Abstract
Background. Tuberculosis (TB) control is a public health priority with 3 million cases unrecognized by the public health system each year. We assessed the impact of improved TB diagnostics and on-site training on TB case detection and treatment outcomes in rural healthcare facilities.
Methods. Fluorescence microscopy, Xpert MTB/RIF, and on-site training were introduced at 10 healthcare facilities. Using quasi-experimental methods, these 10 intervention healthcare facilities were compared with 2 controls and their own performance the previous year.
Results. From January to October 2012, 186 357 and 32 886 outpatients were seen in the 10 intervention and 2 control facilities, respectively. The intervention facilities had a 52.04% higher proportion of presumptive TB cases with a sputum examination (odds ratio [OR] = 12.65; 95% confidence interval [CI], 5.60–28.55). After adjusting for age group and gender, the proportion of smear-positive patients initiated on treatment was 37.76% higher in the intervention than in the control facilities (adjusted OR [AOR], 7.59; 95% CI, 2.19–26.33). After adjusting for the factors above, as well as human immunodeficiency virus and TB retreatment status, the proportion of TB cases who completed treatment was 29.16% higher (AOR, 4.89; 95% CI, 2.24–10.67) and the proportion of TB cases who were lost to follow-up was 66.98% lower (AOR, 0.04; 95% CI, 0.01–0.09). When compared with baseline performance, the intervention facilities had a significantly higher proportion of presumptive TB cases with a sputum examination (64.70% vs 3.44%; OR, 23.95; 95% CI, 12.96–44.25), and these facilities started 56.25% more smear-positive TB cases on treatment during the project period (AOR, 15.36; 95% CI, 6.57–35.91).
Conclusions. Optimizing the existing healthcare workforce through a bundled diagnostics and on-site training intervention for nonphysician healthcare workers will rapidly improve TB case detection and outcomes towards global targets.
Keywords: fluorescence microscopy, health systems strengthening, passive case finding, sub-Saharan Africa, task shifting, tuberculosis, Xpert
Tuberculosis (TB) remains a global epidemic with almost 9 million new cases in 2013 and 1.5 million deaths; 360 000 of these were human immunodeficiency virus (HIV)-associated TB deaths [1]. In sub-Saharan Africa (SSA), 39% of all TB cases are estimated to be coinfected with HIV. Passive TB case detection is defined as detection of active TB disease among symptomatic patients who present to medical service for diagnosis of symptoms [2]. In rural healthcare facilities across much of SSA, many presumptive TB patients are not screened due to high vacancy rates for medical doctor positions [3–6] or absenteeism [7]. Medical doctors are the healthcare cadre most often trained to diagnose TB and initiate treatment. The cascade of TB care and treatment requires strong healthcare systems; healthcare professionals must recognize patients with TB symptoms, order and perform smear microscopy or other tests, link test results to the patient, and give 6–8 months of uninterrupted TB treatment. To meet the human resource needs to address both the TB and the HIV epidemic, nurses and clinical officers will need to be specifically trained to diagnose and treat both HIV and TB. In several randomized trials on HIV care, task shifting (per the World Health Organization [WHO] definition [8]) to nonphysician clinicians resulted in HIV care outcomes that were equivalent to physicians [9–12].
Active case detection outside of healthcare centers has been shown to be effective in finding new TB cases, but it requires additional resources from national TB programs [13–16]. In a Ugandan study, 76.5% of the TB cases detected through home visits had previously visited a healthcare facility for cough [15]. These data suggest that improving healthcare facility-based identification of patients who should be tested for TB could improve TB control. The increased number of patients who would need to be screened, however, would require a more rapid and sensitive screening diagnostic tool than traditional light microscopy. In a meta-analysis, fluorescence microscopy (FM) has been shown to have 10% increased sensitivity with significantly less time required to review each slide [17]. Xpert MTB/RIF, a rapid molecular diagnostic test whose sensitivity is comparable to culture but has a shorter turnaround time (2 hours), is being actively used in many countries and has been proposed for decentralized use. Data from South Africa have shown that Xpert MTB/RIF shortens the time to treatment, but it has limited impact on TB outcomes due to high rates of empirical treatment [18]. Data on rural use in other countries in SSA are still lacking.
We sought to assess the impact of introducing the following bundled intervention: FM and Xpert MTB/RIF diagnostics combined with on-site clinical training for mid-level healthcare practitioners (MLPs) on the number of TB patients identified and treated in rural healthcare facilities.
METHODS
Study Design
This was a prospective, quasi-experimental study of a bundled laboratory diagnostics and on-site training intervention. Using data collected from an electronic data collection system, we compared the 10 healthcare facilities that received the intervention with the 2 healthcare facilities that did not receive the intervention in the following areas: TB case detection, TB treatment outcomes, and a set of TB and TB/HIV quality-of-care indicators. The primary outcomes were TB case detection and treatment outcomes, which were measured using 4 facility performance indicators. The secondary outcomes were quality of TB and TB/HIV diagnosis and care, as measured by 6 facility performance indicators. We also compared each healthcare facility that received the intervention to its own baseline performance in the previous year using deidentified outpatient data and TB data collected by a previous project called the Infectious Diseases Integrated Infectious Diseases Capacity Building Evaluation (IDCAP) program [19].
Setting, Participants, and Eligibility
Twenty cross-border and underserved (as evidenced by having the lowest district TB notification rates) patients were purposively selected from the 36 facilities in the IDCAP intervention. Although 10 control facilities were initially randomized for inclusion, 8 facilities were eliminated by the TB REACH secretariat (sponsor WHO). Two remaining control facilities were selected from the 10 control facilities using a Microsoft Excel random number generator. To address the shortage of control facilities, we also decided to compare each facility to its own baseline historical performance. We were able to accomplish this task because we had routinely collected electronic data from this time period as a result of IDCAP that was available for analysis. In the 12 study facilities (10 intervention, 2 control), electronic data capturing systems, basic laboratory infrastructure, and electrical capacity had already been established (Figure 1) [19, 20]. Healthcare facilities were staffed with MLPs such as clinical officers, nurses, and laboratory staff personnel with 2–3 years tertiary education equivalent to a higher diploma. None of the healthcare facilities had any other active programs to increase TB diagnosis.
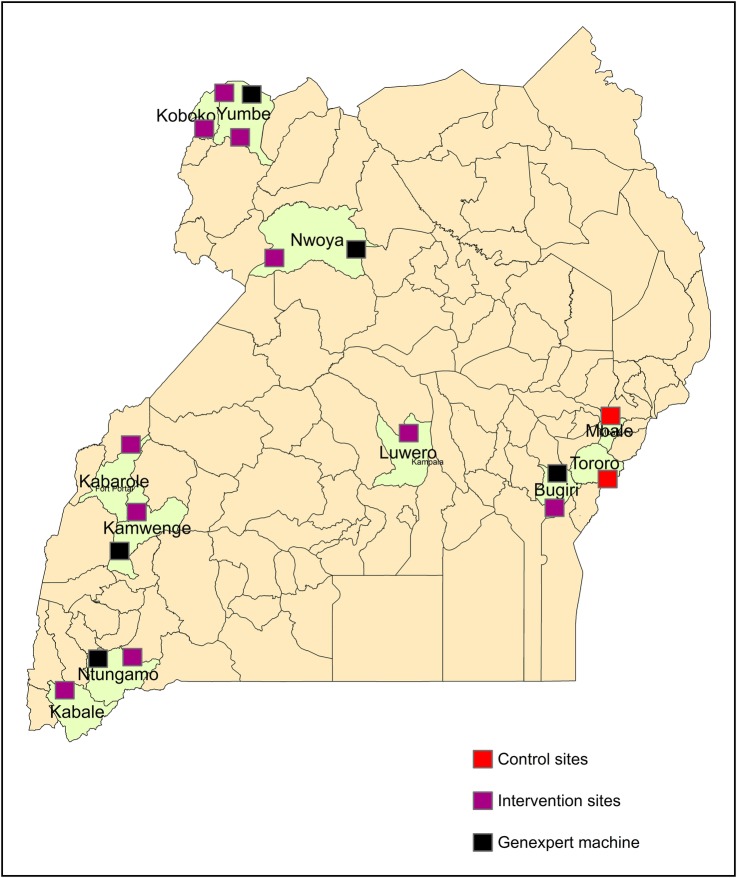
Figure 1.
Map of project health facilities: purple boxes show project healthcare facilities, red boxes show control healthcare facilities, and black boxes show the location of Xpert MTB/RIF machines.
Project Description
The project equipped and improved the capacity of the 10 intervention healthcare laboratories to conduct diagnostic tests for TB using FM (iLEDPrimostar, Zeiss). Laboratory staff personnel, all of whom were qualified microscopists, were trained in FM (Figure 2). Training involved an initial 2-day regional workshop carried out over a weekend, to minimize clinical disruptions, followed by practical hands-on training at the healthcare facilities.
Figure 2.
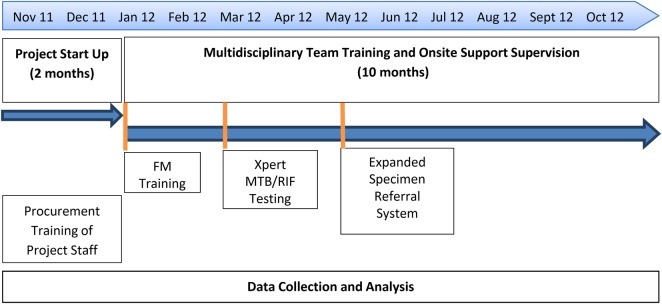
Timeline of the components of the training and health systems strengthening.
On-site support (OSS) visits were conducted over a 9-month period (from January to October 2012) to reinforce the learned laboratory skills and to deliver the clinical training sessions. Each OSS visit lasted 2 days. The first day of each OSS included a multidisciplinary clinical training session followed by a cadre-specific clinical training session. Six modules on TB, which covered key areas of TB diagnosis and case management, were delivered during the OSS visits.
On the second afternoon, a session on data-driven continuous quality improvement was provided for all healthcare center staff. Data from the TB clinical and laboratory registers and outpatient medical encounter form were used to identify bottlenecks to TB diagnosis and treatment and to develop quality improvement work plan solutions.
In March 2012, Xpert MTB/RIF testing (Cepheid, Toulouse, France) was introduced at 5 of the intervention facilities (Figure 1). Introduction of Xpert MTB/RIF testing was delayed because additional electrical support, including additional solar panels and power stabilizers, were required for optimal operation [21]. All personnel at intervention healthcare facilities received on-site training for this technique. In this project, FM was recommended for use on all presumptive TB cases, and serial Xpert MTB/RIF tests were recommended for use only in smear-negative, HIV-positive presumptive TB cases, per WHO policy [22]. To maximize the usage of the machine, laboratory technicians from the 5 intervention facilities without Xpert MTB/RIF were given transport reimbursement to bring specimens to Xpert MTB/RIF facilities, run the test, and take results back to their healthcare facilities in a diagnostic hub approach.
Variable Definitions and Data Sources
The definitions of each of the outcome indicators are in Supplementary Table 1, which reports the numerator, denominator, and data source for each of the indicators. Indicators were chosen based on the WHO guidance for monitoring and evaluating national TB programs [23]. The primary outcomes were TB case detection as measured by (1) the proportion of presumptive TB cases with sputum smear and (2) the proportion of TB cases started on treatment; and TB treatment outcomes as measured by (3) the proportion of TB patients completing TB treatment and (4) the proportion of TB patients who were lost to follow-up, of all those with a TB treatment outcome recorded. Data sources included the following: routinely collected Ministry of Health data from a modified version of the outpatient form (modified medical form 5 (MF5) [24]), the National Tuberculosis and Leprosy Program (NTLP) Laboratory Register, and the NTLP TB Unit Register.
Presumptive TB cases (ie, patients with signs and symptoms of TB based on the Ministry of Health guidelines for intensified TB case finding as recorded on the outpatient encounter form) were defined as patients with cough for more than 2 weeks, or history of cough less than 2 weeks, and either weight loss or night sweats, or a child who had contact with a TB patient.
Six indicators were used to measure the quality of TB and TB/HIV diagnosis and care, the secondary outcomes of the study. These 6 indicators are as follows: (1) proportion of presumptive TB cases with an HIV test, (2) proportion of smear-positive presumptive TB cases, (3) proportion of TB patients tested for HIV, (4) proportion of TB/HIV-coinfected patients started on antiretroviral therapy (ART), (5) proportion of TB patients who are smear or bacteriologically positive, and (6) proportion of TB patients who are sputum smear negative or extrapulmonary TB cases.
For 3 indicators (proportion of presumptive TB cases with a sputum smear, proportion of presumptive TB cases with an HIV test, and proportion of smear or bacteriologically positive TB cases) different data sources were used for the historical IDCAP period and TB REACH. During the IDCAP project, only sputum smear-positive cases were extracted from the NTLP Laboratory Register; therefore, for the proportion of presumptive TB cases with a sputum smear and proportion of smear or bacteriologically positive TB cases, we used the MF5 rather than the NTLP Laboratory Register as the data source for the numerator. Moreover, during the IDCAP project, HIV test results were not recorded in the NTLP Laboratory Register; therefore, for the proportion of presumptive TB cases with an HIV test, we used the MF5 as the data source for the numerator.
Data Collection
For the project period, January–October 2012, routinely collected patient data from the MF5, the NTLP Laboratory Register, and the NTLP Unit register were entered into electronic replicas of these forms or registers by a site data entrant. The timing of data entry and the progression of patients through the healthcare center could also be monitored with the monthly transmission of password-protected data through Blackberry 3G data networks [20, 24]. In June 2013, data entry personnel returned to the sites to collect data on TB treatment outcomes for all those who started treatment between January and October 2012. Historical data collected from September 2010 to August 2011 by the IDCAP project, which used the same forms or registers and data collection processes, were used as the baseline data.
Sample Size
Sample size was calculated to detect a 50% change, from 15% to 65% in proportion of presumptive TB cases with a sputum smear, with a power of 80% and 5% level of significance.
Statistical Methods
The effect of the intervention was assessed by comparing the intervention and control facilities during the intervention period and by comparing the intervention facilities to themselves during the baseline period. Individual level analysis was not possible for 2 indicators (ie, proportion of presumptive TB cases with sputum smear and proportion of presumptive TB cases with an HIV test) because the different data sources used for the numerator and denominator could not be linked (Supplementary Table 1). For these indicators, we used a generalized linear model with an extension to the binomial family, using healthcare facility month as the unit of analysis and study arm as the independent variable, clustering by healthcare facility with robust standard errors to adjust for overdispersion. For the remaining 8 indicators, we used a multivariable logistic regression model, with individual patients as the unit of analysis and study arm as the independent variable. In the adjusted model, we adjusted for age group (over or under 14), gender, HIV status, TB retreatment, and clustering by healthcare facility. The adjustments to demographic variables differed by indicator due to data availability. Specific variables used for each indicator are listed in Supplementary Table 1. These same models were also used to compare proportions among the intervention facilities from the baseline period to the intervention period. Data analysis was done using the Stata statistical package (version 11.2; StataCorp, College Station, TX).
Ethics Statement
This protocol was reviewed and approved by the Scientific Review Committee of the Infectious Diseases Institute, the Institutional Review Boards of Joint Clinical Research Center, Johns Hopkins University, and the Uganda National Council for Science and Technology. A waiver was obtained for individual informed consent because these data are routinely collected by the Ministry of Health, and the electronic database used for analysis did not contain identifying characteristics of patients.
RESULTS
Comparison of Intervention and Control Healthcare Facilities
From January 2012 to October 2012, 186 357 patients were seen in the outpatient department of the 10 project facilities (mean, 1834 patients/month) and 32 886 patients were seen in the 2 control facilities (mean, 1397 patients/month). The outpatient volume, gender, and age mix as well as the baseline TB services provided at the intervention and control facilities are shown in Table 1. Although a similar proportion of outpatients fulfilled the definition for a presumptive TB case in the control facilities compared with the intervention facilities (5.86% vs 3.46%) during the study period, only 12.66% of presumptive TB cases in control facilities had a sputum smear performed compared with 64.70% in the intervention facilities (odds ratio [OR], 12.65; 95% confidence interval [CI], 5.60–28.55) (Table 2). A breakdown by healthcare center (intervention and control) shows that this proportional increase is reflected across all intervention facilities (Supplementary Table 1). The proportion of presumptive TB cases who were tested for HIV was also significantly higher in intervention compared with control facilities (63.37% vs 5.29%; OR, 30.97; 95% CI, 5.11–187.94) (Table 2). Among presumptive TB cases, the proportion of smear-positive patients was lower in the intervention facilities than the control facilities (7.97% vs 19.67%; adjusted OR [AOR], 0.39; 95% CI, 0.20–0.76), suggesting that more smear-negative patients were being recognized and empirically treated. Smear-positive patients in intervention facilities were also more likely to be started on treatment (87.76% vs 50.00%; AOR, 7.17; 95% CI, 1.98–26.03). The proportion of TB cases who completed treatment or were cured was also higher (42.21% vs 13.33%; AOR, 4.89; 95% CI, 2.24–10.67), and the proportion of TB cases who were lost to follow-up was significantly lower (20.38% vs 86.67%; AOR, 0.04; 95% CI, 0.01–0.09). No other process indicator was significantly different between the intervention facilities and control facilities.
Table 1.
Characteristics of Outpatients at Primary Care Facilities in Uganda by Health Facility Month
| Health Facility Characteristics by Health Facility Month |
Intervention Health Facilities (n = 10) |
Control Health Facilities (n = 2) |
Significance Test |
|
|---|---|---|---|---|
| Total Outpatients | Mean (95% CI) | Mean (95% CI) | t Test | P Value |
| Average number of outpatients per facility per month | 1834 (1683; 1985) | 1397 (1213; 1580) | −2.49 | .014 |
| Average percent of outpatients by age group per facility month | Avg. % (95% CI) | Avg. % (95% CI) | ||
| 0–4 | 23% (22%, 25%) | 29% (27%, 30%) | 3.94 | <.001 |
| 5–14 | 17% (17%, 18%) | 15% (14%, 16%) | −3.29 | .001 |
| 15–49 | 48% (47%, 49%) | 45% (44%, 47%) | −2.64 | .009 |
| 50+ | 10% (9%, 10%) | 9% (9%, 10%) | −0.18 | .86 |
| Missing | 2% (1%, 2%) | 2% (1%, 2%) | 0.63 | .53 |
| Average percent of outpatients by sex per facility month | N (95% CI) | N (95% CI) | ||
| Male | 39% (38%, 39%) | 33% (32%, 34%) | −7.65 | <.001 |
| Female | 60% (60%, 61%) | 63% (61%, 64%) | 3.09 | .002 |
| Missing | 1% (0%, 1%) | 4% (2%, 6%) | 5.08 | <.001 |
| Median number of staff (range) | 40 (28–113)a | 44 (36–51) | N/A | N/A |
| Presumptive TB cases and patients | N (95% CI) | N (95% CI) | ||
| Average number of presumptive TB cases per facility per month | 110 (79, 141) | 142 (87, 198) | 0.90 | .37 |
| Average number of TB patients per facility at baseline | 28 (11, 44) | 25 (23, 27) | −0.16 | .88 |
| Proportion presumptive TB cases out of all outpatients | 5.86% | 3.46% | N/A | N/A |
| Proportion with previous TB treatment | 9% | 11% | N/A | N/A |
Abbreviations: Avg, average; CI, confidence interval; N, number; N/A, not applicable; TB, tuberculosis.
a One hospital with a total of 113 staff members was included.
Table 2.
Odds Ratios and Adjusted Odds Ratios of the Comparison Between Intervention and Control Healthcare Facilities Adjusted for Age, HIV Status, TB Retreatment, and Gender and Clustering by Healthcare Facility
| Process/Outcome Indicator | Intervention Sites (n = 10) | Control Sites (n = 2) | OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Sample Size for Regression |
|---|---|---|---|---|---|---|---|
| Presumptive TB Cases | |||||||
| 1 Proportion of presumptive TB cases with a sputum smeara,b | 64.70% (4119/6366) | 12.66% (244/1928) | 12.65 (5.60, 28.55) | <.001 | N/A | ||
| 2 Proportion of presumptive TB cases with an HIV testa | 63.37% (4034/6366) | 5.29% (102/1928) | 30.97 (5.11, 187.94) | <.001 | N/A | ||
| 3 Proportion of smear-positive TB cases | 7.97% (433/5436) | 19.67% (48/244) | 0.35 (0.19-0.66) | <.001 | 0.39 (0.20–0.76) | .005 | 4508 |
| 4 Proportion of smear-positive new TB cases started on treatmentc | 87.76% (380/433) | 50.00% (24/48) | 7.17 (1.98, 26.03) | .003 | 7.59 (2.19, 26.33) | .001 | 478 |
| TB Patients | |||||||
| 5 Proportion of TB patients tested for HIV | 98.75% (475/481) | 97.78% (44/45) | 1.80 (0.38, 8.49) | .458 | 1.78 (0.35, 8.79) | .498 | 509 |
| 6 Proportion of TB/HIV coinfected started on ART | 66.67% (132/198) | 72.73% (8/11) | 0.75 (0.33, 1.72) | .497 | 0.77 (0.34, 1.78) | .545 | 209 |
| 7 Proportion of TB patients who are smear or bacteriologically positive | 71.73% (345/481) | 77.78% (35/45) | 0.72 (0.33, 1.59) | .380 | 0.94 (0.41, 2.18) | .888 | 517 |
| 8 Proportion of TB patients who are sputum smear negative or extrapulmonary TB cases | 10.81% (52/481) | 11.11% (5/45) | 0.97 (0.68, 1.38) | .866 | 0.73 (0.48, 1.12) | .152 | 517 |
| 9 Proportion of new TB patients who completed or cured (of all those with a treatment outcome recorded) | 42.21% (176/417) | 13.33% (6/45) | 4.74 (2.03, 11.12) | <.001 | 4.89 (2.24, 10.67) | <.001 | 453 |
| 10 Proportion of new TB patients who were lost to follow-up (of all those with a treatment outcome recorded) | 20.38% (85/417) | 86.67% (39/45) | 0.03 (0.01, 0.11) | <.001 | 0.04 (0.01, 0.09) | <.001 | 453 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; N/A, not applicable; OR, odds ratio; TB, tuberculosis.
a For indicators 1–2, individual analysis was not possible because these indicators use 2 separate data sources that were not linked at the individual level.
b Laboratory tests conducted during outreach in the intervention arm (N = 1317) were excluded from the numerator because these patients did not have a MF5 completed.
c For proportion of smear-positive patients started on treatment, the analysis did not adjust for HIV status because missing data for this variable dropped the control arm sample size by 60% from 48 to 19.
Comparison of Baseline Period to the Project Period in the Intervention Arm
During the last 12 months of the IDCAP study (from September 2010 to August 2011, the baseline historical period), 6.00% (13 149 of 219 988) fulfilled predefined criteria for presumptive TB cases, higher than the 3.42% (6366 of 186 357) during the intervention period. All process indicators improved in the intervention healthcare facilities, with the exception of proportion of TB patients who are smear or bacteriologically positive (Table 3). Only 3.44% of presumptive TB cases had a sputum smear result in the historical period compared with 64.70% in the intervention period (OR, 51.38; 95% CI, 20.04–131.76). These presumptive TB cases were also more likely to receive an HIV test during the intervention period (63.37% vs 6.74%; OR, 23.95; 95% CI, 12.96–44.25). Among intervention facilities, 6 times as many bacteriologically confirmed cases were identified compared with the IDCAP phase (433 vs 74), despite the fact that the time period was 2 months shorter. There was a 4.8-fold increase in the number of smear-positive patients who were initiated on treatment (380 vs 75), and the proportion of smear-positive patients started on treatment increased significant from 31.51% to 87.76% (AOR, 15.36; 95% CI, 6.57–35.91) during the intervention period. The proportion of HIV-TB coinfected patients started on ART also increased significantly from 23.68% to 66.13% (AOR, 6.24; 95% CI, 2.28–17.06).
Table 3.
Odds Ratios and Adjusted Odds Ratios of the Comparison for Intervention Health Facilities Between Intervention and Historical Time Periods Adjusted for Age, HIV Status, and Gender and Clustering by Health Facility
| Process/Outcome Indicator | IDCAP Project Sept 2010–Aug 2011a | TB REACH Jan–Oct 2012a | OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Sample Size for Regression |
|---|---|---|---|---|---|---|---|
| Presumptive TB Cases | |||||||
| 1 Proportion of presumptive TB cases with a sputum smeard | 3.44% (453/13 151) | 64.70% (4119/6366) | 51.38 (20.04, 131.76) | <.001 | N/A | ||
| 2 Proportion of presumptive TB cases with an HIV testd | 6.74% (886/13 151) | 63.37% (4034 /6366) | 23.95 (12.96, 44.25) | <.001 | N/A | ||
| 3 Proportion of smear-positive TB casesb | 16.34% (74/453) | 7.97% (433/5436) | 0.44 (0.27-0.72) | <.001 | 0.36 (0.18-0.73) | .005 | 4660 |
| 4 Proportion of smear-positive new TB cases started on treatmentb | 31.51% (75/238) | 87.76% (380/433) | 15.58 (7.08, 34.29) | <.001 | 15.36 (6.57, 35.91) | <.001 | 668 |
| TB Patients | |||||||
| 5 Proportion of TB patients tested for HIV | 87.77% (244/278) | 98.75% (473/479) | 11.03 (5.13, 23.70) | <.001 | 11.43 (5.17, 25.25) | <.001 | 757 |
| 6 Proportion of TB/HIV coinfected started on ART | 23.68% (27/114) | 66.67% (132/198) | 6.44 (2.53, 16.44) | <.001 | 6.40 (2.46, 16.70) | <.001 | 312 |
| 7 Proportion of TB patients who are smear or bacteriologically positivec | 69.26% (169/244) | 72.52% (343/473) | 1.15 (0.41, 3.22) | .783 | 1.14 (0.40, 3.29) | .804 | 717 |
| 8 Proportion of TB patients who are sputum smear negative or extrapulmonary TB casesc | 4.10% (10/244) | 10.78% (51/473) | 2.69 (1.33, 5.43) | .006 | 2.97 (1.31, 6.73) | .009 | 717 |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IDCAP, Infectious Diseases Integrated Infectious Diseases Capacity Building Evaluation; N/A, not applicable; OR, odds ratio; TB, tuberculosis.
a IDCAP project period was from September 2010 to August 2011. Project period refers to the 10-month period from January 2012 to October 2012 with outcome follow-up through June 2013.
b For proportion of smear-positive patients started on treatment, the analysis did not adjust for HIV status because missing data for this variable dropped the control arm sample size by 60% from 48 to 19. In addition, the proportion of smear-positive patients started on treatment (denominator) does not match the proportion smear-positive (numerator) in the IDCAP historical control baseline data because 2 different data sources were used. For proportion smear-positive patients, the MF5 was the data source rather than the Tuberculosis Laboratory Register. Only information on sputum smear-positive patients was extracted from the Tuberculosis Laboratory Register. For proportion of smear-positive started on treatment, the Tuberculosis Laboratory linked to the Tuberculosis Unit Register is the data source.
c For the IDCAP historical baseline data, the numerators of smear/bacteriologically positive and sputum smear negative/extrapulmonary TB do not add up to the denominator because some patients did not have a test result recorded in the TB Register.
d For indicators 1–2, individual analysis was not possible because the TB REACH data for these indicators use 2 separate data sources that were not linked at the individual level.
Xpert MTB/RIF Contribution to Tuberculosis Case Finding
From March to October 2012, 28 additional (smear negative, Xpert MTB/RIF positive) cases were diagnosed at the facilities where the Xpert MTB/RIF machines were housed, and 15 additional cases were diagnosed from facilities where laboratory technicians brought specimens to the Xpert MTB/RIF facilities. Therefore, 8.85% (43 of 486) of the total number of cases treated in the 10-month project period or 12.50% (43 of 344) of the number of cases identified in the last 6 months of the project after Xpert MTB/RIF was implemented were confirmed by Xpert MTB/RIF. The total number of patients tested, number of cartridges used (including repeat testing due to errors), and percent yield were 817, 922, and 5.30%, respectively.
DISCUSSION
The proportion of outpatients who were appropriately screened for TB by sputum smear increased dramatically in intervention facilities compared with control facilities and also compared with a baseline historical time period. Significant improvement in treatment outcomes were also measured after our bundled intervention. Despite limited previous training, the existing MLPs in these rural facilities were effectively trained to identify and manage patients with TB and TB-HIV coinfection in the absence of medical officers. These MLPs screened 64% of the presumptive TB cases, increasing the number of TB cases approximately 5-fold, and increased TB completion by 28.85% over the 10-month project period.
Our healthcare systems approach linked clinical and laboratory training—an approach that has been successful in the diagnosis and treatment of other infectious diseases such as malaria [25, 26]. All aspects of TB case finding and treatment were managed by MLPs and supported by simultaneous laboratory strengthening that provided quality diagnostics to accommodate the larger presumptive TB case laboratory volumes. Our study also corroborates previous clinical trials that task-shifting care to MLPs does not compromise outcomes in HIV care [9], including timely initiation of ART in TB-HIV coinfected individuals and in provision of community-based directly observed therapy [27, 28]. In our study, multidisciplinary training followed by intermittent OSS was an effective way to catalyze sustained improvement in passive TB case detection over time and TB outcomes over time.
The project did not offer pay-for-performance rewards, or any other form of additional salary support [29], nor did it offer additional staff at public healthcare facilities; staff at both intervention and control facilities were paid the same salary. Even without such additional incentives, the MLPs in this study were able to identify and treat TB, a critical achievement given that the majority of the Ugandan population is rural and medical doctor absenteeism is pervasive across rural SSA [7].
Fluorescence microscopy detected 90% of the confirmed TB cases during the project period. Fluorescence microscopy has higher sensitivity, shorter turnaround times, and requires only a single reagent for staining compared with Ziehl-Neelsen light microscopy [17, 30]. It allowed for rapid expansion without additional laboratory personnel to accommodate the large increase in the number of patients referred for smear microscopy testing. Previous field implementation studies have demonstrated concerns about specificity despite increases in sensitivity with FM [31, 32], which were overcome in our project through centralized, strong quality assurance systems.
The addition of Xpert MTB/RIF testing at 5 facilities, with transport reimbursement for laboratory technicians to bring sputum samples from HIV-positive, smear-negative patients from the other 5 facilities to Xpert MTB/RIF hubs, did increase the number of microbiologically confirmed cases in the 6 months that the machines were operating. Overall, we found an additional 43 cases using Xpert MTB/RIF. Further implementation science is needed to maximize the benefits of Xpert MTB/RIF through diagnostic hubs to (1) increase the volume of sputa tested at each machine, (2) select the correct patients to test, and (3) solve implementation logistics targeting HIV-infected presumptive TB cases. Other challenges include the need for consistent voltage, electricity, climate control, test errors, and transport for yearly calibration. Maintaining a supply of cartridges, which are still expensive relative to the cost of sputum smear, poses additional challenges as does the cost of machine repair.
One strength of our study is that clinical data from all outpatients were collected, and these data allowed us to accurately determine the number of patients who fulfilled WHO intensive case-finding criteria for screening compared with the total number of outpatients. Our data show that the case detection rate reported by the WHO is likely an overestimate and surveillance will be needed to determine an accurate rate. In rural healthcare centers in Uganda, there are significant missed opportunities to screen symptomatic patients with smear microscopy.
However, our study had several limitations. First, our impact assessment excludes the impact of poor supply chain management because we provided buffer stocks (HIV test kits, antiretroviral and anti-TB treatment, and laboratory reagents). Second, differences in data collection processes between IDCAP and this project, in particular linking of registers, may account for some of the increases from baseline compared with the project period. To address this discrepancy, we produced Supplementary Table 3 to show consistent gains made during TB REACH. Third, we did not verify smear-negative, Xpert-negative cases with TB cultures. In resource-limited, large-scale evaluations of Xpert MTB/RIF, the sensitivity was as low as 57.7% in smear-negative TB [21]. We also did not evaluate whether negative Xpert MTB/RIF results influenced the decision to empirically treat patients. Overall, the proportion of smear-negative TB diagnoses was low, implying a low rate of empiric treatment, but it did increase during the project period, suggesting that MLPs became more confident in diagnosing smear-negative TB as well.
CONCLUSIONS
Although more sensitive TB diagnostics that are deployable at point-of-care are still urgently needed for rural settings in SSA, our bundled intervention of improved diagnostics combined with on-site training and supervision enabled MLPs to recognize, diagnose, and treat TB. This process also rapidly improved TB case finding and TB completion rates in settings without a medical officer. Optimized training of the existing workforce with available diagnostic techniques will improve TB case detection and will require fewer resources than active case-finding strategies.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We gratefully acknowledge Masja Straetemans (external monitor; Royal Tropical Institute, Netherlands), Marcia Weaver and Kelly Willis who kindly shared the IDCAP data, and Dick Chaisson and Larry Moulton for helpful scientific input. We are also grateful to the Health Center staff who participated in this project as well as the staff of the National TB and Leprosy Program who worked with us to implement the study.
Author contributions. Y. C. M., S. N., A. C., and S. Z.-M. designed the study. S. Z.-M. and F. M. oversaw the study. S. Z.-M. and the TB REACH Team gathered the data. S. B., S. Z.-M., and Y. C. M. designed the analysis and interpreted the data. Y. C. M., S. Z.-M., and S. B. drafted the manuscript. All authors read and approved the final manuscript.
Disclaimer. The sponsor did not have a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The sponsor was involved in monitoring overall project performance through quarterly reports and external monitoring.
Financial support. This study was supported by a grant from TB REACH from the Stop TB Partnership through funds from the Canadian International Development Agency.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2014. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 19 March 2015.
- 2.Golub JE, Mohan CI, Comstock GW, et al. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis 2005; 9:1183–203. [PMC free article] [PubMed] [Google Scholar]
- 3.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 2008; 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lienhardt C, Rowley J, Manneh K, et al. Factors affecting time delay to treatment in a tuberculosis control programme in a sub-Saharan African country: the experience of The Gambia. Int J Tuberc Lung Dis 2001; 5:233–9. [PubMed] [Google Scholar]
- 5.Lehmann U, Dieleman M, Martineau T. Staffing remote rural areas in middle- and low-income countries: a literature review of attraction and retention. BMC Health Serv Res 2008; 8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dussault G, Franceschini MC. Not enough there, too many here: understanding geographical imbalances in the distribution of the health workforce. Hum Resour Health 2006; 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaudhury N, Hammer J, Kremer M, et al. Missing in action: teacher and health worker absence in developing countries. J Econ Perspect 2006; 20:91–116. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Task Shifting: Global Recommendations and Guidelines 2008. Available at: http://www.who.int/healthsystems/TTR-TaskShifting.pdf. Accessed 6 February 2015.
- 9.Fairall L, Bachmann MO, Lombard C, et al. Task shifting of antiretroviral treatment from doctors to primary-care nurses in South Africa (STRETCH): a pragmatic, parallel, cluster-randomised trial. Lancet 2012; 380:889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiweewa FM, Wabwire D, Nakibuuka J, et al. Noninferiority of a task-shifting HIV care and treatment model using peer counselors and nurses among Ugandan women initiated on ART: evidence from a randomized trial. J Acquir Immune Defic Syndr 2013; 63:e125–32. [DOI] [PubMed] [Google Scholar]
- 11.Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet 2010; 376:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulle C, Kouanfack C, Laborde-Balen G, et al. Task shifting HIV care in rural district hospitals in Cameroon: evidence of comparable antiretroviral treatment-related outcomes between nurses and physicians in the Stratall ANRS/ESTHER trial. J Acquir Immune Defic Syndr 2013; 62:569–76. [DOI] [PubMed] [Google Scholar]
- 13.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 2013; 382:1183–94. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro AE, Variava E, Rakgokong MH, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med 2012; 185:1110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekandi JN, List J, Luzze H, et al. Yield of undetected tuberculosis and human immunodeficiency virus coinfection from active case finding in urban Uganda. Int J Tuberc Lung Dis 2014; 18:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet 2010; 376:1244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis 2006; 6:570–81. [DOI] [PubMed] [Google Scholar]
- 18.Theron G, Peter J, Dowdy D, et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis 2014; 14:527–32. [DOI] [PubMed] [Google Scholar]
- 19.Weaver MR, Crozier I, Eleku S, et al. Capacity-building and clinical competence in infectious disease in Uganda: a mixed-design study with pre/post and cluster-randomized trial components. PLoS One 2012; 7:e51319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miceli A, Sebuyira LM, Crozier I, et al. Advances in clinical education: a model for infectious disease training for mid-level practitioners in Uganda. Int J Infect Dis 2012; 16:e708–13. [DOI] [PubMed] [Google Scholar]
- 21.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011; 377:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Rapid Implementation of the Xpert MTB/RIF Diagnostic Test Technical and Operational ‘How-to’ Practical Considerations. Available at: http://whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf. Accessed 19 March 2015.
- 23.World Health Organization. Compendium of Indicators for Monitoring and Evaluating National Tuberculosis Programs. Available at: http://whqlibdoc.who.int/hq/2004/WHO_HTM_TB_2004.344.pdf. Accessed 19 March 2015.
- 24.Naikoba S, Colebunders R, Van Geertruyden J, et al. Design of a cluster randomized trial assessing integrated infectious diseases training and onsite support for midlevel practitioners in Uganda. J Clin Care Pathways 2013; 16:152–9. [Google Scholar]
- 25.Namagembe A, Ssekabira U, Weaver MR, et al. Improved clinical and laboratory skills after team-based, malaria case management training of health care professionals in Uganda. Malar J 2012; 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ssekabira U, Bukirwa H, Hopkins H, et al. Improved malaria case management after integrated team-based training of health care workers in Uganda. Am J Trop Med Hyg 2008; 79:826–33. [PubMed] [Google Scholar]
- 27.Van Rie A, Patel MR, Nana M, et al. Integration and task-shifting for TB/HIV care and treatment in highly resource-scarce settings: one size may not fit all. J Acquir Immune Defic Syndr 2014; 65:e110–7. [DOI] [PubMed] [Google Scholar]
- 28.Mafigiri DK, McGrath JW, Whalen CC. Task shifting for tuberculosis control: a qualitative study of community-based directly observed therapy in urban Uganda. Glob Public Health 2012; 7:270–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gertler P, Vermeersch C. Using performance incentives to improve health outcomes. World Bank, June, 2012. Available at: http://elibrary.worldbank.org/doi/pdf/10.1596/1813-9450-6100. Accessed 19 March 2015. [Google Scholar]
- 30.Getahun H, Harrington M, O'Brien R, et al. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 2007; 369:2042–9. [DOI] [PubMed] [Google Scholar]
- 31.Cuevas LE, Al-Sonboli N, Lawson L, et al. LED fluorescence microscopy for the diagnosis of pulmonary tuberculosis: a multi-country cross-sectional evaluation. PLoS Med 2011; 8:e1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albert H, Nakiyingi L, Sempa J, et al. Operational implementation of LED fluorescence microscopy in screening tuberculosis suspects in an urban HIV clinic in Uganda. PLoS One 2013; 8:e72556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.