Abstract
Importance
Millions of Americans are forced to quit smoking as they enter tobacco-free prisons and jails, but most return to smoking within days of release. Interventions are needed to sustain tobacco abstinence after release from incarceration.
Objective
To evaluate the extent to which the WISE intervention (Working Inside for Smoking Elimination), based on motivational interviewing (MI) and cognitive behavioral therapy (CBT), decreases relapse to smoking after release from a smoke-free prison.
Design
Participants were recruited approximately 8 weeks prior to their release from a smoke-free prison and randomized to 6 weekly sessions of either education videos (control) or the WISE intervention.
Setting
A tobacco-free prison in the United States.
Participants
A total of 262 inmates (35% female).
Main Outcome Measure
Continued smoking absti nence was defined as 7-day point-prevalence abstinence validated by urine cotinine measurement.
Results
At the 3-week follow-up, 25% of participants in the WISE intervention (31 of 122) and 7% of the control participants (9 of 125) continued to be tobacco abstinent (odds ratio [OR], 4.4; 95% CI, 2.0-9.7). In addition to the intervention, Hispanic ethnicity, a plan to remain abstinent, and being incarcerated for more than 6 months were all associated with increased likelihood of remaining abstinent. In the logistic regression analysis, participants randomized to the WISE intervention were 6.6 times more likely to remain tobacco abstinent at the 3-week follow up than those randomized to the control condition (95% CI, 2.5-17.0). Nonsmokers at the 3-week follow-up had an additional follow-up 3 months after release, and overall 12% of the participants in the WISE intervention (14 of 122) and 2% of the control participants (3 of 125) were tobacco free at 3 months, as confirmed by urine cotinine measurement (OR, 5.3; 95% CI, 1.4-23.8).
Conclusions and Relevance
Forced tobacco abstinence alone during incarceration has little impact on postrelease smoking status. A behavioral intervention provided prior to release greatly improves cotinine-confirmed smoking cessation in the community.
Trial Registration
clinicaltrials.gov Identifier: NCT01122589
TOBACCO USE CONTRIBUTES to over 400 000 deaths annually.1 It is a major contributor to cancer and heart disease risk and is the leading cause of preventable morbidity, mortality, and health expense in the United States resulting in an estimated $157 billion in related annual health and economic costs.2 Quitting smoking reduces the risk of developing smoking-related illnesses and the morbidity and mortality associated with these illnesses. In 2010, approximately 45.3 million American adults smoked, an overall prevalence of 19.3%.3
One in 8 American smokers pass through prisons and jails annually,4 and since the announcement of the negative health consequences of secondhand smoke, correctional facilities are increasingly becoming tobacco free: approximately 60% have complete smoking bans
See Invited Commentary
(no tobacco products allowed anywhere in the facility by inmates or staff ).5 Despite this, 97% of inmates return to smoking as soon as they are released back into the community.4,6 Smoking among prisoners is approximately 3 times that of the general population,7 and minorities, poor, undereducated, and mentally ill individuals are all overrepresented in correctional facilities.8 Despite the scale of this problem, few studies have addressed the needs of incarcerated smokers.
Many successful interventions have been developed for smoking cessation, but the success of smoking relapse– prevention interventions is limited. Moreover, the available smoking-cessation and relapse-prevention treatments do not address the unique and specific needs of incarcerated men and women who have been tobacco free for months to years (forced-abstinent smokers), who have completed the physical withdrawal from nicotine, and who are returning to environments where tobacco is available.9 Effective smoking relapse–prevention interventions for this population will enhance our ability to attain the Healthy People 2020 goal of decreasing smoking rates to 12% among adults.10
Project WISE (Working Inside for Smoking Elimination)11 is a randomized clinical trial of a smoking abstinence intervention based on motivational interviewing (MI) and cognitive behavioral therapy (CBT), which was developed to target the specific needs of inmates in a smoke-free prison about to be released to the community.
METHODS
Complete details of the methods for this study have been described elsewhere.11 Approvals from the institutional review board and the office for human research protections were obtained prior to any study activities. To further protect study participants, a certificate of confidentiality was obtained.
PARTICIPANTS
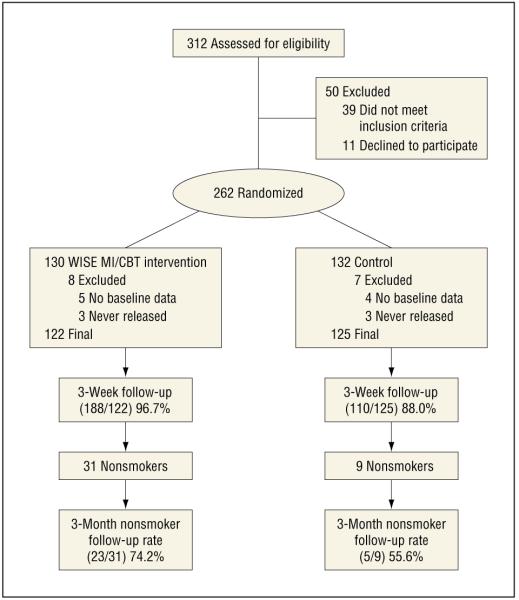
Participants were recruited by research assistants (RAs) from a large state correctional facility in the northeastern United States (Figure 1) in which no tobacco products are allowed on site by inmates or staff, and no pharmacotherapy or behavioral therapy is routinely offered for smoking cessation. Sentenced men and women were eligible for screening if they were to be released within the next 8 weeks. In a confidential setting, RAs explained the study and that participation was completely voluntary. Potential participants were eligible if they were 18 years or older, smoked 10 or more cigarettes per day prior to incarceration, and spoke English. Once a potential participant was determined to be eligible and willing to participate in the study, the informed consent process was completed. All participants received an American Heart Association smoking-cessation pamphlet, a list of community resources, and study contact information.
Figure 1.
Participant flowchart. CBT indicates cognitive behavioral therapy; MI, motivational interviewing; WISE, Working Inside for Smoking Elimination.11
SAMPLE SIZE AND ATTRITION
Of the 312 people screened for the study, 2 did not speak English, 30 did not smoke ≥10 cigarettes/d prior to incarceration, 7 had more than 8 weeks until release, 2 were homeless and could not provide any contact information for follow-up, and 1 was not going to live in the follow-up area). Of the 273 eligible persons, 262 (96.0%) agreed to participate and completed the consent procedure. Of the 262 enrolled and randomized at baseline, 15 were excluded (from this report), 9 because of a computer error that did not save data from the baseline questionnaire and 6 because they were still incarcerated at the end of the study and hence could not be assessed for smoking after release. Of the remaining 247 participants, 228 (92.3%) completed the 3-week postrelease follow-up assessment. Non-smokers at the 3-week follow-up were invited to return for a 3-month follow-up and 70% completed this assessment (28 of 40). Participants lost to follow-up were analyzed as smokers.
PROCEDURES
Following baseline assessment, participants were randomly assigned to either receive the WISE intervention or watch control videos. Randomization was stratified by sex, number of cigarettes smoked in the 30 days prior to incarceration (<20 cigarettes/d vs ≥20 cigarettes/d), and postrelease smoking plans, as previously described.11 Each study condition comprised 6 sessions that took place over approximately 6 weeks prior to release. A 3-week postrelease follow-up, assessment included taking a urine sample for cotinine evaluation, a computerized assessment, and a timeline followback (TLFB) procedure12,13 to determine smoking behaviors on each day following release from prison. Participants who were confirmed tobacco abstinent at 3-week follow-up were asked to return for a 3-month postrelease assessment.
INTERVENTIONS
WISE Intervention
Sessions 1 and 6 of the WISE intervention involved MI, and in sessions 2 through 5, participants received CBT.
Three RAs (with bachelor’s or equivalent degrees) received approximately 24 hours of training, including didactic instruction, role playing, and working with pilot participants. The RAs were matched with sex-concordant participants. Two PhD-level supervisors rated sessions to criteria using the Motivational Interviewing Treatment Integrity system, version 3.1.1,14 and a key elements checklist for CBT sessions prior to RA contact with participants. Supervision was conducted twice per month and when treatment fidelity fell below criteria. Additional coaching was provided until sessions met standard. Sessions were 30 to 60 minutes long, and all were recorded. A random 10% of sessions were coded for fidelity by supervisors, and 10% of those were double coded. There was over 90% agreement in the coding in terms of proficient vs nonproficient, and 85% were proficient.
The research counselors’ therapeutic style and protocol were based on the principles of MI, with a focus on empathy, not arguing, developing discrepancy, self-efficacy, and personal choice.15 The CBT sessions taught participants to recognize specific environmental and affective events (triggers) that occur prior to smoking and to identify behavioral and cognitive strategies to cope with these triggers. Additional brief telephone sessions were conducted at approximately 24 hours and 7 days after the individual’s release. These sessions included elements of both MI and CBT in an effort to maintain and enhance motivation and use of skills after release.
Control Condition
The control videos included a variety of health-related topics (eg, managing chronic pain) but did not target smoking cessation and were matched with the WISE intervention for frequency and duration of contact. To maintain frequency and duration of contact, telephone calls were scheduled for approximately 24 hours and 7 days after release; these calls verified contact information and assessed smoking status.
MEASURES
Full assessments, each taking about 60 minutes, occurred at baseline and at 3 weeks following release. The RAs phoned participants at 24 hours and 7 days after release for brief discussion, and prior to discussion, smoking status was assessed. Assessments at baseline and 3-week follow-up were conducted using audio computer-assisted self-interviews. Assessments included demographics, smoking history and dependence (Fagerström Test for Nicotine Dependence [FTND] modified to reflect time prior to incarceration),16 subjective stress (Perceived Stress Scale),17 presence or absence of smoking-related illnesses (asthma, diabetes, hypertension, stroke, or heart attack), and depressive symptoms (Center for Epidemiologic Studies Depression Scale [CES-D]).18 Participation in a prison drug treatment program was assessed with the question “have you participated in a drug treatment program?” Many prison drug treatment programs use CBT and MI (similar to the WISE intervention).
Intention to remain tobacco free was measured using a 6-point scale and dichotomized: responses of “I plan to smoke when I get out of here and never plan to quit” to “I will probably smoke when I get out of here” were classified as “plans to smoke upon release.” Responses of “I probably won’t smoke when I get out of here” to “I have made plans to not smoke when I get out. and I will never smoke again” were classified as “plans to not smoke upon release.” At the 3-week follow-up, a urine sample was obtained to test for cotinine and other substance use, and a detailed TLFB12,13 was administered to assess tobacco use. An additional urine cotinine measurement was obtained 3 months after release from participants who tested negative for cotinine at the 3-week follow-up. Continued smoking abstinence was defined as testing negative for cotinine (urinary cotinine level, <200 ng/mL) and reporting no smoking in the previous 7 days; all others were considered smokers.19
ANALYSES
We examined baseline differences between conditions on demographic and smoking variables using χ2 tests of proportions for categorical variables and t tests for continuous variables. To be considered having completed the study, a participant had to complete a 3-week follow-up interview and provide a urine sample. We examined other variables that might predict outcome in this sample by comparing those with sustained abstinence to those who smoked. These included sex, age, years of education, race/ethnicity, measures of affective symptoms and vulnerability (CES-D, Perceived Stress Scale), prison drug treatment, and smoking-related variables including FTND score, cigarettes smoked per day prior to incarceration, time since smoked daily, age started smoking daily, number of years of daily smoking, smoking plans after release, presence of smoking-related medical condition, and presence of a spouse/partner who smokes.
Analyses of sustained abstinence at 3 weeks after release used the full intention-to-treat sample and used logistic regression. In the first model, we entered treatment group only. The second model adjusted for other variables related to sustained abstinence with a level of significance set at P<.10. Time since smoked daily was dichotomized (≥6 months vs <6 months) because it was highly skewed.
We next conducted discrete-time survival analysis using Cox proportional hazards regression models to test the hypothesis that the risk of returning to smoking was significantly higher for participants randomized to the control condition compared with participants in the WISE intervention. All analyses were conducted using SPSS statistical software, version 20 (IBM Corp).
RESULTS
PARTICIPANT CHARACTERISTICS
The sample (N = 247) comprised the following racial/ ethnic background: 20.1% Hispanic, 17.6% black, 52.0% white, and 10.2% self-identified as other and did not differ by intervention group (Table 1). Most participants were men (65.2%); mean age was 35.6 years; and the mean time since the last cigarette was 1.5 years. Intervention groups did not differ significantly at baseline on depression, stress, demographic, or smoking variables (Table 1). All 6 WISE intervention sessions were completed by 83.3%, with 11.9% completing 3 or fewer sessions.
Table 1.
Participant Randomization and Baseline Characteristicsa
| Characteristic | Total (n = 247) |
WISEb
(n = 122) |
Control Video (n = 125) |
|---|---|---|---|
| Race/ethnicity | |||
| White | 127 (52.0) | 63 (51.6) | 64 (52.5) |
| Hispanic | 49 (20.1) | 26 (21.3) | 23 (18.9) |
| Black, non-Hispanic | 43 (17.6) | 21 (17.2) | 22 (18.0) |
| Other | 25 (10.2) | 12 (9.8) | 13 (10.7) |
| Sex | |||
| Male | 161 (65.2) | 80 (65.6) | 81 (64.8) |
| Female | 86 (34.8) | 42 (34.4) | 44 (35.2) |
| Smoking plans | |||
| Plan to smoke after release | 126 (51.2) | 65 (53.3) | 61 (49.2) |
| Plan to not smoke after release | 120 (48.8) | 57 (46.7) | 63 (50.8) |
| Education level completed | |||
| <High school | 157 (64.6) | 81 (66.4) | 76 (62.8) |
| High school | 49 (20.2) | 21 (17.2) | 28 (23.1) |
| >High school | 37 (15.2) | 20 (16.4) | 17 (14.0) |
| Reported health status | |||
| Poor-fair | 75 (30.7) | 41 (33.6) | 34 (27.9) |
| Good-excellent | 169 (69.3) | 81 (66.4) | 88 (72.1) |
| In-prison drug treatment | 119 (49.8) | 56 (47.1) | 63 (52.5) |
| Smoking-related medical conditions |
89 (36.0) | 44 (36.1) | 45 (36.0) |
| Age, yc | 35.6 (9.2) | 35.4 (9.4) | 35.7 (9.0) |
| Age started smoking daily, yc | 15.7 (4.5) | 15.6 (4.7) | 15.9 (4.3) |
| Years of smokingc | 19.4 (10.0) | 19.1 (10.0) | 19.7 (10.0) |
| Time since smoked daily, yc | 1.5 (3.4) | 1.6 (3.4) | 1.5 (3.5) |
| Cigarettes/d prior to prison, No.c |
21.7 (11.7) | 20.7 (10.6) | 22.6 (12.8) |
| FTNDc | 5.1 (2.3) | 5.2 (2.2) | 5.1 (2.4) |
| PSSc | 21.8 (6.3) | 21.5 (7.0) | 22.2 (5.6) |
| CES-Dc | 12.7 (5.4) | 12.4 (5.6) | 13.0 (5.3) |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale18; FTND, Fagerström Test for Nicotine Dependence,16 modified to reflect time prior to incarceration; PSS, Perceived Stress Scale17; WISE, Working Inside for Smoking Elimination.11
Unless otherwise indicated, data are reported as number (percentage) of participants.
P > 0.2 for all treatment group differences.
Data reported as mean (SD)values.
Analysis comparing WISE and control participants on confirmed smoking status 3 weeks after release found participants randomized to the WISE intervention were 4.4 (95% CI, 2.0-9.7) times more likely to remain tobacco abstinent than those randomized to the control condition (Table 2). Significant differences (P < .10) were found between smokers and nonsmokers: Those not smoking at the 3-week follow-up started smoking when they were older (age 17.3 vs 15.4 years) (P = .02), smoked for fewer years (16.6 vs 19.9 years) (P = .06), were more likely to have participated in prison drug treatment (63.2% vs 47.3%) (P = .07), had gone longer since smoking regularly (2.5 vs 1.4 years) (P = .06), were more likely to be Hispanic (33.3% vs 17.6%) (P = .02), and planned not to smoke after release from prison (62.5% vs 46.1%) (P = .06). We therefore controlled for these variables in our multivariate logistic regression.
Table 2.
Nonsmokers vs Smokers at 3-Week Follow-upa
| Characteristic | Nonsmoker (n = 40) |
Smoker (n = 207) |
|---|---|---|
| Intervention condition | ||
| WISE intervention | 31 (25.4) | 91 (74.6)c |
| Control video | 9 (7.2) | 116 (92.8)c |
| Female | 14 (35.0) | 72 (34.8) |
| Education level completed | ||
| <High school | 24 (61.5) | 133 (65.2) |
| High school | 9 (23.1) | 40 (19.6) |
| >High school | 6 (15.4) | 31 (15.2) |
| Race/ethnicity | ||
| White | 17 (43.6) | 110 (53.7) |
| Hispanic | 13 (33.3) | 36 (17.6)d |
| Black, non-Hispanic | 5 (12.8) | 38 (18.5) |
| Other | 4 (10.3) | 21 (10.2) |
| Smoking plans | ||
| Plan to smoke upon release | 15 (37.5) | 111 (53.9)e |
| Plan to not smoke upon release | 25 (62.5) | 95 (46.1) |
| Smoking-related medical conditions | 17 (42.5) | 72 (34.8) |
| Spouse/partner smokes | 18 (51.4) | 102 (60.4) |
| Drug use prior to incarceration | 35 (87.5) | 194 (93.7) |
| In prison drug treatment | 24 (63.2) | 95 (47.3)e |
| Age, yb | 35.2 (9.4) | 35.7 (9.2) |
| Age started smoking daily, yb | 17.3 (5.6) | 15.4 (4.2)d |
| Years of smokingb | 16.6 (9.1) | 19.9 (10.1)e |
| Time since smoked daily, yb | 2.5 (2.4) | 1.4 (3.6)e |
| Cigarettes/d prior to prison, No.b |
19.0 (9.5) | 22.1 (12.1) |
| FTNDb | 4.9 (2.3) | 5.2 (2.3) |
| PSSb | 21.5 (6.1) | 21.9 (6.3) |
| CES-Db | 12.3 (4.9) | 12.8 (5.5) |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale18; FTND, Fagerström Test for Nicotine Dependence,16 modified to reflect time prior to incarceration; PSS, Perceived Stress Scale17; WISE, Working Inside for Smoking Elimination.11
Unless otherwise indicated, data are reported as number (percentage) of participants.
Data reported as mean (SD) values.
P < .01 for differences between smokers and nonsmokers.
P < .05 for differences between smokers and nonsmokers.
P< .10 for differences between smokers and nonsmokers.
In the multivariate logistic regression (Table 3), randomization to the intervention group remained a significant predictor of abstinence at 3 weeks (odds ratio [OR], 6.6; 95% CI, 2.5-17.0) compared with those randomized to the control condition. Hispanic ethnicity was also associated with greater tobacco abstinence at 3 weeks (OR, 3.2; 95% CI, 1.1-8.7), as was not smoking for 6 or more months (OR, 4.6; 95% CI, 1.7-12.4) and planning to not smoke (OR, 1.6; 95% CI, 1.2-2.3).
Table 3.
Logistic Regression
| Variable | β (SE) |
P
Value |
OR (95% CI) |
|---|---|---|---|
| WISE intervention | 1.9 (0.5) | <.01 | 6.6 (2.5-17.0) |
| In prison >6 mo | 1.5 (0.5) | <.01 | 4.6 (1.7-12.4) |
| Hispanic | 1.2 (0.5) | .03 | 3.2 (1.1-8.7) |
| Smoking plans | 0.5 (0.2) | <.01 | 1.6 (1.2-2.3) |
| Cigarettes/d prior to prison, No. | −0.03 (0.02) | .10 | 1.0 (0.9-1.0) |
| In-prison drug treatment | 0.6 (0.5) | .16 | 1.9 (0.8-4.6) |
Abbreviations: OR, odds ratio; WISE, Working Inside for Smoking Elimination.11
Participants who were abstinent at their 3-week follow-up (n = 40) were reevaluated at 3 months. In the control group, 2.4% (3 of 125) were cotinine-confirmed abstinent compared with 11.5% (14 of 122) in the intervention group (OR, 5.3; 95% CI, 1.4-23.8). However, follow-up data were obtained on only 28 (70%) of the 40 nonsmokers.
An adjusted survival curve using Cox proportional hazards model is depicted in Figure 2 and includes days to first cigarette from the TLFB data. The first day in the community was the highest-risk day, when most participants relapsed to smoking. After day 1, the rate of relapse declined sharply, with the intervention group maintaining significantly better survival (P = .001). In the survival model examining days to first smoking lapse, the main effect of treatment condition was significant, β (SE), 1.56 (0.16); hazard ratio, 1.75 (P = .001), indicating that the risk of smoking after release was over 1.75 times greater for those in the control condition than for those in the WISE intervention.
Figure 2.
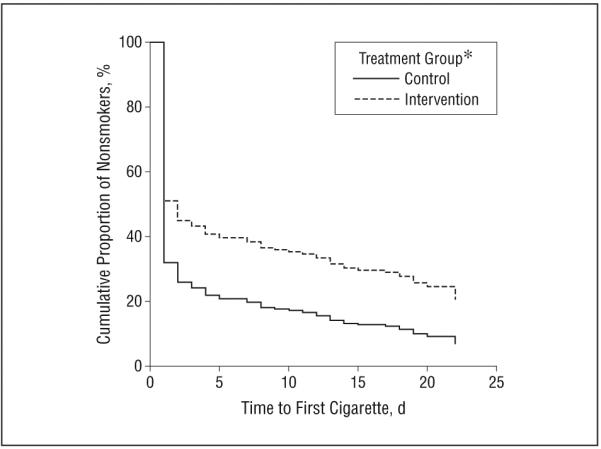
Tobacco abstinence at the 3-week follow-up by the study condition group (adjusted survival curves). *Effect seen in treatment group is significant (P=.001) (β [SE], 0.56 [0.16]; hazard ratio, 1.75), indicating that the risk of smoking after release was over 1.75 times greater for those in the control condition compared with those in the WISE (Working Inside for Smoking Elimination11) intervention.
COMMENT
To our knowledge, this is the first study to demonstrate an effective intervention to prevent smoking relapse after release from a prison with a complete tobacco ban. The abstinence rates were 25.4% and 7.2% at 3 weeks and 11.5% and 2.4% at 3 months in the WISE intervention and control groups, respectively. The situation of forced tobacco abstinence during incarceration is unique in that incarcerated people have no choice about abstaining from tobacco; they have completed nicotine withdrawal; and they face reexposure to tobacco once released. Therefore, comparisons with other interventions are limited in their application and scope. Many smoking-cessation interventions focus on setting a quit date and remaining abstinent; however, in this setting the quit date is forced by someone other than the tobacco user. For those who had smoked prior to incarceration, there is no choice in quit date, and it is only on the date of release when there is a choice to be made about remaining abstinent or returning to smoking.
Results of the WISE intervention are better than those found in many other studies on behavioral intervention for smokers. On average, MI leads to an increase in smoking cessation with a risk reduction (RR) of 1.27.20 Individual counseling is more effective than the control condition with an RR for smoking cessation of 1.39 (95% CI, 1.24-1.57).21 Our results may be greater because of the enforced abstinence prior to the intervention. In addition, participants who had not smoked for 6 months or longer at the time of the intervention were 4.6 times as likely to be abstinent at 3 weeks compared with those who smoked within the past 6 months. This suggests that prolonged forced abstinence can improve smoking outcomes. However, without intervention, only 2.4% of participants remained tobacco free at 3 months after release. Results may also be related to the relatively brief follow-up period; future research should examine this issue. Similar to other studies, our study found the early postrelease period to be an extremely high-risk time,22,23 with more than 60% of control participants relapsing to smoking the first day out.
We chose not to include pharmacotherapy in this intervention because we could find no evidence supporting the use of medications after prolonged tobacco abstinence. Contraband tobacco products exist in prisons, but the majority of inmates will not use them because of the high costs ($10 per cigarette, according to several inmates) and consequences.24 The higher the level of security, the more difficult it is to access cigarettes. Medications have enhanced smoking cessation: varenicline showed an RR of 2.27 (95% CI, 2.02-2.55)25; bupropion RR, 1.69 (95% CI, 1.53-1.85)26; and nicotine replacement therapy RR, 1.58 (95% CI, 1.50-1.66).27 While these studies all had longer follow-up periods, the effect of our behavioral intervention is comparable.
One of the national health objectives for 2020 is to reduce the prevalence of cigarette smoking to less than 12%.28 Effective smoking-cessation programs targeting incarcerated people are necessary to reach this goal because approximately 9 million individuals (>5.4 million smokers) return to the community from correctional facilities annually.4 Tobacco use among prisoners is approximately 3 times that of the general population.7 Smoking-cessation interventions targeting this high-risk and underserved population are instrumental to decrease health disparities and decrease tobacco-related illnesses in this vulnerable population.
Further investigation is needed. Our study’s strengths include a diverse population (52% non-Hispanic whites), inclusion of all smokers regardless of motivation to remain abstinent after release, cotinine verification of smoking status, and a follow-up rate over 90%. The study’s limitations include follow-up after release from prison limited to 3 weeks for all participants; however, participants not smoking at the 3-week follow-up visit were invited to follow up at 3 months after release to assess for continuous abstinence. Because this is the first study of this population, and relapse rates are precipitously high immediately after release, we believed that a brief follow-up period for all participants was appropriate, with longer follow-up of the nonsmokers. Further investigation with longer postrelease follow-up is needed. Also, the study was limited to a sentenced population. Many of the 9 million people released from prisons and jails every year have been imprisoned there for less than 3 months and do not have specific release dates. Evaluations of smoking cessation interventions are needed for inmates at the time of entry to prisons and jails.
In summary, our study shows that an intervention based on MI and CBT can improve continued smoking abstinence after prison release by 6.6 times over that of the control condition. Behavioral intervention for drug use is common in prison settings,29 and so this intervention may be easily integrated into and transported to existing programs in prison settings. Future studies may wish to dismantle the treatment to streamline into the most effective components. Additional work is also needed to examine methods for providing postrelease intervention to help sustain longer-term abstinence.
Acknowledgments
Funding/Support: This work was supported in part by grant R01DA024093 from the National Institutes of Health/National Institute on Drug Abuse (Dr Clarke).
Footnotes
Additional Contributions: We would like to thank the Rhode Island Department of Corrections for their support of this project.
REFERENCES
- 1.Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction. 2001;96(12):1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995-1999. MMWR Morb Mortal Wkly Rep. 2002;51(14):300–303. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Vital signs: current cigarette smoking among adults aged >18 years—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60(35):1207–1212. [PubMed] [Google Scholar]
- 4.Lincoln T, Tuthill RW, Roberts CA, et al. Resumption of smoking after release from a tobacco-free correctional facility. J Correct Health Care. 2009;15(3):190–196. doi: 10.1177/1078345809333388. [DOI] [PubMed] [Google Scholar]
- 5.Kauffman RM, Ferketich AK, Wewers ME. Tobacco policy in American prisons, 2007. Tob Control. 2008;17(5):357–360. doi: 10.1136/tc.2007.024448. [DOI] [PubMed] [Google Scholar]
- 6.Tuthill R, Lincoln T, Conklin T, Kennedy S, Hammett T, Roberts C. Does involuntary cigarette smoking abstinence among inmates during correctional incarceration result in continued abstinence post release?. Presented at the 26th National Conference on Correctional Health Care; Nashville, Tennessee: Oct 21, 2002. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) QuickStats: cigarette smoking prevalence among adults aged >18 years who have ever spent >24 hours on the streets, in a shelter, or in a jail or prison, by sex—United States. 2004 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5510a7.htm. Accessed February 22, 2013.
- 8.Beck AJ, Harrison PM, Karberg JC. Prison and jail inmates at midyear. http://bjs.ojp.usdoj.gov/index.cfm?ty=pbdetail&iid=866. Accessed February 22, 2013.
- 9.Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2009;(1):CD003999. doi: 10.1002/14651858.CD003999.pub3. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Department of Health and Human Services Reduce cigarette smoking by adults. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=41. Accessed February 22, 2013.
- 11.Clarke JG, Martin RA, Stein L, et al. Working Inside for Smoking Elimination (Project W.I.S.E.) study design and rationale to prevent return to smoking after release from a smoke free prison. BMC Public Health. 2011;11:767. doi: 10.1186/1471-2458-11-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobell LC, Sobell M. Timeline followback: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Clifton, NJ: 1992. [Google Scholar]
- 13.Brown RA, Burgess ES, Sales SD, et al. Reliability and validity of a smoking Timeline Follow-Back Interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 14.Moyers TB, Martin T, Manuel JK, Miller WR, Ernst D. Revised global scales: Motivational Interviewing Treatment Integrity 3.1.1 (MITI 3.1.1) http://www.motivationalinterview.org/Documents/miti3_1.pdf. Accessed February 22, 2013.
- 15.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Guilford Press; New York, NY: 2002. [Google Scholar]
- 16.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 19.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 20.Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;(1):CD006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster T, Stead L. Individual behavioural counseling for smoking cessation. Cochrane Database Syst Rev. 2005;18(2):CD001292. doi: 10.1002/14651858.CD001292.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Binswanger IA, Stern MF, Deyo RA, et al. Release from prison: a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke JG, Anderson BJ, Stein MD. Hazardously drinking women leaving jail: time to first drink. J Correct Health Care. 2011;17(1):61–68. doi: 10.1177/1078345810385915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thibodeau L, Seal DW, Jorenby DE, Corcoran K, Sosman JM. Perceptions and influences of a state prison smoking ban. J Correct Health Care. 2012;18(4):293–301. doi: 10.1177/1078345812456019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2012;4:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007;(1):CD000031. doi: 10.1002/14651858.CD000031.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;(1):CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- 28.HealthyPeople.gov, editor. Healthy People 2020: Tobacco Use. http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=41#285_hover. Accessed February 22, 2013.
- 29.Taxman FS, Perdoni ML, Harrison LD. Drug treatment services for adult offenders: the state of the state. J Subst Abuse Treat. 2007;32(3):239–254. doi: 10.1016/j.jsat.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]