We evaluated the efficacy and tolerability of a single-tablet regimen strategy in 304 HIV-1 virologically suppressed patients switching to RPV/FTC/TDF for adverse events or treatment simplification. This strategy maintained virologic suppression and was associated with improved tolerability after 12 months follow-up.
Keywords: HIV-1, STR, switch, tolerability, virologic response
Abstract
Background. The purpose of this study was to assess the efficacy and tolerability of combined antiretroviral therapy (cART) in human immunodeficiency virus (HIV)-1 virologically suppressed patients who switched to rilpivirine (RPV)/tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) as a single-tablet regimen (STR).
Methods. A retrospective multicenter cohort study was performed between September 2012 and February 2014 in Bordeaux University Hospital-affiliated clinics. Patients with a plasma HIV viral load (VL) lower than 50 copies/mL and switching to STR were evaluated at baseline, 3, 6, 9, and 12 months from switch time (M3, M6, M9, M12) for VL and other biological parameters. Change from baseline in CD4 cell counts was evaluated at M6 and M12. Virological failure (VF) was defined as 2 consecutive VL >50 copies/mL.
Results. Three hundred four patients were included in the analysis. Single-tablet regimen switch was proposed to 116 patients with adverse events, mostly efavirenz (EFV)-based (n = 59), and to 224 patients for cART simplification. Thirty of 196 patients with available genotype resistance test results displayed virus with ≥1 drug resistance mutation on reverse-transcriptase gene. After 12 months of follow-up, 93.4% (95.5% confidence interval, 89.9–96.2) of patients remained virologically suppressed. There was no significant change in CD4 cell count. During the study period, 5 patients experienced VF, one of them harboring RPV resistance mutation. Clinical cART tolerability improved in 79 patients overall (29.9%) at M6, especially neurological symptoms related to EFV. Fasting serum lipid profiles improved, but a significant estimated glomerular function rate decrease (−11 mL/min/1.73 m2; P < 10−4) was observed.
Conclusions. Overall, virologic suppression was maintained in patients after switching to RPV/TDF/ FTC. This STR strategy was associated with improved tolerability.
Introduction of combined antiretroviral therapy (cART) has been accompanied by a significant and rapid decline in human immunodeficiency virus (HIV)-related and acquired immune deficiency syndrome-related morbidity and mortality. To date, suppression of HIV type 1 (HIV-1) is the rule for the vast majority of patients with a variety of cART regimens.
Rilpivirine (RPV), a second-generation nonnucleoside reverse-transcriptase inhibitor (NNRTI) (Edurant, Janssen Therapeutics and Janssen-Cilag), is active against wild-type viruses and remains efficient against some NNRTI- resistant HIV-1 strains [1–3]. The efficacy of oral RPV in antiretroviral-naive HIV-1 patients was first evaluated in a phase 2, randomized, dose-ranging study [4, 5], in phase 3 double-blinded studies (ECHO and THRIVE) [6, 7], and in a phase 3 open-label study (STaR) [8]. Pill count, dosing frequency, dietary requirements, and tolerability can impact adherence, virologic efficacy, and quality of life [9–11]. Rilpivirine and its coformulation with emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) as a single-tablet regimen ([STR] RPV/FTC/TDF) is now approved by the European Medicines Agency and the US Food and Drug Administration as a once-daily oral treatment for adults infected with HIV-1 without mutations associated with resistance to TDF, FTC, or the NNRTI class, and harboring a viral load (VL) ≤100 000 HIV-1 RNA copies/mL.
Current antiretroviral treatment guidelines recommend switching therapy in virologically suppressed patients to improve adherence or tolerability or to allow for treatment simplification [12–14]. In the SPIRIT study, a phase 3b randomized, open-label, multicenter, 48-week switch study (immediate switch or delayed switch at week 24), significant fasting serum lipid profile improvements were observed in virologically suppressed HIV-1-infected patients switching to RPV/FTC/TDF from a ritonavir-boosted protease inhibitor (PI/r)-based regimen, compared with those who continued treatment with a PI/r regimen. Moreover, the authors showed a maintained virologic suppression with a low risk of virologic failure (VF) [15]. Thus, switching from efavirenz (EFV)/FTC/TDF to RPV/FTC/TDF was considered a safe, efficacious option for virologically suppressed HIV-infected patients with EFV intolerance [16].
Strategies of treatment simplification have been mostly explored in treatment-experienced patients [15]. In September 2012, the RPV/FTC/TDF coformulation was approved as a STR in France in treatment-naive HIV-infected patients with VL ≤100 000 copies/mL. However, in clinical practice, in accordance with French guidelines, the RPV/FTC/TDF STR is frequently used as a switch drug combination for virologically suppressed treatment-experienced patients with various baseline cART regimens.
The aim of this study was to describe, in a real-world setting, the efficacy of this STR strategy in 304 HIV-1 infected, virologically suppressed patients switching to RPV/FTC/TDF, independently of their previous antiretroviral regimen, compared with baseline characteristics. We also focused on clinical and metabolic tolerability.
METHODS
Design
This secondary data collection and analysis were conducted between September 2012 and February 2014 in Bordeaux University Hospital-affiliated clinics. It was nested within the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS) CO3 Aquitaine Cohort, a prospective hospital-based cohort of HIV-1-infected adults in Southwestern France [17]. Informed consent was obtained from all patients. The Aquitaine Cohort received approval from the Bordeaux University Institutional Review Board.
Participants and Study Drugs
For this study, inclusion criteria were as follows: HIV-1-infected adults (>18 years of age) with 2 sequential plasma HIV-1 VL lower than 50 copies/mL over at least 6 months before switching to STR, and those treated with the same cART for at least 3 months before switching. Patients were included regardless of cART regimen before switch. The STR used was a combination of 200 mg FTC + 300 mg TDF + 25 mg RPV. At least 2 consecutive VL measurements were required during patients' follow-up.
Assessments
Data were collected at time of switch (baseline), at 3 months (M3), 6 months (M6), 9 months (M9), and 12 months (M12) and included total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides (TG), and estimated glomerular function rate (eGFR), using both the modification of diet in renal disease (MDRD) and the Cockroft and Gault equations and CD4+ cell count.
Plasma HIV-1 RNA was determined using the Abbott m2000SP-m2000RT system (limit of quantification: 40 HIV-1 RNA copies/mL). The reverse-transcriptase (RT) and protease sequencing were performed from plasma or blood sample according to VL as described in the ANRS (Paris, France) consensus methods [18]. Drug resistance mutations as well as genotypic resistance (resistance or possible resistance) were defined and interpreted according to the ANRS drug resistance algorithm version 23 [19]. Indications for switch were evaluated using reports retrospectively fulfilled by clinicians. Symptoms-directed physical examinations were also performed at baseline, and all subsequent visits and these data were abstracted from charts.
Endpoints
The primary endpoint was VF during follow-up period (September 2012 to February 2014), defined as 2 consecutive VL measurements >50 copies/mL at least at 2 weeks apart.
Secondary endpoint was the proportion of patients receiving STR who maintained HIV-1 RNA <50 copies/mL at M12. Other secondary endpoints were changes from baseline (M0) in metabolic parameters (fasting serum lipids [TC, LDL, HDL, TG, ratio TC/HDL]) and eGFR at M6; change from baseline in Framingham score [20] at M6; and change from baseline in CD4+ cell counts at M6 and M12. Improvement of tolerability and adverse events were assessed during routine clinic visit at M3 or M6 (ie, first clinical examination after the switch) and retrospectively collected by chart review.
Pharmacology
Rilpivirine plasma concentrations were retrospectively measured at VF using ultraperformance liquid chromatography combined with tandem mass spectrometry (Waters Corporation, Milford, MA) as previously described [21].
Statistical Analysis
Comparisons at M6 or M12 vs baseline were carried out by Student's t test for paired samples for continuous variables, by McNemar's test for dichotomous variables, and by Bowker's test for categorical and ordinal variables. A P value <.05 was considered as statistically significant. The Kaplan–Meier method was used to estimate the probability for a patient to have a VL <50 copies/mL during his or her follow-up after the time of switch. Patients lost to follow-up, stopping STR, or with VF were censored. Data analysis was carried out by SAS 9.3.
RESULTS
Participants
Three hundred four patients were included in this study. Their main characteristics are shown in Table 1. The median age was 47 years (interquartile range [IQR], 39–54), and 73.4% of patients were male. Patients were treated with cART for a median of 7.7 years (IQR, 3.7–12.7) and had VL <50 copies/mL for a median of 4.9 years (IQR, 2.1–8.2) before STR initiation. The baseline median CD4 cell count was 602 cells/mm3 (IQR, 462–788). The switch to the STR was undertaken in 116 patients (38.2%) due to adverse effects with current ART (including 72 central nervous system-related, mostly EFV-treated [n = 59]) and in 224 patients (73.7%) to simplify the cART regimen.
Table 1.
Demographic and Baseline Characteristics of 304 Patients, ANRS CO3, Aquitaine Cohort
| Baseline Parameter | Total (N = 304) |
|---|---|
| Median age, years (IQR) | 47 (39–54) |
| Gender, % | |
| Male | 73.4 |
| Female | 26.3 |
| Transgender | 0.3 |
| Transmission group, n (%) | |
| MSM | 133 (43.8) |
| Heterosexual | 117 (38.5) |
| Intravenous drug use | 22 (7.2) |
| Other | 32 (10.5) |
| AIDS clinical stage,a n (%) | |
| A | 212 (69.7) |
| B | 57 (18.8) |
| C | 35 (11.5) |
| Tobacco consumption, n (%) | |
| Yes | 119 (40.8) |
| No | 173 (59.2) |
| Missing data | 12 (3.9) |
| HT, n (%) | |
| Yes | 125 (41.1) |
| Lipid parameters, median mg/dL (IQR) | |
| TC | 200 (170–227) |
| LDL | 124 (97–148) |
| HDL | 47 (39–58) |
| Triglycerides | 119 (81–178) |
| TC/HDL ratio | 4.1 (3.3–5.1) |
| Creatinine clearance – MDRD (mL/min/1.73 m2), median (IQR), N = 299 | 105 (92–120) |
| CD4+ cell count (cells/mm3), median (IQR) | 602 (462–788) |
| Nadir CD4+ cell count (cells/mm3), median (IQR) | 252 (152–343) |
| Time since first antiretroviral medication (years), median (IQR) | 7.7 (3.7–12.7) |
| Time since HIV plasma viral load <50 copies/mL (years), median (IQR) | 4.9 (2.1–8.2) |
| cART at switch, n (%) | |
| cART containing TDF | 231 (76.0) |
| 2 NRTI + 1 PI/r | 131 (43.1) |
| 2 NRTI + 1 NNRTI | 108 (35.5) |
| With EFV | 86 (28.3) |
| Without EFV | 22 (7.2) |
| 2 NRTI + 1 INI | 29 (9.5) |
| Other third agent | 36 (11.8) |
| Switch reason | |
| cART simplification | 224 (73.7) |
| Adherence improvement | 89 (29.3) |
| Laboratory abnormalities | 101 (33.2) |
| Dyslipidemiab | 75 (24.7) |
| Other | 26 (8.6) |
| Previous ARV side effects | 116 (38.2) |
| Neurological disorders | 72 (23.7) |
| Patients previously on EFV | 59 (19.4) |
| Patients without EFV | 13 (4.3) |
| Digestive disorders | 25 (8.2) |
| Other side effectsc | 19 (6.2) |
| Other reasonsd | 28 (9.2) |
Abbreviations: AIDS, acquired immune deficiency syndrome; ANRS, Agence Nationale de Recherches sur le SIDA et les Hépatites Virales; ARV, antiretroviral; cART, combined antiretroviral therapy; EFV, efavirenz; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HT, arterial hypertension; INI, integrase inhibitor; IQR, interquartile range; LDL, low-density lipoprotein; MDRD, modification of diet in renal disease; MSM, men who have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PI/r, ritonavir-boosted protease inhibitor; TC, total cholesterol; TDF, tenofovir disoproxil fumarate; TG, triglycerides.
a AIDS stage according to the US Centers for Disease Control and Prevention classification.
b TC >2.0 g/L and/or TG >1.5 g/L and/or prescription of lipid-lowering agents.
c Other side effects: dermatological, gynecological, myalgia, urolithiasis, lypodystrophia.
d Other reasons to switch: statin intolerance, post childbirth, cardiovascular risk factors, weight gain, drug interactions.
Baseline Virology
For 64.5% of patients genotype was available at switch time. Among them, 166 (84.7%) were pretherapeutic genotypes and/or test performed at the time of previous VF; 15 (7.6%) genotypes were performed on whole blood HIV-1 DNA before STR initiation, and 15 (7.6%) genotypes had these 2 features. Thirty of 196 patients with available genotype resistance test results displayed virus with ≥1 drug resistance mutation on RT gene (NRTI, n = 11; NNRTI, n = 8; and both, n = 11). Viruses resistant to at least 1 of the STR components were detected in 25 patients (14 to FTC, 8 to RPV, and 3 to FTC and TDF). Baseline resistance data are summarized in Table 2.
Table 2.
Baseline Prevalence of Resistance to Reverse-Transcriptase Inhibitors
| Genotypic resistance data | N (%) |
|---|---|
| Previous genotypic data analysis | 196 (100.0) |
| Resistance to at least one: | |
| NRTI | 11 (5.6) |
| NNRTI | 8 (4.1) |
| RPV | 2 (1.0) |
| Other | 6 (3.1) |
| Resistance to both NRTI + NNRTI | 11 (5.6) |
| NRTI RAMs | |
| None | 174 (88.8) |
| M184V | 14 (7.1) |
| K65R + M184V | 3 (1.5) |
| Other | 5 (2.6) |
| NNRTI RAMs | |
| None | 177 (90.3) |
| RPV-specific | 8 (4.1) |
| H221Y | 1 (0.5) |
| E138A | 2 (1.0) |
| Y181C pathwaya | 5 (2.6) |
| K103N without RPV RAM | 10 (5.1) |
| Other (179E + 190A) | 1 (0.5) |
Abbreviations: NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; RAMs, resistance-associated mutations; RPV, rilpivirine.
a Associated with others mutations (101E; 179DV + 103N; 101KE + 190A; 103N + 179VF; 221HL).
Efficacy and Resistance
The median virologic follow-up time was 8 months (IQR: 3, 11). Five patients (1.6%) developped VF, with the VFs occuring at 3, 6, 7, 8, and 12 months. Virologic success at M3, M6, M9, and M12 was achieved in 97.2% (95% confidence interval [CI], 94.4–98.6), 95.0% (95% CI, 91.5–97.1), 93.4% (95% CI, 89.9–96.2) and 93.4% (95% CI, 89.9–96.2) of patients, respectively (Figure 1). There was no significant change in CD4 cell count between baseline and M6 and M12 (data not shown).
Figure 1.
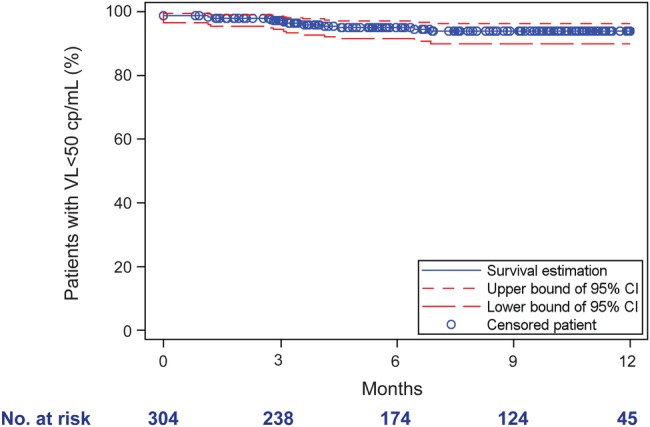
Kaplan–Meier analysis of cumulative rates of patient with a viral load (VL) <50 copies/mL after 12-month follow-up. Kaplan–Meier survival analysis showing the proportion of patients remaining virologically suppressed. The x-axis shows days since initiation of single-tablet regimen (STR): M3 (92 days), M6 (183 days), M9 (274 days), and M12 (365 days). The y-axis shows proportions of participants remaining <50 copies/mL. Numbers at risk and contributing to the analysis at each timepoint are shown below the x-axis.
Regarding VFs, RPV plasma concentration was estimated for 3 of the 5 patients with VF and was shown to be adequate. Their characteristics are reported in Table 3.
Table 3.
Characteristics of Five Patients With Virologic Failure to STR
| Pt | HIV Subtype | NNRTI Prescribed Before Switch | Previous VF Before Switch | cART at Switch | 1st VL >50 (cp/mL) | VL at VF (cp/mL) | Time of VF (Month) | Genotyping Data Before Switch NRTI RAMs; NNRTI RAMs | Genotyping Data at VF NRTI RAMs; NNRTI RAMs | RPV Level at VF (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B | EFV | Yes | FTC/TDF ATV/r |
39 174 | 27 488 | 3.0 | K70R K219K/Q D67N M184V; K103N |
M184V D67N L74V K70R K219Q; L100I K103N H221Ya |
277 |
| 2 | 02_AG | NVP | Yes | FTC/TDF ATV/r |
175 | 119 | 8.4 | L210M; K103KN | Noneb | 109 |
| 3 | 02_AG | No | No | FTC/TDF DRV/r |
168 | 370 | 7.4 | None | None | nd |
| 4 | 02_AG | No | No | FTC/TDF DRV/r |
715 | 89 | 6.0 | None | Noneb | nd |
| 5 | B | No | No | FTC/TDF ATV/r |
64 | 140 | 12.0 | None | Noneb | 122 |
Abbreviations: ART, antiretroviral therapy; ATV, atazanavir; cART, combined antiretroviral therapy; DRV, darunavir; EFV, efavirenz; FTC, emtricitabine; HIV, human immunodeficiency virus; M0, baseline; nd, not determined; NNRTI; nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; r, ritonavir; RAMs, resistance-associated mutations; RPV, rilpivirine; STR, single-tablet regimen; TDF, tenofovir disoproxil fumarate; VF, virological failure; VL, viral load.
a Emergent RAM.
b Genotype was performed on proviral DNA.
Genotypic resistance tests were performed either when physicians prescribed it or retrospectively for the sake of the study. Patient 1, for whom previous historical genotypes showed NNRTI (K103N) and NRTI (K70R, K219K/Q, D67N and M184V) resistance mutations, demonstrated VF at M3 with a genotype showing additional NNRTI resistance mutations (L100I and H221Y). This patient stopped STR and returned to the prior regimen. To date, he remains undetectable. The other patients had no resistance mutations revealed by HIV genotyping at VF or prior STR initiation, except for patient 2 with pre-existing L210M and K103N (Table 3). For this patient, evidence of K103N mutation from historical genotype, probably selected by prior NNRTI therapy (nevirapine), led the clinician to discontinue the STR in favor of a regimen containing FTC/TDF plus boosted-atazanavir. The 3 other patients with VF maintained STR. To date, patients 3 and 4 remain with a VL <50 copies/mL, whereas VF was confirmed for patient 5 (VL = 5004 copies/mL) at M18 with detection of NRTI M184V and K65R and NNRTI E138K mutations on the concomitant HIV RNA genotype. Among 12 patients with a pre-existing K103N mutation in their historical genotypes, 10 maintained virologic suppression, and 2 experienced VF (described above).
Tolerability
Clinical cART tolerability improved in 79 patients (29.9%), particularly neurological side effects in patients switching from an EFV-containing regimen (41 of 86; 47.7%). Twenty-one (6.9%) patients discontinued STR for reasons summarized in Table 4.
Table 4.
Observed Changes at M6 for Patients After Switching and Reasons for STR Discontinuation
| Clinical and biological parameters | N (%) |
|---|---|
| RPV/FTC/TDF improvment (N = 264)a | |
| Clinical tolerance improvement | 79 (29.9) |
| Neurological | 52 (19.7) |
| Patients previously on EFV | 41 (13.5) |
| Patients without EFV | 11 (4.2) |
| Digestive | 19 (7.2) |
| Other | 8 (3) |
| RPV/FTC/TDF discontinuation (N = 304) | 21 (6.9) |
| Virological failureb,c | 2 (0.7) |
| Tolerance | 16 (5.3) |
| Neurological disordersd | 8 (2.6) |
| Digestive disordersb | 7 (2.3) |
| Kidney disorderse | 2 (0.7) |
| Skin rash | 1 (0.3) |
| Nonadherence | 2 (0.7) |
| Pregnancy | 2 (0.7) |
Abbreviations: EFV, efavirenz; FTC, emtricitabine; RPV, rilpivirine; STR, single-tablet regimen; TDF, tenofovir disoproxil fumarate; STR, single-tablet regimen.
a 19 missing data plus 21 STR stop.
b 2 patients presented both neurological and digestive disorders.
c Discontinuation was proposed 3 and 8 months after switching for patients 1 and 2, respectively.
d 1 patient presented both virological failure and neurological disorder.
e 1 Fanconi syndrome and 1 excessive phosphate secretion in the urine.
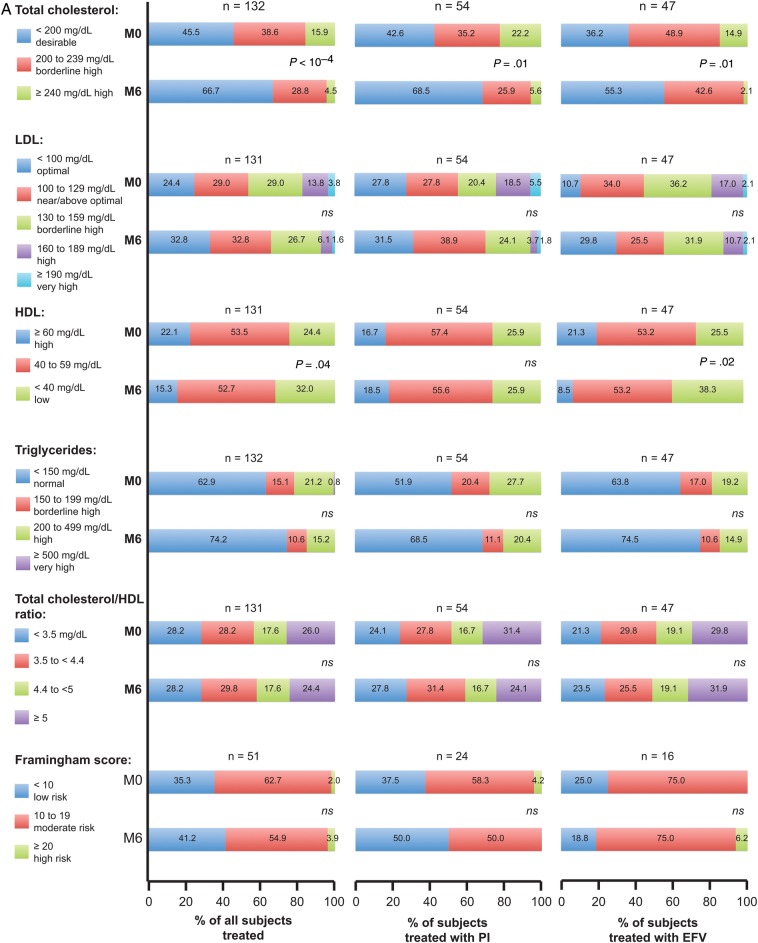
From baseline to M6, there were significant improvements in fasting TC, LDL, and TG. The mean M6 decreases of TC, LDL, and TG were −19, −12, and −27 mg/dL, respectively (P < 10−3). High-density lipoprotein mean level decreased (50 ± 14 mg/dL at baseline vs 46 ± 15 mg/dL at M6 [P < 10−4]), whereas TC/HDL ratio remained stable (4.3 ± 1.2 mg/dL at baseline vs 4.3 ± 1.7 mg/dL at M6 [P = .60]). Categorical analyses of fasting lipids (Figure 2A) demonstrated similar trends, especially for TC. More patients had values in favorable category (<200 mg/dL) at M6 compared with baseline for fasting TC. The differences in TC at M6 remained significant whether the initial third agent was a PI or EFV (P < .05 for both comparisons). A higher percentage of patients who switched off their EFV-containing regimens developed unfavorable HDL levels below the cutoff value of 40 mg/dL at M6 compared with before the switch (38.3% vs 25.5%; P = .03).
Figure 2.
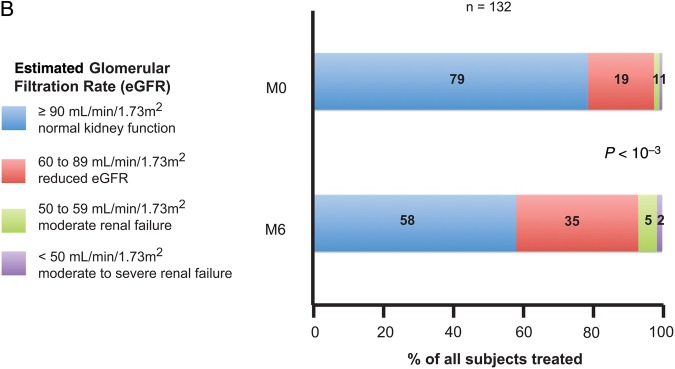
(A) Summary of fasting lipids and Framingham score at baseline and M6: all antiretroviral (ARV), protease inhibitor (PI) regimen, and efavirenz (EFV) regimen before single-tablet regimen (STR) switch. P values for fasting lipid and Framingham parameters compared at baseline vs M6. (B) Summary of creatinine parameters at baseline and M6 for all ARV. Abbreviation: ns, not significant.
When a PI had been previously prescribed, the mean M6 decreases of TC, HDL, LDL, and TG were −20, −1, −13, and −36 mg/dL, respectively (P < 10−4, P = .31, P = .01, P < 10−3, respectively). For patients previously treated with an EFV-based regimen, the median M6 decreases of TC, HDL, and LDL were −22, −7, −12, and −21 mg/dL, respectively (P < 10−4, P < 10−4, P < 10−3, P = .03, respectively).
However, no significant change was observed for Framingham scores between baseline and M6 (Figure 2A), with a median score of 11 (IQR, 8–15) and 10 (6–16), respectively (P = .81). Overall, lipid-lowering agents were prescribed in 52 patients (20.3%) before switching vs 43 (16.8%) on STR regimen (P = .07).
For 132 analyzed patients at M6, the mean decrease of eGFR was −11 mL/min/1.73 m2 (P < 10−4). The median eGFR was 106 ± 22 mL/min/1.73 m2 at baseline and was 94 ± 23 mL/min/1.73 m2 at M6. A statistical trend in favor of this decrease was observed in patients not previously treated with TDF at baseline (−10 ± 14 mL/min/1.73 m2 in TDF treated patients at baseline vs −15 ± 16 mL/min/1.73 m2, respectively; P = .07).
We obtained similar eGFR decrease using a Cockroft and Gault estimation (−9 ± 14 mL/min/1.73 m2; P < 10−4).
An eGFR above 90 mL/min/1.73 m2 was observed for 104 mL/min patients (78.8%) at baseline and 77 patients (58.3%) at M6 (P < 10−4) (Figure 2B). Of 136 patients with an eGFR measurement available at M6, 10 patients (7.3%) had an eGFR < 60 mL/min/1.73 m2, 3 of them having an eGFR <50 mL/min/1.73 m2, which is the recommended threshold to discontinue this STR. For 1 of these 3 patients, the initial eGFR was 46 mL/min/1.73 m2 at baseline, and he was diagnosed with Fanconi's syndrome 10 months after the switch. In addition, 1 patient was diagnosed with phosphate wasting 3 months after the switch. These 2 patients received TDF before the switch for 4 and 10 years, respectively.
DISCUSSION
Our study evaluated the efficacy and tolerability of a STR strategy in 304 HIV-1 virologically suppressed patients switching to RPV/FTC/TDF. Ninety-percent of these patients remained virologically suppressed after 12 months of follow up. Only 5 patients (1.6%) experienced VF.
Our real-world virologic results are comparable with those of the SPIRIT randomized trial that showed a maintained virologic suppression at W48 for 89.3% of patients switching to RPV/FTC/TDF with a low risk of VF (8 VFs (2.5%) at W48) [15]. Thus, switching to this STR is a possible and effective strategy if physicians take into account previous antiretroviral therapy (ART) regimens and historical plasma genotypes preceding the switch. In the absence of documented therapeutic history and/or previous plasma genotypes, the use of resistance genotyping of proviral DNA is possible, but its limitations (missing mutations and limited concordance with plasma genotype) must be taken into account [22, 23]. Results of a previous RNA genotype was available for 84.7% of patients, and DNA genotype was only prospectively determined for 15 subjects. Furthermore, 10 of 12 patients with a pre-existing K103N mutation in their historical genotypes maintained virologic suppression. In the SPIRIT trial, 57% of patients did not harbor any NNRTI mutation after NNRTI failure, and among patients with at least 1 NNRTI mutation only 22% of patients had RPV/FTC/TDF-susceptible viruses. Moreover, among the 24 patients with historical genotypes showing a K103N mutation, 18 were virologically suppressed at week 24 in the immediate switch arm [15]. Similar results were observed in a study evaluating the resistance to RPV/FTC/TDF in pretreated patients with viruses with at least 1 NNRTI mutation (other than for rilpivirine); 22% of the sequences were susceptible to the combination RPV/FTC/TDF [24]. A recent pilot study demonstrated the successful switch to RPV/FTC/TDF of 3 HIV-1-infected women who acquired an isolated K103N mutation during previous NNRTI-based therapy [25]. Altogether, these results suggest that the RPV/FTC/TDF combination may be prescribed after previous VF on an NNRTI-based therapy. However, it is still unclear whether the presence of drug-resistant minority HIV-1 variants that were not detected by bulk sequencing will impact the virologic response in these patients, as has been described for first-generation NNRTIs [26].
Antiretroviral regimen tolerability and convenience are important determinants of adherence [11]. In previous studies with RPV/FTC/TDF, there were no signature toxicities or treatment-limiting side effects associated with the switch. Rilpivirine is a usually well tolerated NNRTI. In our study, the main reason for clinicians to propose a switch to RPV/FTC/TDF was cART simplification. Switching strategy was associated with improved clinical tolerability in 79 patients (29.9%) and mainly neurological side effects in patients switching from an EFV-containing regimen (41 of 86; 47.7%). The nonblinded and nonrandomized design could introduce bias on the part of the participants who may have overstated the symptomatic improvements they experienced after the switch.
Discontinuation of STR was reported for 21 (6.9%) patients, a greater frequency than in the SPIRIT trial in which 7 participants of 297 (2.4%) in the RPV/FTC/TDF immediate switch arm discontinued their treatment due to adverse events [15].
Improvements in fasting lipid level profiles (TC, LDL, and TG) were observed after switching from PI- or NNRTI-regimens to RPV/FTC/TDF. Categorical analyses of fasting lipids demonstrated similar trends, especially for TC. For pretreated patients, cART modification can be an important component of overall cardiovascular risk reduction. This strategy may also allow the discontinuation of lipid-lowering agents, leading to reduced cost and pill burden. These 2 issues need to be considered in the antiretroviral decision-making process [12]. A significant HDL decrease was reported under STR, especially in patients switching from an EFV-based regimen. This decrease was previously reported in the SPIRIT study [15] and in metabolic pooled data of ECHO and THRIVE trials [27]. This HDL level decline associated with TC level decrease explains the TC/HDL ratio stability in our study. Framingham score and Framingham categorical analyses remained stable at M6. However, more data may be needed (only 51 patients were evaluated) as well as longer follow-up to precisely evaluate this dimension.
A greater decrease in eGFR (Cockroft and Gault) was observed in our study at M6 than in the SPIRIT trial (−9.0 mL/min vs −4.4 mL/min) [15]. Our mean eGFR changes were similar to those described in another switch study (FTC/TDF + nevirapine switched to RPV/FTC/TDF) published by Allavena et al [28] with a median decrease in eGFR of −14 mL/min/1.73 m2 (MDRD) at M3. In THRIVE and ECHO phase 3 trials, maximum mean decreases in eGFR were −5 to 9 and −8 to 11 mL/min/per 1.73 m2 from baseline during treatment with rilpivirine to week 48, respectively [6, 7]. In our study, 104 patients (78.8%) had an eGFR above 90 mL/min/1.73 m2 at baseline vs 77 patients (58.3%) at M6 (P < 10−4) (Figure 2B). This difference is very substantial and to date, to the best of our knowledge, had never been previously reported. This decrease does not seem to be linked to TDF prescription because 76% of patients were under TDF at baseline. Moreover, the mean decrease was comparable whether patients were receiving TDF at baseline or not: −10 and −15 mL/min/1.73 m2, respectively. However, a trend in favor of this decrease in patients not previously treated with TDF at baseline was observed (P = .07), although it did not reach statistical significance, possibly due to insufficient sample size. These findings are consistent with the known effects of RPV on the renal Organic Cation Transporter 2 [29]. Inhibition of creatinine secretion by the proximal renal tubule with such transporter inhibitors (rilpivirine, cobicistat, dolutegravir, etc) will require further efforts in estimating the renal GFR. Changes in calculated GFR do not reflect changes in true GFR, as calculated by iohexol clearance [30, 31]. Finally, we reported 2 cases of renal-related discontinuation, both being tubular dysfunctions and both with a history of TDF use. Once again, these cases highlight the need to carefully screen tubular function with appropriate blood and urinary tests. Rilpivirine/emtricitabine/tenofovir disoproxil fumarate should not be started or continued when eGFR is below 50 mL/min/1.73 m2.
As previously discussed, the evaluation of ART tolerability after switching is limited by the retrospective and non-randomized nature of this study. The main limitation of our study is the data analysis of a relatively small group of patients for longitudinal metabolic parameters, 132 of 304 patients for fasting lipid and eGFR parameters at M0 and M6. Moreover, we could anticipate more VFs because the median duration of follow-up was only 8 months. The main strength is the relatively large number of enrolled patients, and, to date, we report on of the largest cohort of patients switching to this STR.
CONCLUSIONS
In summary, switching to an RPV/FTC/TDF regimen in patients with suppressed VL is an effective strategy. Before switching, a careful analysis of the cART history and of available resistance genotypes, along with regular adherence assessment and counseling, will be key contributors to this STR success using antiretroviral agents with a low genetic barrier of resistance such as RPV.
Acknowledgments
We thank all patients who participated in this study. We dedicate this work to Bernard Masquelier (1963–2013), a bright virologist and true mentor in the field. We also want to thank all the members of The Groupe d'Epidémiologie Clinique du SIDA en Aquitaine (GECSA) and all the participants of the ANRS CO3 Aquitaine Cohort.
Financial support. This work was supported by a 5-year grant from the French Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) within the Coordinated Action no. 7 (AC7). Other sources of support include the Bordeaux University Hospital through the COREVIH Aquitaine, INSERM (U897) and the Bordeaux School of Public Health (ISPED).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Azijn H, Tirry I, Vingerhoets J, et al. TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother. 2010;54:718–27. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guillemont J, Pasquier E, Palandjian P, et al. Synthesis of novel diarylpyrimidine analogues and their antiviral activity against human immunodeficiency virus type 1. J Med Chem. 2005;48:2072–9. doi: 10.1021/jm040838n. [DOI] [PubMed] [Google Scholar]
- 3.Janssen PA, Lewi PJ, Arnold E, et al. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J Med Chem. 2005;48:1901–9. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 4.Pozniak AL, Morales-Ramirez J, Katabira E, et al. Efficacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial. AIDS. 2010;24:55–65. doi: 10.1097/QAD.0b013e32833032ed. [DOI] [PubMed] [Google Scholar]
- 5.Wilkin A, Pozniak AL, Morales-Ramirez J, et al. Long-term efficacy, safety, and tolerability of rilpivirine (RPV, TMC278) in HIV type 1-infected antiretroviral-naive patients: week 192 results from a phase IIb randomized trial. AIDS Res Hum Retroviruses. 2012;28:437–46. doi: 10.1089/AID.2011.0050. [DOI] [PubMed] [Google Scholar]
- 6.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378:229–37. doi: 10.1016/S0140-6736(11)60983-5. [DOI] [PubMed] [Google Scholar]
- 7.Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378:238–46. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 8.Porter DP, Kulkarni R, Fralich T, et al. Characterization of HIV-1 drug resistance development through week 48 in antiretroviral naive subjects on rilpivirine/emtricitabine/tenofovir DF or efavirenz/emtricitabine/tenofovir DF in the STaR study (GS-US-264–0110) J Acquir Immune Defic Syndr. 2014;65:318–26. doi: 10.1097/QAI.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 9.Stone VE, Jordan J, Tolson J, et al. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral therapy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36:808–16. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 10.Langebeek N, Sprenger HG, Gisolf EH, et al. A simplified combination antiretroviral therapy regimen enhances adherence, treatment satisfaction and quality of life: results of a randomized clinical trial. HIV Med. 2014;15:286–90. doi: 10.1111/hiv.12112. [DOI] [PubMed] [Google Scholar]
- 11.Gandhi M, Gandhi RT. Single-pill combination regimens for treatment of HIV-1 infection. N Engl J Med. 2014;371:248–59. doi: 10.1056/NEJMct1215532. [DOI] [PubMed] [Google Scholar]
- 12.Prise en charge médicale des personnes vivant avec le VIH. Recommandations du groupe d'experts. Rapport 2013. La documentation française. Paris: 2013. p. 476. Available at: http://www.sante.gouv.fr/IMG/pdf/Rapport_Morlat_2013_Mise_en_ligne.pdf . Accessed 30 March 2014. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/0/ Accessed 19 February 2013. [PubMed]
- 14.European AIDS Clinical Society. EACS Guidelines Version 7.02 – June 2014. Available at: http://www.eacsociety.org/Portals/0/140601_EACS%20EN7.02.pdf . Accessed 14 October 2014.
- 15.Palella FJ, Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS. 2014;28:335–44. doi: 10.1097/QAD.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 16.Mills AM, Cohen C, Dejesus E, et al. Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. HIV Clin Trials. 2013;14:216–23. doi: 10.1310/hct1405-216. [DOI] [PubMed] [Google Scholar]
- 17.Thiebaut R, Morlat P, Jacqmin-Gadda H, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d'Epidemiologie du SIDA en Aquitaine (GECSA) AIDS. 2000;14:971–8. doi: 10.1097/00002030-200005260-00008. [DOI] [PubMed] [Google Scholar]
- 18.Agence Nationale de Recherche sur le SIDA et les Hépatites Virales French recommendation for HIV-1 genotyping. Available at: http://www.hivfrenchresistance.org . Accessed 15 January 2015.
- 19.Agence Nationale de Recherche sur le SIDA et les Hépatites Virales French recommendations for antiretroviral drug resistances. Available at: http://www.hivfrenchresistance.org/2013/Algo-sep-2013.pdf . Accessed 14 October 2014. [DOI] [PubMed]
- 20.Jung BH, Rezk NL, Bridges AS, et al. Simultaneous determination of 17 antiretroviral drugs in human plasma for quantitative analysis with liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2007;21:1095–104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 22.Delaugerre C, Braun J, Charreau I, et al. Comparison of resistance mutation patterns in historical plasma HIV RNA genotypes with those in current proviral HIV DNA genotypes among extensively treated patients with suppressed replication. HIV Med. 2012;13:517–25. doi: 10.1111/j.1468-1293.2012.01002.x. [DOI] [PubMed] [Google Scholar]
- 23.Wirden M, Soulie C, Valantin MA, et al. Historical HIV-RNA resistance test results are more informative than proviral DNA genotyping in cases of suppressed or residual viraemia. J Antimicrob Chemother. 2011;66:709–12. doi: 10.1093/jac/dkq544. [DOI] [PubMed] [Google Scholar]
- 24.Lambert-Niclot S, Charpentier C, Storto A, et al. Rilpivirine, emtricitabine and tenofovir resistance in HIV-1-infected rilpivirine-naive patients failing antiretroviral therapy. J Antimicrob Chemother. 2014;69:1086–9. doi: 10.1093/jac/dkt463. [DOI] [PubMed] [Google Scholar]
- 25.Rokx C, Verbon A, Rijnders B. Successful switch to rilpivirine/tenofovir/emtricitabine in HIV-1-infected patients with an isolated K103N mutation acquired during prior nonnucleoside reverse transcriptase inhibitor therapy. HIV Med. 2014 doi: 10.1111/hiv.12157. doi:10.1111/hiv.12157. [DOI] [PubMed] [Google Scholar]
- 26.Li JZ, Paredes R, Ribaudo HJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA. 2011;305:1327–35. doi: 10.1001/jama.2011.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tebas P, Sension M, Arribas J, et al. Lipid levels and changes in body fat distribution in treatment-naive, HIV-1-infected adults treated with rilpivirine or efavirenz for 96 weeks in the ECHO and THRIVE trials. Clin Infect Dis. 2014;59:425–34. doi: 10.1093/cid/ciu234. [DOI] [PubMed] [Google Scholar]
- 28.Allavena C, Dailly E, Reliquet V, et al. Switching from tenofovir/emtricitabine and nevirapine to a tenofovir/emtricitabine/rilpivirine single-tablet regimen in virologically suppressed, HIV-1-infected subjects. J Antimicrob Chemother. 2014;69:2804–8. doi: 10.1093/jac/dku187. [DOI] [PubMed] [Google Scholar]
- 29.Moss DM, Liptrott NJ, Curley P, et al. Rilpivirine inhibits drug transporters ABCB1, SLC22A1, and SLC22A2 in vitro. Antimicrob Agents Chemother. 2013;57:5612–8. doi: 10.1128/AAC.01421-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi P, Montinaro V, Rusconi S, et al. The problem of renal function monitoring in patients treated with the novel antiretroviral drugs. HIV Clin Trials. 2014;15:87–91. doi: 10.1310/hct1503-87. [DOI] [PubMed] [Google Scholar]
- 31.Capetti A, Rizzardini G. Cobicistat: a new opportunity in the treatment of HIV disease? Expert Opin Pharmacother. 2014;15:1289–98. doi: 10.1517/14656566.2014.920008. [DOI] [PubMed] [Google Scholar]