Abstract
Background. Longitudinal data on liver disease in human immunodeficiency virus (HIV) mono-infection are scarce. We used noninvasive serum biomarkers to study incidence and predictors of hepatic steatosis and fibrosis.
Methods. Hepatic steatosis was diagnosed by hepatic steatosis index ≥36. Advanced liver fibrosis was diagnosed by fibrosis-4 index >3.25. Kaplan–Meier analysis was used to estimate incidences. Cox regression analysis was used to explore predictors of hepatic steatosis and fibrosis development.
Results. In this retrospective observational study, 796 consecutive HIV mono-infected patients were observed for a median of 4.9 (interquartile range, 2.2–6.4) years. Incidence of hepatic steatosis was 6.9 of 100 per person-years (PY) (95% confidence interval [CI], 5.9–7.9). Incidence of advanced liver fibrosis was 0.9 of 100 PY (95% CI, 0.6–1.3). Development of hepatic steatosis was predicted by black ethnicity (adjusted hazard ratio [aHR] = 2.18; 95% CI, 1.58–3; P < .001) and lower albumin (aHR = 0.94; 95% CI, 0.91–0.97; P < .001). Development of advanced liver fibrosis was predicted by higher glucose (aHR = 1.22; 95% CI, 1.2–1.3; P < .001) and lower albumin (aHR = 0.89; 95% CI, 0.84–0.93; P < .001).
Conclusions. Incident hepatic steatosis is frequent in HIV mono-infected patients, particularly in those of black ethnicity. These patients can also develop advanced liver fibrosis. Identification of at-risk individuals can help early initiation of hepatological monitoring and interventions, such as targeting euglycemia.
Keywords: hepatic steatosis, HIV mono-infection, liver fibrosis, serum biomarkers
After the introduction of effective combination antiretroviral therapy (cART), more than 50% of deaths among persons infected with human immunodeficiency virus (HIV) are from causes other than acquired immune deficiency syndrome (AIDS) [1]. Overall, liver-related death, mainly due to chronic infection with hepatitis C virus (HCV) or hepatitis B virus, represents the most frequent cause of non-AIDS-related death [2]. Human immunodeficiency virus mono-infected persons are also at risk for liver disease from multiple cofactors, including insulin resistance, long-term use of cART, and HIV hepatotoxicity [3, 4]. Hepatic steatosis is a common liver disease in Western countries associated with metabolic abnormalities [5, 6]. Cross-sectional studies reported it in up to 40% of HIV mono-infected patients [3]. Hepatic steatosis is the first pathophysiological step for nonalcoholic steatohepatitis (NASH), a progressive liver disease leading to cirrhosis and related complications [6]. Liver fibrosis is the hallmark of a progressive disease that can lead to cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC) [7]. Liver fibrosis may be as frequent as 8% in HIV mono-infected patients [8]. Liver biopsy has long been the gold standard to assess hepatic steatosis and fibrosis. However, this procedure is invasive, costly, impractical as a screening tool, and prone to sampling error [9]. The burden of liver disease in HIV mono-infected persons may not be fully appreciated because of the drawbacks of liver biopsy and its underutilization. As such, data on incident hepatic steatosis and fibrosis and associated predictors in HIV mono-infection are lacking. Recently, noninvasive serum biomarkers have been implemented in place of liver biopsy. Validated fibrosis biomarkers include fibrosis-4 (FIB-4) index, based on aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelets, and age, as well as AST-to-Platelet Ration Index (APRI) [10, 11]. These biomarkers are based on routine parameters and have been validated in the setting of HIV infection, where their accuracy to assess liver fibrosis ranges from 65% to 90% [7, 12]. Lee et al [13] have proposed an index named hepatic steatosis index (HSI), based on transaminases, body mass index (BMI), gender, and absence or presence of diabetes. This biomarker has been validated in more than 10 000 apparently healthy individuals, with a sensitivity of 93.1% and a specificity of 92.4% to diagnose hepatic steatosis when compared with ultrasound [13].
Most of the previous studies on hepatic steatosis and fibrosis in HIV mono-infected patients had a cross-sectional design, so they captured prevalence and associated risk factors at a single time point. Because hepatic steatosis and fibrosis are chronic processes, longitudinal studies are more suitable to capture dynamic changes. Moreover, no study has employed a simple serum biomarker to evaluate hepatic steatosis in HIV mono-infected patients.
The aim of this study was to investigate incidence and predictors of hepatic steatosis and fibrosis in a large cohort of well characterized HIV mono-infected persons by using validated serum biomarkers. We also validated for the first time HSI versus liver ultrasound in the specific context of HIV mono-infection. To our knowledge, this is the first large-scale longitudinal study designed to investigate incident hepatic steatosis in HIV mono-infection.
PATIENTS AND METHODS
Study Design and Population
The study was conducted at a single site, the Chronic Viral Illness Service of the McGill University Health Centre, which included all eligible adult patients with documented HIV mono-infection (positive enzyme-linked immunosorbent assay with Western blot confirmation) in active follow-up. The Chronic Viral Illness Service is a university-based clinic serving more than 2000 active HIV-infected patients. Since 1989, a computerized database on all patients has been maintained into which demographic data, clinical diagnosis, laboratory results, and prescription information have been prospectively entered. To be included, patients had to fulfill the following criteria: (1) age >16 years; (2) availability of relevant serum parameters for the calculation of noninvasive biomarkers on the same date; (3) and at least 2 study visits between 2007 and 2013. Exclusion criteria were (1) positivity for HCV antibody; (2) positivity for hepatitis B surface antigen; (3) any of the outcome measures (hepatic steatosis, advanced liver fibrosis) present at baseline; (4) history or evidence at entry of HCC; (5) liver transplantation; (6) last follow-up visit antecedent to January 2011. For the purpose of the present study, information on patients' demographics and laboratory results was based on data recorded at their last follow-up visit. Patients were observed until November 2013 or were censored either when they died or at their last clinic visit. All of the participants provided informed consent. The Institutional Research Ethic Board approved the study, which was conducted according to the Declaration of Helsinki.
Outcome Measures and Noninvasive Biomarkers for Hepatic Steatosis and Liver Fibrosis
The outcome measures were hepatic steatosis and advanced liver fibrosis (histologically defined as stage 3–4 by METAVIR staging system [14]). Advanced fibrosis is a clinically meaningful threshold because it is the hallmark of a progressive liver disease that will rapidly lead to liver cirrhosis [15]. Hepatic steatosis index, defined as 8 × AST/ALT + BMI (+2, if female; +2, if diabetes), was applied to diagnose hepatic steatosis [13]. Fibrosis-4 was used to diagnose advanced liver fibrosis and was calculated as age (years) × AST/platelet count (109/L) × AST ½ [10]. The outcome was defined as the second consecutive event diagnosed by standard cutoff values: HSI ≥36 for hepatic steatosis and FIB-4 >3.25 for advanced liver fibrosis.
Performance of Hepatic Steatosis Index Versus Liver Ultrasound to Diagnose Hepatic Steatosis in Human Immunodeficiency Virus Mono-Infected Patients
Given that HSI was never specifically validated in HIV mono-infection, we conducted a cross-sectional validation in 355 HIV mono-infected patients. These patients were extracted from the cohort of the present study by including all consecutive HIV mono-infected patients who had a liver ultrasound available within 6 months from the HSI and without excluding those with the outcome (HSI) at baseline. We adopted liver ultrasound as reference standard because prior studies have shown a good sensitivity and specificity for ultrasonography to diagnose hepatic steatosis in HIV-positive patients [16]. The validation cohort was a subgroup of our study cohort with available ultrasound and before excluding patients with the outcome at baseline and without a follow-up visit (see Figure 1). Abdominal ultrasound was performed by 2 experienced radiologists at the Department of Radiology of the McGill University Heath Center. Hepatic steatosis was diagnosed by the radiologist on the basis of characteristic imaging findings, namely, bright liver pattern, liver-kidney contrast, vascular blurring, and deep hepatic attenuation.
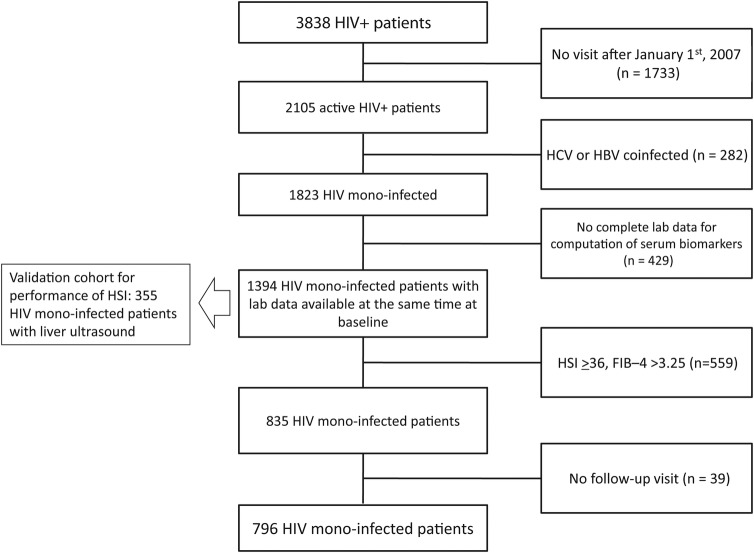
Figure 1.
Flow chart displaying selection of study participants for analysis. Of 3838 patients with human immunodeficiency virus (HIV) infection present in the Chronic Viral Illness Service database, 1733 patients were excluded because they were not actively observed (last follow-up visit antecedent to January 1, 2007), 282 patients were excluded because of hepatitis C virus or hepatitis B virus co-infection, and 429 were excluded because of missing data. After further exclusion of 559 patients who had the outcome at baseline and 39 cases without a follow-up visit, the remaining sample of 796 consecutive patients with HIV mono-infection and in active follow-up was included in the present study. Abbreviations: HIV, human immunodeficiency virus; HCV, hepatitis C virus; HBV, hepatitis B virus; HSI, hepatic steatosis index; FIB-4, fibrosis-4.
Clinical and Biological Parameters
Clinical parameters included age, gender, ethnicity, BMI, history of hypertension or diabetes, risk factor for HIV infection, time since HIV diagnosis, and cART. Biological parameters included platelets, AST, ALT, albumin, total cholesterol, glucose, CD4 cell count, and HIV viral load. Obesity was defined as BMI ≥30 [17]. The diagnosis of diabetes was based on treatment with antidiabetic drugs. The duration of HIV infection was obtained using the date of HIV diagnosis. A HIV viral load value less than 50 copies/mL was considered undetectable (COBAS Amplicor, Roche; lower limit of detection, 50 copies/mL).
Statistical Analysis
Baseline (time zero) corresponded to the first visit after January 1, 2007 when relevant serum parameters were available simultaneously for the calculation of all noninvasive biomarkers. Continuous variables were expressed as median (interquartile range [IQR]), and categorical variables were presented as numbers (percentage). We compared characteristics of participants by outcome status using Kruskall-Wallis test for continuous variables and Pearson's χ² or Fisher's exact test for categorical variables. All tests were 2-tailed and with a significance level of α = 0.05. The performance of HSI to diagnose steatosis compared with liver ultrasound was measured with the following: sensitivity, specificity, positive predictive value, negative predictive value, accuracy, positive and negative likelihood ratios (LR + and LR−, respectively), and area under the receiver operating characteristic curve (AUC). We estimated incidence rates of hepatic steatosis (HSI ≥36) and advanced liver fibrosis (FIB-4 >3.25) by dividing the number of participants developing the outcome by the number of person-years (PY) of follow-up. Poisson count models were used to calculate confidence intervals (CIs) for incidence rates. Kaplan–Meier plots for time to development of hepatic steatosis were calculated and stratified by ethnicity category. Multivariate time-dependent Cox regression models were constructed to assess predictors of fibrosis and steatosis progression and included covariates that were determined a priori to be clinically important. Final models were adjusted for gender, black ethnicity, time since HIV diagnosis, history of hypertension, and time-updated cART, CD4 cell count, albumin, and glucose. Albumin was selected because it is a protein synthetized by the liver and a sensitive marker of liver function. Robust variance estimation was used in all Cox regression analyses to account for the correlation of data contributed by the same participant at multiple visits. Statistical analyses were performed using R program for Windows Release 2.13.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
After applying exclusion criteria (Figure 1), 796 patients with HIV mono-infection and in active follow-up were included. The main demographic, clinical, and serological characteristics of the study population are summarized in Table 1. Overall, there were 667 males and median age was 43.5 years (IQR, 36–49.7). The most represented ethnicities were white/Caucasian (57%) and black (26%). The majority of black patients were Africans (57%) and from Caribbean countries (41%). The most frequent risk factor for HIV infection was men who have had sex with men (60%). The median BMI was 23.4 kg/m2 (IQR, 21.6–25.2), and 111 (14%) patients were obese. Most of the patients (76%) were on cART. Median CD4 cell count was 354 cells/µL (IQR, 217–532), and median time since HIV diagnosis was 6.3 years (IQR, 1.7–13.3).
Table 1.
Baseline Characteristics of Study Population and Univariate Analysis of Those Who Developed the Outcomes During Follow-up
| Characteristics | Total (n = 796) | Developed Steatosis (HSI ≥36) (n = 194) | Developed Advanced Fibrosis (FIB-4 >3.25) (n = 31) |
|---|---|---|---|
| Age (median years, IQR) | 43.5 (36–49.7) | 42.3 (34.6–49.5) | 49.2 (44–55)** |
| Male gender (%) | 667 (84) | 125 (64)** | 26 (84) |
| Ethnicity | |||
| White/Caucasian | 456 (57) | 91 (47)* | 22 (71) |
| Black | 210 (26) | 82 (42)** | 9 (29) |
| MSM (%) | 471 (60) | 77 (40)** | 20 (65) |
| IVDU (%) | 12 (2) | 9 (5)** | 0 |
| Diabetes (%) | 30 (4) | 16 (8)** | 4 (13)* |
| Hypertension (%) | 58 (7) | 21 (11)* | 4 (13) |
| BMI (median Kg/m², IQR) | 23.4 (21.6–25.2) | 24.4 (22.3–26.4)** | 23.3 (20.8–24.8) |
| Time since HIV diagnosis (median years, IQR) | 6.3 (1.7–13.3) | 6.3 (1.7–13.2) | 16 (5.4–18.8)* |
| HIV viral load <50 copies/mL (%) | 318 (40) | 52 (27)* | 8 (26) |
| CD4 count (median cells/μL, IQR) | 353.5 (216.5–531.8) | 276.5 (162.5–436)* | 222 (126.2–373.8)* |
| On cART (%)a | 602 (76) | 139 (72) | 21 (68) |
| PI + NRTI | 356 (59) | 92 (66) | 14 (67) |
| NNRTI + NRTI | 265 (44) | 56 (40) | 9 (43) |
| Other + NRTI | 35 (6) | 5 (4) | 2 (10) |
| DDX | 26 (4) | 10 (7) | 3 (14) |
| Platelet count (median 109/L, IQR) | 229 (190–276.2) | 227.5 (184–275) | 209 (162–250) |
| AST (median IU/L, IQR) | 25 (21–31) | 26 (21–33) | 29 (24.5–41.5)* |
| ALT (median IU/L, IQR) | 26 (20–36) | 26 (19.3–38) | 27 (21.5–39.5) |
| Albumin (median g/L, IQR) | 41 (39–44) | 40.5 (37–43)** | 41 (34.5–43.5) |
| Glucose (median mmol/L, IQR) | 5.1 (4.7–5.6) | 5.1 (4.7–5.7) | 5.2 (4.9–5.9) |
| Cholesterol (median mmol/L, IQR) | 4.5 (3.6–5.7) | 4.5 (3.9–5.8) | 4.7 (3.7–6) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; cART, combination antiretroviral therapy; DDX, didanosine/stavudine/zalcitabine; HIV, human immunodeficiency virus; HSI, hepatic steatosis index; IQR, interquartile range; IU, international units; IVDU, intravenous drug use; MSM, men who have sex with men; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitors.
a Percentage based on number of patients on cART.
* P < .05; **P < .001. The P values refer to t test or χ2 test between patients who developed the outcomes (steatosis and fibrosis, here shown) and the correspondent counterpart of patients who did not develop the outcomes (data not shown).
Performance of Hepatic Steatosis Index to Diagnose Hepatic Steatosis in 355 Human Immunodeficiency Virus Mono-Infected Patients
The characteristics of the validation cohort to study HSI performance to diagnose steatosis are depicted in Table 2. Overall, HSI showed an AUC of 0.88 and an accuracy of 84.5% when compared with liver ultrasound (Table 3). These figures are in line with those reported in the original study about HSI for diagnosis of hepatic steatosis in apparently healthy individuals [13].
Table 2.
Characteristics of Validation Cohort of 355 HIV Mono-Infected Patients for the Performance Study of HSI vs Liver Ultrasound
| Age (median years, IQR) | 45 (40–50.3) |
| Male gender (%) | 238 (67) |
| Diabetes (%) | 11 (3) |
| BMI (median kg/m², IQR) | 24.5 (22.6–28.1) |
| Time since HIV diagnosis (median years, IQR) | 11.2 (6.6–16.1) |
| HIV viral load <50 copies/mL (%) | 231 (65) |
| CD4 count (median cells/µL, IQR) | 516 (355–675.5) |
| On cART (%) | 337 (95) |
| Platelet count (median 109/L, IQR) | 225 (193.5–266.3) |
| AST (median IU/L, IQR) | 24 (21–28) |
| ALT (median IU/L, IQR) | 24 (19–36) |
| Albumin (median g/L, IQR) | 41 (39–44) |
| Glucose (median mmol/L, IQR) | 5.2 (4.4–5.8) |
| Cholesterol (median mmol/L, IQR) | 4.6 (4.1–5.4) |
| HSI ≥ 36 (%) | 117 (33) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; HSI, hepatic steatosis index; IQR, interquartile range; IU, international units; mono, mononucleosis.
Table 3.
Diagnostic Performance of HSI for Hepatic Steatosis Compared With Liver Ultrasound in the Validation Cohort of 355 HIV Mono-Infected Patients
| AUC (95% CI) | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|
| 0.88 (0.80–0.95) | 84.5 | 85.7 | 84.1 | 63.2 | 94.9 | 5.4 | 0.2 |
Abbreviations: AUC, area under the receiving operating characteristic curve; CI, confidence interval; HIV, human immunodeficiency virus; HSI, hepatic steatosis index; LR+, positive likelihood ratio; LR−, negative likelihood ratio; mono, mononucleosis.; NPV, negative predictive value; PPV, positive predictive value.
Incidence of Hepatic Steatosis and Advanced Liver Fibrosis
Patients were observed for a median of 4.9 (IQR, 2.2–6.4) years. Overall, 194 (24%) achieved the outcome of hepatic steatosis, accounting for an incidence of 6.9 of 100 PY (95% CI, 5.9–7.9). For advanced liver fibrosis, 31 (4%) achieved the outcome, accounting for an incidence of 0.9 of 100 PY (95% CI, 0.6–1.3).
Predictors of Development of Hepatic Steatosis and Advanced Liver Fibrosis
The results of the multivariate Cox regression analysis are shown in Table 4. Overall, development of hepatic steatosis was predicted by black ethnicity (adjusted hazard ratio [aHR], 2.18; 95% CI 1.58–3; P < .001) and lower albumin (aHR, 0.94; 95% CI, 0.91–0.97; P < .001). Development of advanced liver fibrosis was predicted by higher glucose levels (aHR, 1.22; 95% CI, 1.2–1.3; P < .001) and lower albumin (aHR, 0.89; 95% CI, 0.84–0.93; P < .001). Figure 2 shows development of hepatic steatosis (as measured by HSI ≥36) during the study period by ethnicity category (black vs non-black). Patients of black ethnicity had a higher incident rate of hepatic steatosis compared with non-black ones (log rank: P < .001).
Table 4.
Multivariate Cox Regressions Analyses of Predictors of Development of Hepatic Steatosis (HSI ≥36) and Advanced Liver Fibrosis (FIB-4 >3.25)a
| Predictor | Hepatic Steatosis (HSI ≥ 36) | Advanced Liver Fibrosis (FIB-4 >3.25) |
|---|---|---|
| Time-independent baseline covariates | ||
| Male gender | – | 1.18 (0.4–3.48) |
| Black ethnicity | 2.18 (1.58–3)** | 1.25 (0.44–3.57) |
| Hypertension | 0.99 (0.59–1.68) | – |
| Time since HIV diagnosis (per 5 years) | 1.01 (0.89–1.15) | 1.42 (0.98–2.04) |
| Time updated covariates | ||
| CD4 count (per 100 cells/mL) | 1.01 (0.95–1.06) | 0.91 (0.75–1.11) |
| Albumin (g/L) | 0.94 (0.91–0.97)** | 0.89 (0.84–0.93)** |
| Glucose (mmol/L) | – | 1.22 (1.2–1.3)** |
| On cART | 0.91 (0.56–1.45) | 0.39 (0.10–1.44) |
Abbreviations: cART, combination antiretroviral therapy; FIB-4, fibrosis-4; HIV, human immunodeficiency virus; HSI, hepatic steatosis index.
a Adjusted hazard ratio and 95% confidence intervals are shown for predictors analyzed in Cox regression analysis. The hepatic steatosis model is based on 176 outcomes instead of 194 due to missingness.
* P < .05; **P < .001.
Figure 2.
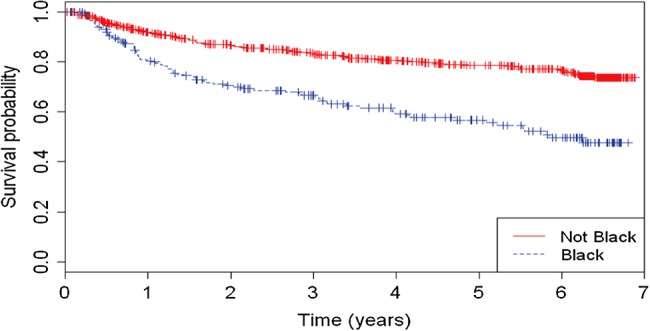
Development of hepatic steatosis (as measured by hepatic steatosis index [HSI] ≥36) during the study period by ethnicity category (black vs non-black).
DISCUSSION
This longitudinal study, based on a large cohort of well characterized HIV mono-infected patients, shows that development of incident hepatic steatosis is a frequent clinical event in this population. Human immunodeficiency virus mono-infected patients also develop advanced liver fibrosis, which is the hallmark of a progressive disease eventually leading to cirrhosis and its end-stage complications. To our knowledge, this is the first study to dissect incidence and predictors of hepatic steatosis and to evaluate advanced liver fibrosis in the same study population during a long follow-up period. We used simple steatosis and fibrosis biomarkers, which can be easily integrated as screening tools in the busy setting of HIV clinics.
Development of hepatic steatosis was frequent in our cohort, with a cumulative incidence of 24% over a median of 4.9 (IQR, 2.2–6.4) years. In the general population, the incidence rate of nonalcoholic fatty liver disease (NAFLD) ranges from 0.029 to 3.1 of 100 PY [18–20]. In our experience, the incidence rate of hepatic steatosis in HIV mono-infected patients was 6.9 of 100 PY. Because HSI left 40% of cases unclassified (those falling in between the lower, 30, and higher, 36, cutoff value; data not shown), the incidence could have been even higher. Our results suggest that HIV mono-infected patients are at risk for incident hepatic steatosis compared with the general population. No previous study has investigated the incidence of hepatic steatosis in HIV mono-infected patients. A number of longitudinal studies exist in the setting of HIV/HCV co-infection, but their results are not applicable to HIV mono-infection due to the critical confounding effect of HCV, which carries increased risk of steatosis [21]. Cross-sectional studies reported a prevalence of hepatic steatosis of 13%–40% in HIV mono-infected patients [3]. More importantly, these studies used either liver biopsy or time-consuming and expensive radiologic techniques, which are unlikely to be implemented in HIV clinics. In this study, we provide evidence that HSI can accurately predict hepatic steatosis in HIV mono-infected patients, with 84.5% accuracy and 0.88 AUC compared with liver ultrasound. Prior studies have shown a good sensitivity and specificity for ultrasonography to diagnose hepatic steatosis in HIV-positive patients [16]. Hepatic steatosis index is a cheap biomarker providing an immediate result and can be used for screening and serial monitoring [13]. Moreover, HSI is easy to integrate in the daily busy setting of HIV clinics because it is not time-consuming and does not require access to radiologic facilities. The fact that a significant proportion of HIV mono-infected patients develop hepatic steatosis translates into relevant clinical implications. Hepatic steatosis and NASH may progress to cirrhosis and end-stage liver complications [5]. Nonalcoholic steatohepatitis could have accounted for a significant proportion of idiopathic cirrhosis in HIV mono-infected patients, which has been underrecognized in the past due to underutilization of liver biopsy. Hepatic steatosis not only causes liver disease, but it has also been associated with cardiac disease and decreased survival in the general population [22]. Considering that liver disease is often asymptomatic until end-stage complications occur, identification of at-risk individuals and early diagnosis of hepatic steatosis is critical in order to initiate interventions [5, 6].
We used a referenced serum biomarker to diagnose liver fibrosis [5, 7, 12, 23]. We also conducted the analysis with another validated biomarker for liver fibrosis, APRI, and we found similar results as for FIB-4 (data not shown) [11]. Only 1 longitudinal study has investigated the incidence of advanced liver fibrosis in HIV mono-infected patients by biomarkers [24]. This is a clinically meaningful endpoint because patients with advanced fibrosis have a fast progression to liver cirrhosis and an overall 10-year mortality rate of 18.5% [15]. We found an incidence rate of 0.9 of 100 PY, which is in line with the figure of 1.3 of 100 PY reported in the study by Mendeni et al [24].
Development of hepatic steatosis was predicted by black ethnicity in our cohort. This finding is peculiar to HIV infection given that black ethnicity is considered protective in the general population [25]. Susceptibility to hepatic steatosis in the general population is associated to a single-nucleotide polymorphism in the patatin-like phospholipase domain-containing 3 gene, which is less frequent in black people, thus suggesting a protective effect of this ethnicity [26]. Only 2 studies investigated ethnicity as predictor of hepatic steatosis in HIV mono-infected persons, and no effect protective effect was found [26, 27]. Hepatic steatosis in HIV-infected persons has already been associated with factors other than those classically known for HIV-seronegative patients, including lower BMI [28]. We speculate that the multifactorial contribution of viral factors, hepatotoxicity, and dysmetabolisms could overcome the susceptibility conferred by genetics in HIV mono-infected persons. Because Canada's black HIV+ population are frequently foreign-born, mostly from sub-Saharan African and Caribbean countries, they may have had poorer control of HIV disease in the past, and this can lead to faster progression to hepatic steatosis [29].
Higher glucose was associated with development of advanced liver fibrosis. Poorer glycemic control predicts fibrosis progression in patients with NAFLD and HIV/HCV co-infection [30, 31]. This observation suggests that glycemic control may help attenuate liver fibrosis progression. We could not account for effect of hyperglycemia on hepatic steatosis given that diabetes is encompassed in the formula of HSI. Lower albumin was a predictor of development of both hepatic steatosis and fibrosis. Albumin is primarily synthesized by hepatocytes and is a sensitive marker of liver function. Dropping values may indicate progressive liver impairment [32]. Lower albumin has also been associated with faster HIV disease progression and higher rates of all-cause mortality [33]. It can be argued that lower albumin may be a marker for poor nutritional status. However, in the study by Mehta et al [33], adjustment for BMI did not affect the relationship between albumin and mortality. On the same line, in our cohort, there was no correlation between BMI and albumin (data not shown). We hypothesize that lower albumin reflects faster progression of HIV disease and chronic inflammation induced by HIV, which could in turn synergize in promoting liver disease.
This study has limitations that should be acknowledged. First, we estimated the incidence of hepatic steatosis and fibrosis based on surrogate serological markers. However, large-scale studies using liver biopsy in HIV mono-infected persons are unlikely to be performed and ethically questionable given augmented risk of bleeding, costs, and lack of a clinical indication [9]. Moreover, FIB-4 showed suboptimal accuracy in HIV/HCV coinfected patients with normal ALT, and this might imply also a lower accuracy in HIV mono-infected patients [34]. Second, other noninvasive methods, such as transient elastography, may outperform serum biomarkers for diagnosis of hepatic steatosis and fibrosis [35, 36]. However, transient elastography is unlikely to be readily available in HIV clinical settings. In a nationwide Canadian survey, we have recently shown that difficult access to transient elastography can be a limiting factor for liver fibrosis screening, particularly in the setting of HIV infection [37]. Simple serum biomarkers are easy to implement by HIV clinicians in daily clinics and could be used as first-line tests in regions with limited healthcare resources. Third, because data about alcohol intake was not available, we could not account for its effect. Hepatic steatosis index incorporates in its formula a number of metabolic characteristics (BMI, diabetes) and ALT/AST ratio, which has been used for noninvasive separation of alcoholic and nonalcoholic liver disease [38]. Thus, we suggest that HSI likely reflects more a metabolic than an alcoholic fatty liver. Fourth, because BMI is encompassed in the formula of HSI, we could not assess this variable as predictor of hepatic steatosis. However, cross-sectional studies showed that prevalence of obesity is low in HIV+ patients with hepatic steatosis, thus suggesting that hepatic steatosis is related to other factors in this population [28, 39]. On the same line, prevalence of obesity was low in our population (14%). Fifth, some of the predictors we found, including higher glucose, could also have been affected by exposure to cART or uncontrolled HIV, for which we did not account.
CONCLUSIONS
In conclusion, in this first longitudinal study we showed that development of incident hepatic steatosis by a serum biomarker is frequent in HIV mono-infected patients. This population also develops advanced liver fibrosis. As such, noninvasive screening and serial monitoring by simple biomarkers should be implemented. At-risk HIV mono-infected patients, including black people and those with higher glucose and lower albumin, could particularly benefit from early initiation of hepatological monitoring and interventions, such as targeting euglycemia. This may in turn translate into reduced progression to cirrhosis and liver-related outcomes. This finding is relevant given that liver transplantation, the only definitive treatment for end-stage liver disease, has been limited in patients infected with HIV due to concerns about potential higher rates of complications, fear of HIV transmission, and restricted criteria for inclusion in the waiting list [40]. Prospective studies aimed at investigating the effect of screening strategies by means of simple biomarkers and the impact of interventions on the burden of liver disease in HIV mono-infected patients are needed.
Acknowledgments
G. S. holds a Chercheur-Boursier career award from the Fonds de la Recherche en Santé du Quebéc (FRSQ). M. B. K. holds a Chercheurs Nationaux career award from the FRQS.
Author contributions. G. S. contributed to the conception, study design, data, and interpretation of the data and first draft of the article. K. C. R.-K. contributed to statistical analysis and interpretation of the data. M. B. K. contributed to the conception, study design, data, and interpretation of the data. All authors approved the final version of the article. M. B. K. is the guarantor.
Potential conflicts of interest. G. S. has acted as speaker for Merck, Vertex, Gilead, Echosens, served as an advisory board member for Boheringer Ingelheim and Novartis, and has received research funding from Vertex, ViiV, and Merck. M. B. K. has received honoraria and acted as a consultant for ViiV, Gilead, Janssen, and Merck and received research funding from Merck.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Puoti M, Moioli MC, Travi G, et al. The burden of liver disease in human immunodeficiency virus-infected patients. Semin Liver Dis. 2012;32:103–13. doi: 10.1055/s-0032-1316473. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Lemoine M, Serfaty L, Capeau J. From nonalcoholic fatty liver to nonalcoholic steatohepatitis and cirrhosis in HIV-infected patients: diagnosis and management. Curr Opin Infect Dis. 2012;25:10–6. doi: 10.1097/QCO.0b013e32834ef599. [DOI] [PubMed] [Google Scholar]
- 4.Rockstroh JK, Mohr R, Behrens G, et al. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr Opin HIV AIDS. 2014;9:365–70. doi: 10.1097/COH.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372–84. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Sebastiani G, Castera L, Halfon P, et al. The impact of liver disease aetiology and the stages of hepatic fibrosis on the performance of non-invasive fibrosis biomarkers: an international study of 2411 cases. Aliment Pharmacol Ther. 2011;34:1202–16. doi: 10.1111/j.1365-2036.2011.04861.x. [DOI] [PubMed] [Google Scholar]
- 8.DallaPiazza M, Amorosa VK, Localio R, et al. Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49:1017–44. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 10.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 11.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 12.Hasson H, Merli M, Galli L, et al. Non-invasive fibrosis biomarkers - APRI and Forns - are associated with liver stiffness in HIV-monoinfected patients receiving antiretroviral drugs. Liver Int. 2013;33:1113–20. doi: 10.1111/liv.12159. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–8. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 15.Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–54. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 16.Ryan P, Blanco F, Garcia-Gasco P, et al. Predictors of severe hepatic steatosis using abdominal ultrasound in HIV-infected patients. HIV Med. 2009;10:53–9. doi: 10.1111/j.1468-1293.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Geneva: World Health Organization; 2000. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 18.Bedogni G, Miglioli L, Masutti F, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387–91. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 19.Whalley S, Puvanachandra P, Desai A, et al. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med. 2007;7:119–24. doi: 10.7861/clinmedicine.7-2-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology. 2005;41:64–71. doi: 10.1002/hep.20543. [DOI] [PubMed] [Google Scholar]
- 21.Woreta TA, Sutcliffe CG, Mehta SH, et al. Incidence and risk factors for steatosis progression in adults coinfected with HIV and hepatitis C virus. Gastroenterology. 2011;140:809–17. doi: 10.1053/j.gastro.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 24.Mendeni M, Foca E, Gotti D, et al. Evaluation of liver fibrosis: concordance analysis between noninvasive scores (APRI and FIB-4) evolution and predictors in a cohort of HIV-infected patients without hepatitis C and B infection. Clin Infect Dis. 2011;52:1164–73. doi: 10.1093/cid/cir071. [DOI] [PubMed] [Google Scholar]
- 25.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price JC, Seaberg EC, Latanich R, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109:695–704. doi: 10.1038/ajg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crum-Cianflone N, Collins G, Medina S, et al. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8:183–91. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammed SS, Aghdassi E, Salit IE, et al. HIV-positive patients with nonalcoholic fatty liver disease have a lower body mass index and are more physically active than HIV-negative patients. J Acquir Immune Defic Syndr. 2007;45:432–8. doi: 10.1097/QAI.0b013e318074efe3. [DOI] [PubMed] [Google Scholar]
- 29.Baidoobonso S, Bauer GR, Speechley KN, et al. HIV risk perception and distribution of HIV risk among African, Caribbean and other Black people in a Canadian city: mixed methods results from the BLACCH study. BMC Public Health. 2013;13:184. doi: 10.1186/1471-2458-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haukeland JW, Konopski Z, Linnestad P, et al. Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2005;40:1469–77. doi: 10.1080/00365520500264953. [DOI] [PubMed] [Google Scholar]
- 31.Konerman MA, Mehta SH, Sutcliffe CG, et al. Fibrosis progression in human immunodeficiency virus/hepatitis C virus coinfected adults: prospective analysis of 435 liver biopsy pairs. Hepatology. 2014;59:767–75. doi: 10.1002/hep.26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Child CG, Turcotte JG. In: Surgery and portal hypertension. The liver and portal hypertension. Child CG, editor. Philadelphia: Saunders; 1964. pp. 50–64. [Google Scholar]
- 33.Mehta SH, Astemborski J, Sterling TR, et al. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]
- 34.Shah AG, Smith PG, Sterling RK. Comparison of FIB-4 and APRI in HIV-HCV coinfected patients with normal and elevated ALT. Dig Dis Sci. 2011;56:3038–44. doi: 10.1007/s10620-011-1710-2. [DOI] [PubMed] [Google Scholar]
- 35.de Ledinghen V, Douvin C, Kettaneh A, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–9. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 36.Macias J, Gonzalez J, Tural C, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28:1279–87. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 37.Sebastiani G, Ghali P, Wong P, et al. Physicians’ practices for diagnosing liver fibrosis in chronic liver diseases: a nationwide, Canadian survey. Can J Gastroenterol Hepatol. 2014;28:23–30. doi: 10.1155/2014/675409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowa JP, Atmaca O, Kahraman A, et al. Non-invasive separation of alcoholic and non-alcoholic liver disease with predictive modeling. PLoS One. 2014;9:e101444. doi: 10.1371/journal.pone.0101444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–7. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 40.Cherian PT, Alrabih W, Douiri A, et al. Liver transplantation in human immunodeficiency virus-infected patients: procoagulant, but is antithrombotic prophylaxis required? Liver Transpl. 2012;18:82–8. doi: 10.1002/lt.22449. [DOI] [PubMed] [Google Scholar]