Despite its public health impact, there are relatively few classes of drugs in use for the treatment of heart failure (HF) and left ventricular dysfunction.1 HF pharmacology is based upon relatively few signal transduction pathways – most prominently the sympathetic nervous system and the renin angiotensin aldosterone system.1 As such the quest for additional therapeutic targets remains critically important, and in this regard oxidative stress/nitroso-redox imbalance is a potential target of long standing interest.2
Several lines of evidence support that this pathway may be of pathophysiologic relevance. First, serum uric acid (SUA) is a biomarker of oxidative stress in several cardiovascular diseases including heart failure.3–5 This elevation of SUA is primarily due to the increased amounts of available xanthine and hypoxanthine after cellular damage, which is then catalyzed into uric acid via xanthine oxidase (XO).6 XO uses oxygen as a potential electron acceptor, thus forming reactive oxygen species (ROS) resulting in oxidative stress.6
Several observational studies and meta-analysis have identified elevations of SUA as an independent marker of poor cardiac function, mortality, poor functional capacity as well as the development of atrial arrhythmias in heart failure.7–10 Thus an active hypothesis is that SUA may not only represent a prognostic biomarker of heart failure but may also represent a potential target for intervention.
A second line of evidence emerges from experimental studies exploring the role of XO in heart failure, showing first and foremost an upregulation of this enzyme in the cardiovascular system.6 Furthermore, preclinical animal data supported the use of XO inhibitors in heart failure showing greater survival, improved left ventricular function, enhanced mechanoenergetic coupling, attenuation of ventricular remodeling, decrease in myocardial oxygen consumption, reduced afterload and improved ventricular vascular coupling.11,12 In humans, intracoronary and intravenous allopurinol improved myocardial efficiency and increased the concentration of high-energy phosphates within the heart.3,13 Therefore, XO inhibitors in animals and humans improve cardiac function enhancing mechanoenergetic coupling while reducing myocardial oxygen consumption and improving afterload. An important insight however is that the enhancement of mechanoenergetic coupling depends on the degree XO overexpression in heart failure animal models.6
A third line of evidence is supported by nested case-control and retrospective cohort studies showing a decrease in heart failure readmissions as well as all-cause mortality in patients with gout who receive allopurinol.14,15
Together these findings have prompted a series of clinical trials examining XO inhibition in patients with HF. In this issue of Circulation, Givertz and colleagues16 report the results of the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) trial, a double blind, multicenter randomized trial that compared guideline adherent therapy plus allopurinol to guideline adherent therapy alone in a high risk HF population with elevated SUA. In this study XO inhibition with allopurinol did not improve functional capacity, clinical status or left ventricular ejection fraction. Other randomized studies have reached similar conclusions and are summarized in Table 1.17–21 The randomized studies of XO inhibition in HF consistently fail to show improvement in clinical composite outcomes. It is important to note however that two studies, including the EXACT-HF trial, do show trends toward improvement of secondary outcomes like hospitalizations and ejection fraction.16,21 The results seem to be independent of the severity of the HF, patients enrolled, the use of active metabolites of XO inhibitors and dosages to decrease uric acid, as well as the use of different clinical composite outcomes. Another potential caveat from the randomized trials is that long-term effects of these medications remain unknown since the trials had relatively short-term follow-up.
Table 1.
Comparison of randomized studies using xanthine oxidase inhibition in heart failure.
| Author | Heart failure Population |
Xanthine oxidase inhibitor |
Follow- up in weeks |
Primary Outcome definition |
Primary outcome result |
|---|---|---|---|---|---|
| Givertz et al. 2015 | 253 with SUA >9.5 mg/dl with one more high risk marker | Allopurinol 300–600 mg/day | 24 | Clinical status: Outcomes, medication change and patient global assessment. | 13% improved in both allopurinol and placebo arms. |
| Greig et al. 2011 | 32 NYHA II–III | Allopurinol 300 mg/day | 4 | 6-minute walk test and oxidative stress markers | No difference in 6-minute walk test and improved oxidative markers |
| Nasr et al. 2010 | 59 NYHA III–IV | Allopurinol 300 mg/day | 36 | Composite endpoint: Global cardiac function and mortality/morbidity | Allopurinol did not improve composite endpoint |
| Hare et al. 2008 | 405 with a median SUA of 7.8 mg/dl and NYHA III–IV | Oxypurinol 600 mg/day | 24 | Clinical status: Outcomes, medication change, patient global assessment or NYHA | 43% improved in the oxypurinol arm compared to 45% in the placebo arm. Improved primary outcome in patients with higher uric acid levels |
| Cingolani et al. 2006 | 60 NYHA II–III | Oxypurinol 600 mg/day | 4 | Ejection fraction | 4.7+/− 2.6 % higher EF between oxypurinol and placebo arms |
| Gavin et al. 2005 | 50 NYHA II–III | Allopurinol 300 mg/day | 12 | Exercise stress test and 6 minute walk test | No difference in exercise performance with a decrease in plasma BNP. |
SUA: Serum uric acid, NYHA: New York Heart Association, EF: ejection fraction, BNP: Brain natriuretic peptide
The study by Givertz is based in part on the Oxypurinol Therapy for Congestive Heart Failure (OPT-CHF) trial results which compared xanthine oxidase inhibitors to guideline therapy. In post-hoc analysis of this study oxypurinol showed a potential benefit in HF patients with elevated SUA and this benefit correlated to the degree of SUA reduction. This study contributed to the rationale for the present study, which employed elevated SUA as an enrollment criteria in order to select for a group of patients with elevated XO.
Potential epidemiological explanations for the negative findings reported by Givertz16 and others include the possibility that SUA might be just a marker of disease severity and prognosis and not a target for therapy. Also, a combination of sample size, low event rates and short follow-up time could have limited the ability to detect a real long-term effect shown as a trend towards lower hospitalizations in the allopurinol group reported in this study. Another potential but unlikely explanation could lie in the use of oral medications subject to first pass effect metabolism of the liver since the available experimental results in humans used parental allopurinol.3,13 This is an unlikely theory because there was a significant decrease in SUA.
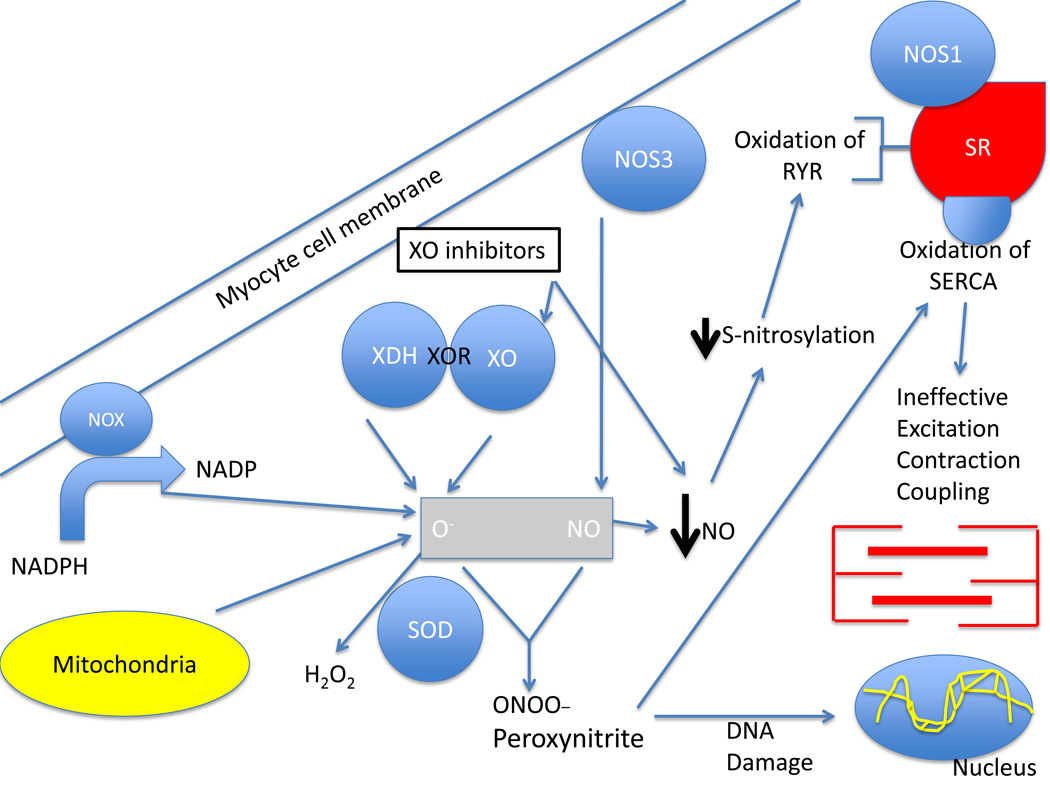
The non-significant findings of EXACT-HF and other studies prompt an examination of the pathophysiological mechanisms that are the basis for this novel therapeutic strategy. A leading possibility explaining the lack of response to XO inhibition could be the fact that this pharmacologic strategy only addressed one of two limbs underlying nitroso-redox imbalance (Figure 1). In HF, not only are ROS generating pathways upregulated but important aspects of reactive nitrogen species (RNS) production are downregulated.2
Figure 1.
Effect of XO inhibition on the nitroso-redox balance. The underlying mechanistic basis for the use of xanthine oxidase inhibitors in the failing heart. As depicted xanthine oxidase inhibitors (XOI) act on one key enzyme – XO/XDH – to inhibit ROS production but has other actions that might detract from full restoration of nitroso-redox balance. XOI decrease serum uric acid and superoxide production by inhibiting xanthine oxidase. Importantly, however, there are other sources of ROS production in the failing heart, including mitochonrdrial respiration and NADPH oxidases, that are not affected by XOI. In addition, NOS activity may be impaired in heart failure, or further disrupted by XOI. In states of inadequate NO production, oxidation or diminished S-nitrosylation of the RYR receptor and other key proteins involved in excitation-contraction coupling impairs calcium cycling which drives optimal myocardial performance. Persistent ROS production also consumes NO and leads to peroxynitrite formation which can cause DNA, protein and lipid damage. Peroxynitrite oxidizes the calcium ATPase SERCA (responsible for calcium reuptake into the SR). Thus, NO continues to be depleted by XO inhibition perpetuating nitroso-redox imbalance and causing ineffective excitation-contraction coupling. Abbreviations: XOR: xanthine oxidoreductase, XO: xanthine oxidase, XDH: xanthine dehydrogenase, O−: Superoxide, NO: nitric oxide, NADPH: nicotine adenine dinucleotide phosphate, NOX: NADPH oxidases, 2NOS: nitric oxide synthase, SOD: superoxide dismutase, SR: sarcoplasmic reticulum, SERCA: sarcoplasmic reticulum calcium ATPase, RyR: ryanodine receptor.
Three important factors related to the nitroso-redox balance might have bearing on the findings by Givertz and colleagues.16 First, other enzymes and metabolic pathways contribute to nitroso-redox imbalance, including other enzymes that produce ROS (NADPH oxidase enzymes and the respiratory chain in the mitochondria), superoxide dismutase that neutralizes superoxide, and the family of nitric oxide synthases (NOSs) which produces nitric oxide (NO). Selective XO inhibition might be inadequate to curtail the cascade of ROS accumulated in HF and, very importantly, this is supported by the present study because myeloperoxidase levels did not change. Second, we now know that the nitroso-redox balance is intimately interconnected. This is supported by a series of experiments that found that NO binds superoxide to produce peroxynitrite, NO modulates the expression of XO, NOS inhibitors abolish the contractile effect of XO inhibitors and XO inhibition can actually decrease NO production.6,22 NOS1 deficient animal models have proven an increase in mortality, left ventricular remodeling, and ventricular arrhythmias after myocardial infarction.23,24 Thus, inhibition of XO in the failing circulation may fail to have beneficial effects if NOS activity and/or signaling is also depressed; XO inhibition may affect only one limb of the nitroso-redox imbalance.
Thus, the results of EXACT-HF add another important data point in the quest to unravel whether aspects of nitroso-redox imbalance have potential as a therapeutic target. The trial suggests that XO inhibition even in high SUA heart failure patients alone is inadequate to improve clinical outcomes. As this field progresses it will be crucial to examine other limbs of this balance and to ask whether augmenting NO production concomitantly with inhibition ROS production will have clinical benefits in HF.
Acknowledgments
Funding Sources: JMH is supported by NIH grants R01 HL110737, R01 HL107110, R01HL084275, 5UMHL113460 and grants from the Starr Foundation and Soffer Family Foundation.
Footnotes
Disclosures: JMH disclose a relationship with Vestion that includes equity, board membership, and consulting. LT has no disclosures.
References
- 1.Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. doi: 10.1016/S0140-6736(14)61889-4. [DOI] [PubMed] [Google Scholar]
- 2.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 3.Cappola TP, Kass DA, Nelson GS, Berger RD, Rosas GO, Kobeissi ZA, Marban E, Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 4.Tsukimori K, Yoshitomi T, Morokuma S, Fukushima K, Wake N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens. 2008;21:1343–1346. doi: 10.1038/ajh.2008.289. [DOI] [PubMed] [Google Scholar]
- 5.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ. Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation. 2003;107:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 6.Zimmet JM, Hare JM. Nitroso-redox interactions in the cardiovascular system. Circulation. 2006;114:1531–1544. doi: 10.1161/CIRCULATIONAHA.105.605519. [DOI] [PubMed] [Google Scholar]
- 7.Tamariz L, Harzand A, Palacio A, Verma S, Jones J, Hare J. Uric acid as a predictor of all-cause mortality in heart failure: a meta-analysis. Congest Heart Fail. 2011;17:25–30. doi: 10.1111/j.1751-7133.2011.00200.x. [DOI] [PubMed] [Google Scholar]
- 8.Tamariz L, Agarwal S, Soliman EZ, Chamberlain AM, Prineas R, Folsom AR, Ambrose M, Alonso A. Association of serum uric acid with incident atrial fibrillation (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;108:1272–1276. doi: 10.1016/j.amjcard.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamariz L, Hernandez F, Bush A, Palacio A, Hare JM. Association between serum uric acid and atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm. 2014;11:1102–1108. doi: 10.1016/j.hrthm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Jankowska EA, Ponikowska B, Majda J, Zymlinski R, Trzaska M, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Ponikowski P. Hyperuricaemia predicts poor outcome in patients with mild to moderate chronic heart failure. Int J Cardiol. 2007;115:151–155. doi: 10.1016/j.ijcard.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Naumova AV, Chacko VP, Ouwerkerk R, Stull L, Marban E, Weiss RG. Xanthine oxidase inhibitors improve energetics and function after infarction in failing mouse hearts. Am J Physiol Heart Circ Physiol. 2006;290:H837–H843. doi: 10.1152/ajpheart.00831.2005. [DOI] [PubMed] [Google Scholar]
- 12.Stull LB, Leppo MK, Szweda L, Gao WD, Marban E. Chronic treatment with allopurinol boosts survival and cardiac contractility in murine postischemic cardiomyopathy. Circ Res. 2004;95:1005–1011. doi: 10.1161/01.RES.0000148635.73331.c5. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. J Am Coll Cardiol. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508–2516. doi: 10.1161/CIRCULATIONAHA.106.651117. [DOI] [PubMed] [Google Scholar]
- 15.Gotsman I, Keren A, Lotan C, Zwas DR. Changes in uric acid levels and allopurinol use in chronic heart failure: association with improved survival. J Card Fail. 2012;18:694–701. doi: 10.1016/j.cardfail.2012.06.528. [DOI] [PubMed] [Google Scholar]
- 16.Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, Tamg W, Dunlap ME, LeWinter MM, Mann D, Felker M, O'Connor C, Goldsmith SR, Ofili EO, Saltzberg MT, Marguiles KB, Cappola TP, Konstam MA, Semigran MJ, McNulty SE, Lee KL, Shah MR, Hernandez AF. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the EXACT-HF study. Circulation. 2015;131:XX–XXX. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare JM, Mangal B, Brown J, Fisher C, Jr, Freudenberger R, Colucci WS, Mann DL, Liu P, Givertz MM, Schwarz RP. OPT-CHF Investigators. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol. 2008;51:2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 18.Gavin AD, Struthers AD. Allopurinol reduces B-type natriuretic peptide concentrations and haemoglobin but does not alter exercise capacity in chronic heart failure. Heart. 2005;91:749–753. doi: 10.1136/hrt.2004.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greig D, Alcaino H, Castro PF, Garcia L, Verdejo HE, Navarro M, Lopez R, Mellado R, Tapia F, Gabrielli LA, Nogerol C, Chiong M, Godoy I, Lavandero S. Xanthine-oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant. 2011;30:408–413. doi: 10.1016/j.healun.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nasr G, Maurice C. Allopurinol and global left myocardial function in heart failure patients. J Cardiovasc Dis Res. 2010;1:191–195. doi: 10.4103/0975-3583.74262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cingolani HE, Plastino JA, Escudero EM, Mangal B, Brown J, Perez NG. The effect of xanthine oxidase inhibition upon ejection fraction in heart failure patients: La Plata Study. J Card Fail. 2006;12:491–498. doi: 10.1016/j.cardfail.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction: evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry. 2003;42:1150–1159. doi: 10.1021/bi026385a. [DOI] [PubMed] [Google Scholar]
- 23.Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- 24.Burger DE, Lu X, Lei M, Xiang FL, Hammoud L, Jiang M, Wang H, Jones DL, Sims SM, Feng Q. Neuronal nitric oxide synthase protects against myocardial infarction-induced ventricular arrhythmia and mortality in mice. Circulation. 2009;120:1345–1354. doi: 10.1161/CIRCULATIONAHA.108.846402. [DOI] [PubMed] [Google Scholar]