Abstract
The diagnosis of histoplasmosis in patients with human immunodeficiency virus in southern Africa is complicated by the nonspecific presentation of the disease in this patient group and the unavailability of sensitive diagnostics including antigen assays. Treatment options are also limited due to the unavailability of liposomal amphotericin and itraconazole, and the inability to perform therapeutic drug monitoring further confounds management. We present 3 clinical cases to illustrate the limits of diagnosis and management in the southern African context, and we highlight the need for additional diagnostic tools and treatment options in resource-limited settings.
Keywords: AIDS, developing countries, disseminated progressive histoplasmosis, fluconazole, HIV, itraconazole, liposomal amphotericin B, low- and middle-income country, opportunistic infection, resource-limited settings
Histoplasmosis, although not endemic in southern Africa, has emerged, in the context of the human immunodeficiency virus (HIV) epidemic, as an opportunistic infection (OI) of potential importance [1–3]. Histoplasmosis in patients with advanced HIV most commonly manifests as disseminated progressive disease (DPH), usually with a subacute presentation [4], but it can also present fulminantly with shock and altered mental status [5]. In the absence of skin or mucus membrane lesions, the presenting symptoms and signs of DPH closely mimic OIs such as tuberculosis which because of its high prevalence is often treated empirically in the developing world. Importantly, unless DPH is promptly diagnosed and treated, in persons with HIV it is associated with a high mortality [4].
The actual burden of histoplasmosis in patients infected with HIV in southern Africa remains largely unknown, owing to at least 2 factors: (1) the often nonspecific presentation when skin lesions are absent and (2) the lack of access to sensitive diagnostic tests, notably the Histoplasma capsulatum antigen assay [6]. As a result, DPH is an OI that is likely underrecognized [7]. To underscore the challenges associated with the diagnosis and management of DPH among patients with HIV in southern Africa, we present cases encountered in routine clinical care.
Patient 1
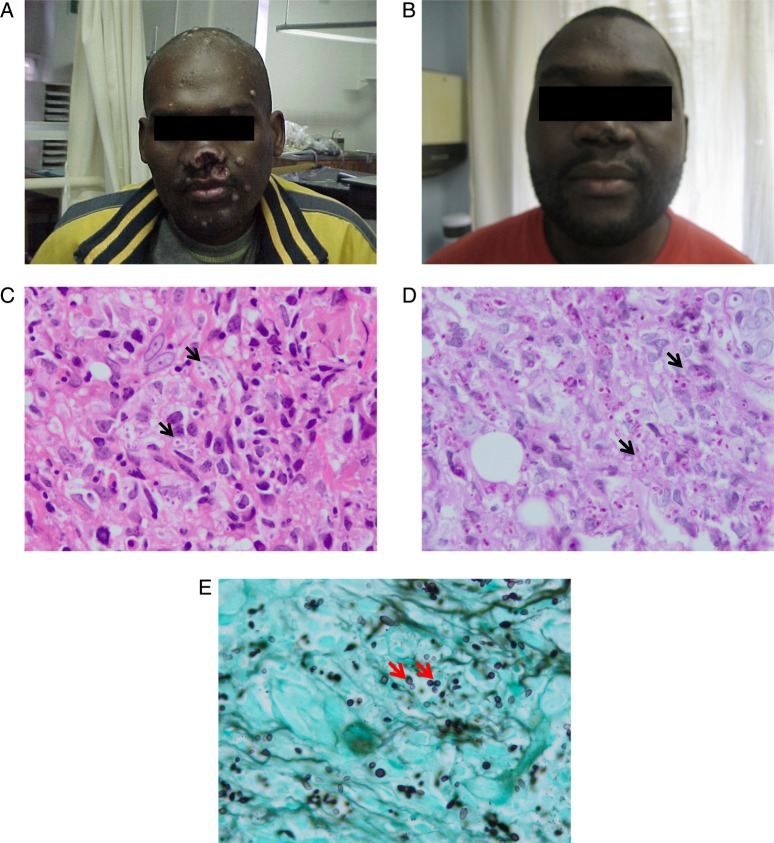
A 36-year-old male with advanced HIV (CD4 cell count 14 cells/mm3) was admitted to a hospital in KwaZulu-Natal, South Africa with skin lesions for 4 weeks and altered mental status and vomiting for 1 week. The patient began antiretroviral therapy (ART) 12 weeks before admission, when he was clinically well. In the hospital, he was disorientated, febrile, and had oral candidiasis. His skin exam revealed flesh-colored nodules. Smaller nodules were scattered throughout his chin and chest, and 1 erosive lesion was on his forehead (Figure 1A). An abdominal ultrasound revealed abdominal lymphadenopathy and hepatosplenomegaly. Cerebrospinal fluid (CSF) pressure, chemistry, and cell count were normal, and a CSF cryptococcal antigen test was negative. The patient was placed on 200 mg daily dose of fluconazole (for candidiasis), and he received high-dose cotrimoxazole as treatment for suspected central nervous system toxoplasmosis. A skin biopsy was obtained. The patient deteriorated rapidly within 24 hours of admission, with the development of hypotension and worsening mental status, and he died. A shave biopsy later revealed an infiltrate of histiocytes containing innumerable small ovoid yeast-like organisms measuring 2–4 µm in diameter. Clear halos surrounded many of the organisms both within histiocytes and free in the dermis. Admixed lymphocytes and plasma cells were also noted (Figure 1B).
Figure 1.
A, Profile and forehead upon hospital presentation. B, A shave biopsy later revealed a diffuse infiltrate of histiocytes within which innumerable small ovoid yeast-like organisms measuring 2–4 µm in diameter. Clear halos surrounded many of the organisms both within histiocytes and free in the dermis. Admixed lymphocytes and plasma cells were noted.
Patient 2
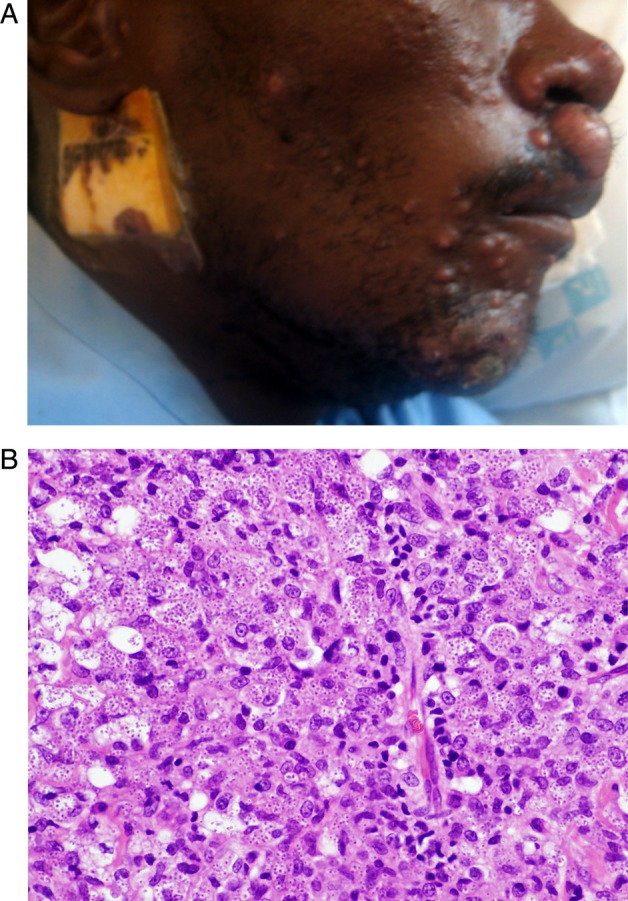
A 35-year-old male was admitted to a teaching hospital in KwaZulu-Natal with a 2-month history of pruritic skin ulcerations. At admission, the patient was in his fourth month of therapy for tuberculous lymphadenitis. Exam revealed oral candidiasis, an ulcer involving the tongue, several ulcerated nodules over the face, legs, and hands, and destructive lesions of the nasal septum and nostrils (Figure 2A). He was found to have a CD4 cell count of 14 cells/µL. Liver function tests showed an alkaline phosphatase of 172 U/I and γ-glutamyl transpeptidase of 118 U/I. Cerebrospinal fluid was normal. Histology of the skin biopsy showed intracellular yeast forms (Figure 2C–E). Gram stain of the skin biopsy demonstrated yeast cells identified as H capsulatum var. duboisii by culture. The patient was commenced on itraconazole 200 mg/day 3 times daily. Rifampicin was stopped due to the potential interaction with itraconazole. He completed 1 year of itraconazole at 200 mg twice daily after which he was switched to fluconazole 200 mg twice daily. He initiated stavudine, lamivudine, and efavirenz 2 months after HIV diagnosis. The cutaneous lesions healed within 9 months (Figure 2B).
Figure 2.
A and B, Front profile before (A) and after (B) antifungal treatment sections of the skin biopsy demonstrate a diffuse infiltrate of histiocytes with voluminous cytoplasm and scattered uninucleate and multinucleate giant cells (C, haematoxylin & eosin). Conspicuous intracellular yeast forms, measuring approximately 8–12 µm with prominent, refractile cell walls were conspicuous on haematoxylin and eosin stains (arrows, C) and were highlighted on periodic acid Schiff staining (arrows, D). Gomori Grocott methenamine silver staining confirmed budding yeast forms with a ‘figure-of-eight’ configuration (arrows, E).
Patient 3
A 45-year-old male with HIV (CD4 cell count 29 cells/µL) was admitted to a teaching hospital in Durban, KwaZulu-Natal, South Africa for management of biopsy-proven histoplasmosis. Five months earlier, the patient had presented to a local hospital with epigastric pain, vomiting, and bloody diarrhea and an endoscopy had revealed a perforated duodenal ulcer. The current examination was notable for cervical lymphadenopathy, hepatomegaly, and splenomegaly. There were no oral or skin lesions. Ultrasound of the abdomen revealed left paraortic and splenic hilar lymphadenopathy. In addition, there were multiple splenic hypodensities. A solid mass was noted in the hepatic flexure of the colon. These findings were believed to be suggestive of extrapulmonary tuberculosis, and the patient began empiric antituberculous therapy followed by combination ART. To confirm the diagnosis, the left cervical lymph node was biopsied and a colonoscopy was performed, revealing a 1.5 cm deep ulcer with hypertrophic edges in the hepatic flexure. The biopsy showed ulcerated colonic mucosa with the lamina propria expanded by histiocytes, containing yeasts compatible with histoplasmosis. Lymph node biopsy revealed a histiocyte-rich inflammatory cell infiltrate with numerous fungi morphologically consistent with histoplasmosis. The patient was given amphotericin B for 14 days followed by maintenance therapy with oral fluconazole 800 mg/daily for a period of 30 days. Antituberculous treatment was continued. However, the patient died before treatment completion from sepsis, reportedly from Acinetobacter baumannii bacteremia.
DISCUSSION
For febrile patients with advanced HIV in southern African—with or without characteristics skin lesions—DPH must be considered as a potentially lethal systemic infection. However, as a result of major gaps in diagnostics, DPH is likely an underrecognized cause of death in this region, as it is in many parts of the world. According to some authors, a vicious cycle has emerged in resource-limited settings whereby the absence of diagnostics and clinical awareness of histoplasmosis in the setting of acquired immune deficiency syndrome has led to under diagnosis and missed opportunities to treat this potentially important OI [7].
Without characteristic skin lesions, the diagnosis of DPH is particularly challenging, because the presentation is nonspecific and difficult to distinguish from more common OIs such as extrapulmonary tuberculosis, a disease which may also be present as a copathogen [8]. Mucocutaneous manifestations of DPH, although common in some series, are not a sensitive sign in DPH and may be present in as few as 10% of patients. This makes the diagnosis of DPH highly reliant on laboratory investigations that are not readily available. Furthermore, low autopsy rates may allow many cases of HIV-associated DPH to go unrecognized, further contributing to the underestimation of the burden of DPH as an OI in resource-limited settings. Most importantly, although histopathology and culture are the diagnostic gold standards, delays in obtaining positive laboratory confirmation impact negatively on patient management.
Histoplasma capsulatum antigen detection assays, which are inaccessible in southern Africa, are central to the rapid diagnosis of DPH in patients infected with HIV. These assays can be applied to urine or serum and, among patients with HIV infection, have reported sensitivities of more than 85% [9]. Antigen detection assays offer a rapid turnaround time (usually 24 hours) compared with histology or culture, which allows for early initiation of treatment [6]. Additional advantages of quantitative antigen assays include avoiding invasive biopsies, monitoring response to treatment, and determining the timing of treatment termination. Advantages also include the early detection of relapse, which is of particular concern in patients with HIV.
In resource-limited settings, an additional challenge is that key agents in the optimal treatment of moderate or severe DPH are not readily available, specifically liposomal amphotericin and itraconazole. The treatment of choice for moderate to severe DPH in patients with HIV is liposomal amphotericin for at least 14 days, with some experts advocating a longer course in patients with a slow clinical response [10]. Although liposomal amphotericin B is associated with a faster clinical response and less toxicity than amphotericin B deoxycholate (0.7–1 mg/kg per day), outcomes with deoxycholate are comparable. Due to its accessibility, amphotericin B deoxycholate is the drug of choice for moderate to severe disease in southern Africa. For mild to moderate disease, the current recommendation places itraconazole above fluconazole. Itraconazole demonstrated an 85% response in 1 study whereas fluconazole showed a 74% response in a separate study [11, 12]. In the absence of a head-to-head comparison, and considering the excellent pharmacokinetic properties of fluconazole, which eliminates the need for therapeutic drug monitoring, fluconazole arguably becomes a reasonable choice in resource-constrained settings.
Maintenance antifungal therapy is important to prevent relapse in HIV-associated DPH and should be provided in combination with ART. As maintenance therapy, fluconazole is inferior to itraconazole with drug resistance accounting for some fluconazole treatment failure [13]. Nonetheless, because it is less costly and more readily availability, fluconazole is used as maintenance therapy in resource-limited settings. In the absence of itraconazole, it might be prudent to consider higher doses of fluconazole, up to 800 mg/daily, as maintenance therapy given the reassuring experience with the use of high-dose fluconazole in the treatment of cryptococcal meningitis [14].
Monitoring the response of DPH to treatment in patients infected with HIV in resource-limited settings involves careful attention to clinical parameters (such as weight gain) and resolution of clinical signs attributable to the disease. Guidelines recommend that serum or urine histoplasma antigen levels be used as sensitive measures of response to treatment and to detect early relapse [15, 16]. In the absence of these tools, discontinuation of treatment and early detection of relapse become extremely challenging.
CONCLUSIONS
In conclusion, although HIV-associated DPH has been described in southern Africa and other resource-limited settings, the unavailability of sensitive diagnostic tools make the actual burden of HIV-associated DPH unknown. Difficulty in differentiating DPH from more common OIs, such as tuberculosis, potentially leads to misdiagnosis and inappropriate treatment. The lack of optimal treatment options should encourage critical interrogation of guidelines to facilitate optimal treatment within resource constraints. There is an urgent need to make diagnostic, therapeutic, and monitoring tools available for this disease in areas of the world where histoplasmosis is known to be an HIV-associated OI of potential importance. Finally, studies are required to evaluate the benefit of high-dose fluconazole in management of DPH.
Acknowledgments
We gratefully acknowledge the generosity of the patients who participated in this research.
Author contributions. R. A. M. and M.-Y. S. M. conceived the study. R. A. M., L. G., T. C. M., and C. C. collected the primary data. P. K. R. and M.-Y. S. M. provided the pathological descriptions. R. A. M. and C. C. drafted the manuscript. R. A. M. and M.-Y. S. M. critically revised the manuscript for intellectual content. All authors read and approved the final manuscript. R. A. M. and M.-Y. S. M. are guarantors of the paper.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.K Ramdial P, Mosam A, Dlova NC, et al. Disseminated cutaneous histoplasmosis in patients infected with human immunodeficiency virus. J Cutan Pathol 2002; 29:215–25. [DOI] [PubMed] [Google Scholar]
- 2.Pillay T, Pillay DG, Bramdev A. Disseminated histoplasmosis in a human immunodeficiency virus-infected African child. Pediatr Infect Dis J 1997; 16:417–8. [DOI] [PubMed] [Google Scholar]
- 3.Gumbo T, Just-Nubling G, Robertson V, et al. Clinicopathological features of cutaneous histoplasmosis in human immunodeficiency virus-infected patients in Zimbabwe. Trans R Soc Trop Med Hyg 2001; 95:635–6. [DOI] [PubMed] [Google Scholar]
- 4.Wheat LJ, Kauffman CA. Histoplasmosis. Infect Dis Clin North Am 2003; 17:1–19, vii. [DOI] [PubMed] [Google Scholar]
- 5.Vathesatogkit P, Goldenberg R, Parsey M. A 27-year-old HIV-infected woman with severe sepsis and pulmonary infiltrates. Disseminated histoplasmosis with severe sepsis and acute respiratory failure. Chest 2003; 123:272–3, 274–276. [DOI] [PubMed] [Google Scholar]
- 6.Durkin MM, Connolly PA, Wheat LJ. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J Clin Microbiol 1997; 35:2252–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nacher M, Adenis A, Mc Donald S, et al. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis 2013; 7:e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agudelo CA, Restrepo CA, Molina DA, et al. Tuberculosis and histoplasmosis co-infection in AIDS patients. Am J Trop Med Hyg 2012; 87:1094–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams B, Fojtasek M, Connolly-Stringfield P, et al. Diagnosis of histoplasmosis by antigen detection during an outbreak in Indianapolis, Ind. Arch Pathol Lab Med 1994; 118:1205–8. [PubMed] [Google Scholar]
- 10.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58:1–207; quiz CE201–204. [PubMed] [Google Scholar]
- 11.Wheat J, MaWhinney S, Hafner R, et al. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Acquired Immunodeficiency Syndrome Clinical Trials Group and Mycoses Study Group. Am J Med 1997; 103:223–32. [DOI] [PubMed] [Google Scholar]
- 12.Wheat J, Hafner R, Korzun AH, et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. AIDS Clinical Trial Group. Am J Med 1995; 98:336–42. [DOI] [PubMed] [Google Scholar]
- 13.Wheat LJ, Connolly P, Smedema M, et al. Emergence of resistance to fluconazole as a cause of failure during treatment of histoplasmosis in patients with acquired immunodeficiency disease syndrome. Clin Infect Dis 2001; 33:1910–3. [DOI] [PubMed] [Google Scholar]
- 14.Pappas PG, Chetchotisakd P, Larsen RA, et al. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV-associated cryptococcal meningitis. Clin Infect Dis 2009; 48:1775–83. [DOI] [PubMed] [Google Scholar]
- 15.Goldman M, Zackin R, Fichtenbaum CJ, et al. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin Infect Dis 2004; 38:1485–9. [DOI] [PubMed] [Google Scholar]
- 16.Hay RJ. Mycotic Infections. In: Cook GC, Alimuddin IZ, eds. Manson's Tropical Diseases. 22nd ed Saunders; 2009. [Google Scholar]