To make a working BDNF stock, thaw a 200-μL aliquot of master stock on ice. At the same time, chill 3.8 mL of sterile 0.2% BSA solution on ice. Once chilled, add the master BDNF stock to the 0.2% BSA solution and mix well, but gently, to avoid foaming. Make 20-μL working aliquots and flash-freeze in liquid nitrogen. Store at −80°C.
DRG Base Medium
| Reagent | Volume | Final concentration |
|---|---|---|
| DMEM (Gibco 11960-044) | 37 mL | 50% |
| Neurobasal (Gibco 21103-049) | 37 mL | 50% |
| Insulin (0.5 mg/mL) <R> | 800 μL | 5 μg/mL |
| Sodium pyruvate (Gibco 11360-070) | 800 μL | 1 mM |
| Penicillin/streptomycin (100×; Gibco 15140-122) | 800 μL | 1× |
| L-Glutamine (Gibco 25030-081) | 800 μL | 1 mM |
| SATO supplement, NB-based (100×) <R> | 800 μL | 1× |
| T3 stock (4 μg/mL) <R> | 800 μL | 40 ng/mL |
| NS21a (50×) <R> | 1.6 mL | 1× |
| NAC stock (5 mg/mL) <R> | 80 μL | 5 μg/mL |
| Final volume | 80 mL |
Filter-sterilize the medium through a 0.22-μm filter and store at 4°C for up to 1 wk. Add growth factors (NGF, BDNF, NT-3, and forskolin) immediately before use.
Alternatively, use N21-MAX Media Supplement (R&D Systems AR008) or B-27 (Invitrogen 17504-044). If using NS21 or B27 that contains T3, it is unnecessary to add T3 to DRG Base Medium.
Forskolin Stock (4.2 mg/mL)
To prepare, add 1 mL of sterile DMSO to a 50-mg bottle of forskolin (Sigma-Aldrich F6886) and pipette up and down until the powder is fully resuspended. Transfer to a 15-mL conical tube and add an additional 11 mL of DMSO to achieve a final concentration of 4.2 mg/mL. Store in 20- and 80-μL aliquots at −20°C.
High-Ovomucoid Stock Solution (6×)
BSA (Sigma-Aldrich A8806)
DPBS (Thermo Scientific HyClone SH3026401)
NaOH (1 N)
Trypsin inhibitor (Worthington LS003086)
Add 6 g of BSA to 150 mL D-PBS.
Add 6 g of trypsin inhibitor and mix to dissolve.
Add at least 1.5 mL of 1 N NaOH to adjust the pH; continue adding NaOH as necessary to bring up the pH to 7.4.
Bring the volume to 200 mL with D-PBS.
Filter-sterilize through a 0.22-μm filter.
Make 1.0-mL aliquots and store at −20°C.
Insulin (0.5 mg/mL)
Working under a sterile tissue culture hood, prerinse a 0.22-μm filter with sterile H2O. Discard the flowthrough.
Add 50 mg of insulin (either recombinant human insulin [Sigma-Aldrich I2643] or bovine pancreas insulin [Sigma-Aldrich I6634]) to 100 mL of sterile H2O in a 200-mL beaker. Immediately add 500 μL of 1 N HCl to adjust the pH.
Stir the mixture with a sterile pipette to dissolve the insulin completely; avoid creating bubbles.
Filter the solution through the pre-rinsed 0.22-μm filter.
Make 5-mL aliquots and store at 4°C for up to 4–6 wk. For use in media, dilute 100× to a concentration of 5 μg/mL.
Low-Ovomucoid Stock Solution (10×)
To prepare, add 3 g of BSA (Sigma-Aldrich A8806) to 150 mL D-PBS. Mix well. Add 3 g of trypsin inhibitor (Worthington LS003086) and mix to dissolve. Add ~ 1 mL of 1 N NaOH to adjust the pH to 7.4. Bring the volume to 200 mL with D-PBS. Filter-sterilize through a 0.22-μm filter. Make 1.0-mL aliquots and store at −20°C.
NAC Stock (5 mg/mL)
To prepare, dissolve 50 mg of N-acetyl-L-cysteine (NAC) powder (Sigma-Aldrich A8199) in 10 mL of Neurobasal Medium (Gibco/Life Technologies 21103). (The solution will be yellowish.) Filter through a 0.22-μm filter. Prepare 20- and 80-μL aliquots and store them at −20°C.
NS21 (50×) (Chen et al. 2008)
Add 12.5 g of BSA (Sigma-Aldrich A4161) to 100 mL of Neurobasal medium (Gibco 21103-049). Stir at room temperature for ~ 2 h to dissolve the BSA.
In a cold room at 4°C or in a beaker on ice, add each of the reagents in the table in the order that they are listed. Continue stirring the solution during the additions. Add all liquid stocks dropwise, and add all powders directly to the Neurobasal medium.
Aliquot the solution into 400-μL or 800-μL volumes and store at −30°C.
-
Dilute the NS21 stocks 1/50 in growth medium.
Sigma-Aldrich catalog no. Stock (mg/mL) Final in 1× NS2L (μg/mL) Amount stock add to 100 mL neurobasal Notes on stock solution Aqueous stocks (store at 4°C) L-Carnitine C7518 2.0 2.0 5 mL Resuspend all 10 mg in 5 mL dH20, add all of this Ethanolamine E0508 1.0 1.0 5 mL Add 5 μL to 5 mL dH20, add all of this D+-Galactose G0625 15 15 5 mL Weigh 75 mg and add to 5 mL dH20, add all of this Putrescine P5780 16.1 16.1 5 mL Weigh 80 mg and add to 5 mL dH20, add all of this Sodium selenite S9133 0.01435 0.01435 5 mL Resuspend all 1 mg in 70 mL dH20, add 5 mL of this Ethanolic stocks (store under nitrogen at −70°C to minimize oxidation) Corticosterone C2505 2.0 0.02 50 μL Linoleic acid L1012 100 10 50 μL Linolenic acid L2376 100 1.0 50 μL Lipoic acid (thioctic acid) 4.7 0.047 50 μL Progesterone 0.63 0.0063 50 μL Retinol acetate R7882 10 0.1 50 μL Retinal, all trans (vit. A) 10 0.1 50 μL D,L-α-tocopherol (vit. E) B5240 100 1.0 50 μL D,L-α-tocopherol acetate T3001 100 1.0 50 μL Add fresh Albumin, bovine A4161 100 2500 12.5 g Weigh out 12.5 g and add directly to neurobasal, first step in the process Catalase C40 16 2.5 80 mg Weigh out 80 mg and add directly Glutathione (reduced) G6013 1.0 1.0 5 mg Weigh out 5 mg and add directly Insulin I6634 4.0 4.0 5 mL Weigh out 20 mg, dissolve in 5 mL of 0.1% acetic acid, add all of this Superoxidase dismutase S5395 2.5 2.5 12.5 mg Calculate 12.5 mg from weight on three bottles and add directly (mix of new and old lots) Transferrin T5391 2.0 5.0 10 mg Add all 10 mg directly (rinse bottle with the B27 mix) T3 (triiodol-L-thyronine) T6397 0.002 0.002 5 μL Weigh out 10 mg, dissolve in 5 mL of 0.1 M NaOH, add all of thisa aWe typically omit T3 from the recipe to enable making media lacking T3 for some cell types. If T3 is omitted, it will need to be added to DRG Base Medium separately.
NT-3 Stock (1 μg/mL)
Prepare a master stock of neurotrophin-3 (NT-3; Peprotech 450-03) at 1–0.1 mg/mL according to manufacturer’s instructions. (Instructions may vary from lot to lot; generally, NT-3 is dissolved in buffer [e.g., Dulbecco’s phosphate-buffered saline] plus 0.2% BSA.) Store at −80°C.
Prepare 0.2% BSA (Sigma-Aldrich A4161) in Dulbecco’s phosphate-buffered saline (D-PBS; HyClone SH30264.01). Filter-sterilize and chill.
Dilute an aliquot of NT-3 master stock to 1 μg/mL in sterile, chilled 0.2% BSA prepared in Step 2.
Aliquot the NT-3 working stock from Step 3 (e.g., 20 μL/tube) and snap-freeze in liquid nitrogen.
Store aliquots at −80°C.
Poly-D-Lysine (PDL) Stock (1 mg/mL)
Resuspend poly-D-lysine (PDL; Sigma-Aldrich P6407; molecular weight 70–150 kDa) at 1 mg/mL in borate buffer by combining 50 mg of PDL and 50 mL of 0.15 M boric acid (pH 8.4).
Filter to sterilize, and then aliquot (e.g., 100 μL/tube). Store aliquots at −20°C.
SATO Supplement, NB-Based (100×)
-
Prepare the following stock solutions (these should be made fresh; do not reuse):
Combine 2.5 mg of progesterone (Sigma-Aldrich P8783) and 100 μL of ethanol to make a progesterone stock solution.
Combine 4.0 mg of sodium selenite (Sigma-Aldrich S5261), 10 μL of 1 N NaOH, and 10 mL of Neurobasal (NB, Gibco 21103-049) to make a sodium selenite stock solution.
-
Add the following to 80 mL of Neurobasal medium:
Reagent Quantity Final concentration in medium (1×) BSA (Sigma-Aldrich A4161) 800 mg 100 μg/mL Transferrin (Sigma-Aldrich T1147) 800 mg 100 μg/mL Putrescine dihydrochloride (Sigma-Aldrich P5780) 128 mg 16 μg /mL Progesterone stock solution 20 μL 60 ng/mL (0.2 μM) Sodium selenite stock solution 800 μL 40 ng/mL Mix well, and filter-sterilize through a prerinsed 0.22-μm filter. Make 200-μL or 800-μL aliquots, and store at −20°C.
T3 Stock (4 μg/mL)
To prepare, dissolve 3.2 mg of 3,3′,5-triiodo-L-thyronine sodium salt (T3; Sigma-Aldrich T6397) in 400 μL of 0.1 N NaOH. Add 10 μL to 20 mL of Dulbecco’s phosphate-buffered saline (D-PBS; HyClone SH30264.01). Filter through a 0.22-μm filter, discarding the first 10 mL. Make 200-μL aliquots, and store at −20°C.
Acknowledgments
We thank Jonah Chan, Ye Zhang, Amanda Brosius Lutz, Jack Wang, and Arun Thottumkara for helpful discussions and advice and Adiljan Ibrahim for excellent technical assistance. J.B.Z. is a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation. This protocol was developed in Ben Barres’ laboratory. We thank the Myelin Repair Foundation and the National Institutes of Health (NIH) for –grant R01 EY10257 for support.
References
- Chen Y, Stevens B, Chang J, Milbrandt J, Barres BA, Hell JW. NS21: Re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB. Purification and culture of dorsal root ganglion neurons. Cold Spring Harb Protoc. 2014 doi: 10.1101/pdb.top073965. [DOI] [PMC free article] [PubMed] [Google Scholar]