Abstract
Background. Histoplasmosis causes severe disease in patients with defects of cell-mediated immunity. It is not known whether outcomes vary related to the type of immunodeficiency or class of antifungal treatment.
Methods. We reviewed cases of active histoplasmosis that occurred at Vanderbilt University Medical Center from July 1999 to June 2012 in patients with human immunodeficiency virus (HIV) infection, a history of transplantation, or tumor necrosis factor (TNF)-α inhibitor use. These groups were compared for differences in clinical presentation and outcomes. In addition, outcomes were related to the initial choice of treatment.
Results. Ninety cases were identified (56 HIV, 23 transplant, 11 TNF-α inhibitor). Tumor necrosis factor-α patients had milder disease, shorter courses of therapy, and fewer relapses than HIV patients. Histoplasma antigenuria was highly prevalent in all groups (HIV 88%, transplant 95%, TNF-α 91%). Organ transplant recipients received amphotericin B formulation as initial therapy less often than other groups (22% vs 57% HIV vs 55% TNF-α; P = .006). Treatment failures only occurred in patients with severe disease. The failure rate was similar whether patients received initial amphotericin or triazole therapy. Ninety-day histoplasmosis-related mortality was 9% for all groups and did not vary significantly with choice of initial treatment.
Conclusions. Histoplasmosis caused milder disease in patients receiving TNF-α inhibitors than patients with HIV or solid organ transplantation. Treatment failures and mortality only occurred in patients with severe disease and did not vary based on type of immunosuppression or choice of initial therapy.
Keywords: histoplasmosis, HIV, TNF-α inhibitors, transplant
Patients with defects of cell-mediated immunity are more vulnerable than immunocompetent patients to infection with Histoplasma capsulatum and more frequently develop disseminated disease and die from the infection [1–10]. We performed a retrospective, 13-year review at Vanderbilt University Medical Center of patients who had human immunodeficiency virus (HIV) infection, had undergone transplantation, or received tumor necrosis factor (TNF)-α inhibitor therapy and were also diagnosed with histoplasmosis. The aim of our review was to determine whether there were differences in clinical presentation, laboratory values, or outcomes related to the type of underlying immunodeficiency. In addition, we wanted to evaluate whether the initial choice of treatment had any impact on outcome.
PATIENTS AND METHODS
All patients diagnosed with active histoplasmosis at Vanderbilt University Medical Center between July 1, 1999 and June 30, 2012 were identified. Criteria for diagnosis were positive urine or serum Histoplasma antigens, positive cultures, or histologic evidence of histoplasmosis together with clinical signs and symptoms compatible with active infection [7]. Patients were only included in the study if they had HIV infection, a history of solid organ or stem cell transplantation, or had received TNF-α inhibitor therapy and were 18 years or older.
Patient records were reviewed for demographic information, presenting signs and symptoms, baseline laboratory data, and radiographic findings. Other data collected were Histoplasma serologies, urine and serum Histoplasma antigen levels (Miravista, Indianapolis, IN; or ARUP Laboratories, Salt Lake City, UT), fungal cultures, relevant pathological studies, CD4 counts and HIV viral loads, and type and duration of antifungal therapy. In transplant patients, we recorded the time since transplantation, type of transplant, history of recent (<6 months) rejection treatment, and immunosuppressive therapy. In recipients of TNF-α inhibitors, the underlying diagnosis and other immunosuppressive therapies were documented. Severe illness was defined as initial intensive care unit (ICU) care; moderate illness was defined as initial hospital care outside the ICU; and mild illness was defined as initial care in the clinic [11]. Outcomes of the study included overall and histoplasmosis-related mortality, relapse, and treatment failure. Treatment failure was defined as either death from histoplasmosis, or in the case of individual antifungal agents, the attending clinician′s decision to switch from one agent to another based on lack of appropriate clinical response. Relapse was defined as re-emergence of signs and symptoms compatible with active histoplasmosis and/or an increase in urine or serum antigen levels that had previously been down-trending, either during continuing therapy or after discontinuation of antifungal therapy.
Data Analysis
Information was entered into a computer database, and statistics were performed using Stata software, version 12 (Statacorp, College Station, TX). Proportions were compared using Fisher's exact test, and continuous variables were compared using the Mann-Whitney U test. To evaluate mortality, a time-to event analysis was used, with follow-up beginning from the day of presentation for medical care of the histoplasmosis. Survival curves were constructed using the Kaplan-Meier method and compared using log-rank statistics. A 2-sided P value of < .05 was considered significant. Differences were compared between HIV patients and transplant patients, as well as HIV patients and TNF-α patients. Due to small numbers, we did not compare differences between transplant patients and TNF-α patients.
RESULTS
During the 13-year study period, 90 immunosuppressed patients met our case definition. Of these, 56 (62%) had HIV/acquired immune deficiency syndrome (AIDS), 23 (26%) had received a solid organ transplant, and 11 (12%) were on a TNF-α inhibitor. Table 1 shows the patient characteristics of the 3 populations. Patients with HIV/AIDS were more likely to be African American and reside in an urban area. All patients with HIV infection met criteria for AIDS with CD4 counts between 3 and 180 cells/µL (median 31 cells/µL). Only 27% of the HIV patients had been on antiretroviral therapy (ART) within the last 6 months; of those, only one third had been adherent. Most of the transplant group had received a kidney transplant (16 kidney, 2 kidney/pancreas, 1 kidney/heart, 2 liver, 2 heart). No patient who had received a lung or hematopoietic stem cell transplant was diagnosed with histoplasmosis during the study period. The median time from transplantation to onset of illness was 36 months (range, 2 months to 29 years). Five transplant recipients (22%) had been treated for acute rejection within the preceding 6 months. Patients on TNF-α inhibitors had varying diseases, but inflammatory bowel disease predominated (55%). The administered TNF-α inhibitors were either adalimumab (64%) or infliximab (36%). The median time from initiation of TNF-α inhibitor to infection was 26 months.
Table 1.
Patient Characteristics
| Variable | HIV/AIDS N = 56 | Transplant N = 23 | P Value HIV/AIDS vs Transplant | TNF-α inhibitor N = 11 | P Value HIV/AIDS vs TNF-α inhibitor |
|---|---|---|---|---|---|
| Male | 46 (82%) | 8 (35%) | <.001 | 7 (64%) | .2 |
| Agea | 42 (26–74) | 49 (20–67) | .08 | 43 (23–65) | .7 |
| White | 26 (46%) | 17 (74%) | .02 | 10 (91%) | .03 |
| African American | 26 (46%) | 5 (22%) | 1 (9%) | ||
| Latino | 4 (7%) | 0 (0%) | 0 (0%) | ||
| Rural environment | 13 (27%) | 14 (61%) | .003 | 5 (45%) | .2 |
| CD4 (cells/µL)a | 31 (3–180) | ||||
| On ART | 15 (27%) | ||||
| Time since transplanta | 36 months (2–348) | ||||
| Rejection in last 6 months | 5 (22%) | ||||
| Organ | |||||
| Kidney | 16 | ||||
| Heart | 2 | ||||
| Liver | 2 | ||||
| Kidney/Pancreas | 2 | ||||
| Kidney/Heart | 1 | ||||
| TNF-α inhibitor indication | |||||
| IBD | 6 | ||||
| RA | 2 | ||||
| Psoriasis | 2 | ||||
| Seronegative spondylarthropathy | 1 | ||||
Abbreviations: AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; HIV, human immunodeficiency virus; IBD, inflammatory bowel disease; RA, rheumatoid arthritis; TNF, tumor necrosis factor.
a Median (range).
Table 2 shows clinical, laboratory, and radiological aspects of the patients' illnesses. Clinical presentations of HIV patients and transplant recipients were similar, but more patients taking TNF-α inhibitors had mild disease when compared with HIV patients (27% vs 2%; P = .01). The median duration of symptoms before presentation was similar among all groups (2–3 weeks). No difference in presenting symptoms was found among the 3 groups. Fever was the most common presenting symptom (82%–87%). Human immunodeficiency virus patients had more leukopenia and thrombocytopenia than the other 2 groups and more anemia than patients on TNF-α inhibitors. Transplant recipients had more renal dysfunction on presentation. All 3 groups had a high prevalence of urine Histoplasma antigen positivity (88%–95%), but HIV patients were more likely to have positive fungal blood cultures (51% HIV vs 27% transplant vs 0% TNF-α). Of the 8 patients who had negative urine Histoplasma antigen levels, 3 had positive fungal blood cultures, 1 had a positive serum Histoplasma antigen, 2 patients grew H capsulatum in culture (1 cerebrospinal fluid, 1 sputum), and 2 patients had H capsulatum identified by histopathology of tissue. Only 6 patients had Histoplasma serologies performed, of which 4 were positive. Fifteen patients had serum Histoplasma antigens performed, and 12 were positive (88% in HIV, 75% in transplant, 67% in TNF-α; P = .8). Patients infected with HIV were more likely to have adenopathy present on chest imaging, and TNF-α patients had a miliary pattern reported more frequently. Of note, all of the 7 patients who had a miliary pattern identified were classified as moderate infection (none had severe infection).
Table 2.
Clinical Characteristics
| Variable | HIV/AIDS N = 56 | Transplant N = 23 | P Value HIV/AIDS vs Transplant | TNF-α Inhibitor N = 11 | P Value HIV/AIDS vs TNF-α Inhibitor |
|---|---|---|---|---|---|
| Severity of illness | |||||
| Mild | 2% | 4% | .5 | 27% | .01 |
| Moderate | 70% | 61% | 45% | ||
| Severe | 29% | 35% | 27% | ||
| Fever (T ≥ 38°C) | 82% | 87% | .8 | 82% | 1.0 |
| Cough | 57% | 39% | .2 | 64% | .8 |
| Diarrhea | 36% | 35% | 1.0 | 18% | .3 |
| Duration of symptoms (days)a | 19 (0–120) | 14 (3–180) | .3 | 14 (4–60) | .8 |
| WBCb (thousand/mL) | 3.2 (0.7–8.9) | 4.3 (1.6–10.8) | .008 | 5.3 (3.8–14.2) | .0004 |
| Hematocrit (%) | 31 (14–47) | 31 (23–39) | .7 | 40 (31–44) | .0003 |
| Platelet count (thousand/mL)c | 127 (14–451) | 191 (51–538) | .04 | 262 (93–489) | .01 |
| Creatinine (mg/dL)c | 0.9 (0.4–12) | 2 (0.9–6.2) | <.0001 | 0.9 (0.7–2.2) | .9 |
| Total bilirubin (mg/dL)c | 0.7 (0.1–5.6) | 1 (0.3–5.6) | .04 | 0.6 (0.4–2.7) | 1.0 |
| AST (U/L)d | 75 (15–1100) | 31 (3–318) | .003 | 72 (20–259) | .2 |
| LDH (U/L)e | 550 (113–15940) | 336 (112–3593) | .07 | 682 (289–871) | .8 |
| Positive blood culturesf | 18/35 (51%) | 4/15 (27%) | .10 | 0/5 (0%) | .04 |
| Positive urine Histo Agg | 43/49 (88%) | 21/22 (95%) | 1.0 | 10/11 (91%) | 1.0 |
| Abnormal CXRh | 32/53 (60%) | 16/23 (70%) | .6 | 9/11 (82%) | .3 |
| Abnormal CTi chest | 27/29 (93%) | 14/16 (87%) | .6 | 8/9 (89%) | 1.0 |
| Adenopathy on CT | 19/29 (66%) | 4/16 (25%) | .01 | 2/9 (22%) | .05 |
| Miliary pattern on CT | 2/29 (7%) | 1/16 (6%) | 1.0 | 4/9 (44%) | .02 |
| Interstitial infiltrates on CT | 8/29 (28%) | 4/16 (25%) | 1.0 | 4/9 (44%) | .4 |
| Alveolar infiltrates on CT | 5/29 (17%) | 6/16 (38%) | .2 | 3/9 (33%) | .4 |
Abbreviations: AIDS, acquired immune deficiency syndrome; AST, aspartate aminotransferase; CT, computed tomography; CXR, chest radiograph; Histo Ag, Histoplasma antigen; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; TNF, tumor necrosis factor; WBC, white blood cells.
a Duration of symptoms at time of presentation for medical care, median (range).
b WBC at presentation, median (range).
c Median (range).
d AST at presentation, median (range).
e LDH at presentation, median (range).
f Denominator represents number of patients who had fungal blood cultures performed.
g Denominator represents number of patients who had urine antigens performed.
h Denominator represents number of patients who had CXR performed.
i Denominator represents number of patients who had CT performed.
Treatment and outcomes are summarized in Table 3. Only 22% of transplant patients initially received an amphotericin B formulation compared with 57% of HIV-infected and 55% of TNF-α inhibitor recipients. Median duration of amphotericin B formulation therapy was 11 days (range, 2–17). Overall, a triazole was used as initial therapy in 47 (52%) of the 90 patients (11 severe, 31 moderate, 5 mild cases). Itraconazole was the predominant triazole chosen, but 7 patients received voriconazole and 1 received posaconazole. The failure rate of initial triazole therapy was 16% in patients with HIV infection, 22% in transplant recipients, and 0% in patients on TNF-α inhibitors. The frequency of triazole failure was similar among patients with severe infection compared with those with mild to moderate infection (3 of 11 vs 5 of 36; P = .4). Three patients initially treated with triazoles died of histoplasmosis within 90 days of presentation, as did 4 patients initially treated with amphotericin B. One patient died without having received antifungal therapy. All histoplasmosis-related deaths occurred in severe cases. The histoplasmosis-related 90-day mortality was 9% and did not differ based on initial choice of therapy (Figure 1), underlying immunosuppressed state (Figure 2), or duration of symptoms before diagnosis. Six patients died from histoplasmosis more than 90 days after initial presentation; all of these patients had AIDS and 4 of the patients were not compliant with their antifungal therapy. Relapse of histoplasmosis was seen in 12% of patients (8 HIV patients, 2 transplant patients, no TNF-α patient). The median duration of therapy was lower in the TNF-α inhibitor group compared with HIV patients, but the treatment duration was similar between transplant and HIV patients. The duration of therapy was similar between patients who relapsed versus those that did not relapse (median, 18 vs 17 months; P = .3). All TNF-α patients had discontinuation of their TNF-α inhibition therapy after their diagnosis of histoplasmosis.
Table 3.
Treatment and Outcomes
| Variable | HIV/AIDS N = 56 | Transplant N = 23 | P Value HIV/AIDS vs Transplant | TNF-α Inhibitor N = 11 | P Value HIV/AIDS vs TNF-α Inhibitor |
|---|---|---|---|---|---|
| Initial therapy | |||||
| Amphotericin Ba | 57% | 22% | .006 | 55% | 1.0 |
| Triazole | 43% | 78% | 45% | ||
| Triazole failure | 4/24 (16%) | 4/18 (22%) | .7 | 0/5 (0%) | 1.0 |
| Duration of therapy (months)b | 19 (1–139) | 14 (2–84) | .4 | 8 (1–12) | .0007 |
| Infection Relapse | 8/51 (16%) | 2/21 (10%) | .7 | 0/10 (0%) | .3 |
| Histoplasma related 90-day mortality | 5/56 (9%) | 2/23 (9%) | 1.0 | 1/11 (9%) | 1.0 |
| Overall Histoplasma related mortality | 11/56 (20%) | 2/23 (9%) | .3 | 1/11 (9%) | .7 |
Abbreviations: AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus; TNF, tumor necrosis factor.
a Includes amphotericin B, amphotericin B lipid complex, liposomal amphotericin B.
b Median (range).
Figure 1.
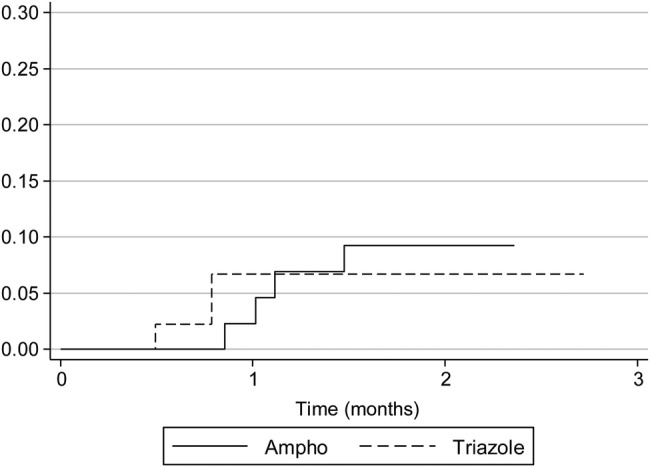
Ninety-day mortality by initial therapy. Actuarial curves representing histoplasmosis-related mortality in patients grouped by initial antifungal therapy. Log-rank statistics; P = .7.
Figure 2.
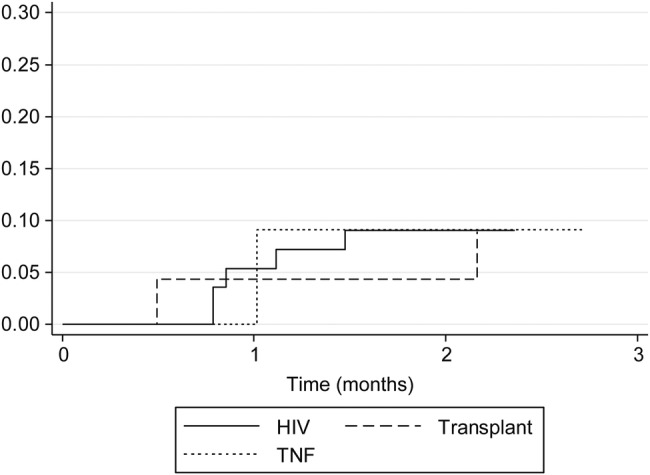
Ninety-day mortality by immunodeficiency. Actuarial curves representing histoplasmosis-related mortality in patients grouped by immunodeficient state. Log-rank statistics; P = 1.0.
DISCUSSION
The goal of this study was to compare the clinical course of histoplasmosis in 3 groups of immunocompromised patients managed at a single institution in an endemic area. All groups had a 2- to 3-week duration of symptoms before presentation and had fever as their predominant symptom as had previously been described in HIV patients [4]. All 3 groups had a high prevalence of abnormal chest imaging, especially if computed tomography scanning was performed. Patients receiving TNF-α inhibitors had milder disease than HIV patients. Fewer patients with TNF-α inhibition were classified as having severe disease on presentation; they received shorter courses of antifungal therapy; and none had positive blood cultures. The initial presentation of transplant patients resembled that of HIV-infected patients in having a low frequency of mild disease. The worse renal function in the transplant recipients likely reflected the predominance of kidney recipients in the population and the well documented nephrotoxicity of calcineurin inhibitors.
Diagnosis of histoplasmosis has been greatly facilitated by the development of Histoplasma antigen testing. As in previous studies, the great majority of immunosuppressed patients had positive urine antigen tests, with no difference in frequency between the 3 groups [11]. Too few serum antigen tests or serologies were obtained to be analyzed. Serum antigen testing is useful in diagnosing acute histoplasmosis in immunocompetent patients, and future studies should analyze the utility of serum antigen testing in immunosuppressed populations [11].
A second aim of our study was to evaluate whether the initial choice of antifungal therapy affected outcomes. Published guidelines recommend lipid formulations of amphotericin B for initial treatment of moderately severe to severe histoplasmosis in immunocompromised patients [12]. In this series, triazoles were the initial treatment in more than half of the patients, 11 of whom had severe disease. A clear preference for triazole therapy was seen in transplant recipients, of whom only 22% received initial amphotericin B therapy, compared with over half of the patients in the other groups. This result appears to diverge from practice elsewhere. In a recent large multicenter study, 73% of 152 transplant patients with histoplasmosis received amphotericin B formulations as initial therapy. Yet the death rate from histoplasmosis in our study (9%) was virtually identical to that in the multicenter study (10%) [7]. Further studies are needed to identify which patients would be optimally treated with initial triazole versus amphotericin B therapy. Such a study might reasonably be restricted to transplant recipients because of their greater risk for nephrotoxicity.
An issue that requires further resolution is how long to treat immunosuppressed patients with disseminated histoplasmosis [7, 12]. This approach has not been rigorously studied and may vary based on the type and degree of immunosuppression. In our study, patients receiving TNF-α inhibitors were successfully treated with a shorter course of therapy than patients with HIV infection. Guidelines suggest that antifungal therapy should be continued in patients with AIDS who have CD4 counts less than 150 cells/µL, but that discontinuation may be considered in patients on ART who have received therapy for at least 1 year and have immune reconstitution and low urine or serum antigen levels [13]. Data on when to stop treatment in transplant recipients is limited, and the level of immunosuppression cannot be objectively quantified. Assi et al [7] suggested that a reasonable duration of therapy would be 1 year, especially if the urine antigen has fallen below 2 ng/mL. The majority of relapses in this population seem to occur during the first 2 years after discontinuation of triazole therapy; thus, enhanced clinical and laboratory monitoring has been recommended during this period. Ultimately, prospective studies will be required to provide solid evidence for clinical decision making.
Our study of histoplasmosis is limited by its retrospective nature and the lack of consistency in treatment across patient groups. The subgroup sample sizes, particularly the TNF-α group, were small, and this may have made it more difficult to identify significant differences that may exist if the sample sizes were larger. However, the data suggest that histoplasmosis is more severe in patients with HIV infection than in patients who received TNF-α inhibitors. The HIV patients in our series all had AIDS, and most were not on ART at the time of diagnosis. Prior studies show that the outcome for such patients is highly dependent on successful treatment of the HIV infection as well as patient cooperation [4]. Patients receiving TNF-α inhibitors seemed to have the mildest disease. Although there was 1 death in this group, patients on TNF-α inhibitors were more likely to have mild disease, were cured with shorter treatment courses, and had no relapses. Some of the differences that were noted between HIV patients and TNF-α patients could potentially be explained by access to healthcare, because TNF-α patients may be more likely to have health insurance and to be more closely monitored. Previous studies have shown that most of these patients can be safely restarted on TNF-α inhibitors after they have been treated, and that was also true for 2 patients in this study [10].
Efforts to improve outcomes in the future might focus on earlier evaluation of symptoms and more rapid diagnosis, perhaps by point-of-care testing for Histoplasma antigen. Once patients have responded to treatment, they should have regular clinical and laboratory follow-up to ensure that they are taking their medications and achieving adequate serum levels. Finally, because only a small percentage of the immunocompromised patients in endemic areas develop histoplasmosis, identification of environmental and genetic risk factors may help to target subpopulations for special attention.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Tobon AM, Agudelo CA, Rosero DS, et al. Disseminated histoplasmosis: a comparative study between patients with acquired immunodeficiency syndrome and non-human immunodeficiency virus-infected individuals. Am J Trop Med Hyg. 2005;73:576–82. [PubMed] [Google Scholar]
- 2.Deodhar D, Frenzen F, Rupali P, et al. Disseminated histoplasmosis: a comparative study of the clinical features and outcome among immunocompromised and immunocompetent patients. Natl Med J India. 2013;26:214–5. [PubMed] [Google Scholar]
- 3.Wheat LJ, Connolly-Stringfield PA, Baker RL, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69:361–74. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Baddley JW, Sankara IR, Rodriquez JM, et al. Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis. 2008;62:151–6. doi: 10.1016/j.diagmicrobio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Cuellar-Rodriguez J, Avery RK, Lard M, et al. Histoplasmosis in solid organ transplant recipients: 10 years of experience at a large transplant center in an endemic area. Clin Infect Dis. 2009;49:710–6. doi: 10.1086/604712. [DOI] [PubMed] [Google Scholar]
- 6.Freifeld AG, Iwen PC, Lesiak BL, et al. Histoplasmosis in solid organ transplant recipients at a large Midwestern university transplant center. Transpl Infect Dis. 2005;7:109–15. doi: 10.1111/j.1467-8365.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 7.Assi M, Martin S, Wheat LJ, et al. Histoplasmosis after solid organ transplantation. Clin Infect Dis. 2013;57:1542–9. doi: 10.1093/cid/cit593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsiodras S, Samonis G, Boumpas DT, et al. Fungal infections complicating tumor necrosis factor alpha blockade therapy. Mayo Clin Proc. 2008;83:181–94. [PubMed] [Google Scholar]
- 9.McKinsey DS, Spiegel RA, Hutwagner L, et al. Prospective study of histoplasmosis in patients infected with human immunodeficiency virus: incidence, risk factors, and pathophysiology. Clin Infect Dis. 1997;24:1195–203. doi: 10.1086/513653. [DOI] [PubMed] [Google Scholar]
- 10.Hage CA, Bowyer S, Tarvin S, et al. Recognition, diagnosis, and treatment of histoplasmosis complicating tumor necrosis factor blocker therapy. Clin Infect Dis. 2010;50:85–92. doi: 10.1086/648724. [DOI] [PubMed] [Google Scholar]
- 11.Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis. 2011;53:448–54. doi: 10.1093/cid/cir435. [DOI] [PubMed] [Google Scholar]
- 12.Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–25. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]
- 13.Goldman M, Zackin R, Fichtenbaum CJ, et al. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin Infect Dis. 2004;38:1485–9. doi: 10.1086/420749. [DOI] [PubMed] [Google Scholar]


