Abstract
Primary brain tumor patients have multiple risk factors for Pneumocystis jiroveci and may require prophylaxis with TMP-SMZ or dapsone. Although dapsone is generally safe and efficacious, we present a case of a patient diagnosed with a brain stem glioblastoma who developed methemoglobinemia and haemolytic anemia after presenting with worsening confusion and cardiopulmonary system dysfunction. This case highlights one of the potentially severe complications associated with dapsone therapy. Although this illustrates an unusual toxicity of dapsone, a high index of suspicion should be given to high-risk patients due to ethnic heritage, anemia, or advanced age. Furthermore, given the toxicities of TMP-SMZ and dapsone, further work is needed to determine the threshold CD4+ count at which empiric prophylaxis should be initiated.
Keywords: Glioblastoma, Dapsone, Methemoglobinemia, Anemia
Introduction
Immunosuppression is a well-documented and predictable outcome for many patients with glioblastoma treated with corticosteroids and radiation. In one series, over 20% of patients treated in this manner have CD4+ counts lower than 200/mm3 which was associated with an increased risk of hospitalization for infection [1]. Temozolomide is known to induce significant lymphopenia and preferentially affects the CD4+ subpopulation [2]. The addition of concurrent daily temozolomide during radiation therapy and “dose dense” temozolomide schedules may induce an even greater degree of immune suppression putting patients at risk of opportunistic infections [3]. Patients with primary brain tumors undergoing chemoradiation are routinely given prophylaxis for Pneumocystis jiroveci pneumonia (PJP). Opportunistic infections have less commonly been described in patients receiving adjuvant “standard dose” temozolomide, although cases have been reported [4]. Trimethoprim-sulfamethoxazole (TMP-SMX), dapsone and pentamidine can be used for prophylaxis against P. jiroveci. Although the recommended and most commonly used drug is TMP-SMX, 30–40% of patients discontinue this therapy due to complications such as nausea/vomiting, rash, lymphopenia, and thrombocytopenia [5, 6]. Although atovaqone and pentamidine are effective alternatives, there is an increase in the use of dapsone for P. jiroveci prophylaxis in patients with glioblastoma. Dapsone can cause a self-limiting hemolytic anemia, but in patients with glucose-6-phosphate dehydrogenase (G6PD)-deficiency hemolysis can be severe. Dapsone therapy can also cause methemoglobinemia, a condition where ferrous iron (Fe2+) in the heme groups becomes oxidized to ferric iron (Fe3+). In effect methemoglobin results in a functional anemia, unable to efficiently bind oxygen in O2 rich environments (lungs) and unable to effectively deliver oxygen to hypoxic tissues. In acquired cases of methemoglobinemia, the relatively acute change in oxygen carrying capacity can have important clinical consequences. Cyanosis can be detected when MetHb levels reach 8–12% [7, 8]. Healthy individuals without anemia or cardiovascular compromise may have few symptoms at MetHb levels less than 15%, but at higher levels may develop headaches, fatigue, dyspnea, confusion, syncope, and tachycardia [9]. Here, we present a patient with a brainstem glioblastoma who developed dapsone-induced methemoglobinemia during chemoradiation in an effort to increase awareness of neuro-oncology practitioners of the risks of dapsone-induced complications in this patient population.
Case report
The patient was a 50 year old male with a history of common bile duct adenocarcinoma in complete remission after treatment with chemoradiation and gemcitabine 5 years earlier who presented with a one year history of progressive left greater than right sided weakness, clumsiness, and unsteady gait. Magnetic resonance imaging (MRI) of the brain revealed a 2.4 × 1.6 × 2.3 cm expansile mass of the medulla with extension into the left brachium pontis and cerebellar hemisphere and rostral extension to the level of C5. Enhancement was noticed within the C2 region. The patient started treatment with conformal radiation therapy with concurrent temozolomide. Dapsone was prescribed for PJP prophylaxis at 100 mg daily. Four days into treatment, the patient presented to the emergency room with intractable back pain, tachycardia, and dyspnea. On admission, his absolute lymphocyte count was 0.27 thousand per microliter. The patient was transferred to the ICU where an initial arterial blood gas (ABG) revealed PH 7.47, pCO2 27, pO2 71, HCO3 19, and oxygen saturation of 95% on 100% oxygen by face mask. Despite non-invasive positive pressure ventilation with 100% oxygen, his oxygen saturation by noninvasive pulse oximetry remained at 85–90%. Bedside spectrometry was done which measured methemoglobin levels at 16% (normal less than 1–3%), thus confirming a diagnosis of methemoglobinemia. Given the association with initiation of dapsone and the absence of another explanation, methemoglobinemia was thought to be due to dapsone which was discontinued. Methylene blue 100 mg IV was administered with improvement in respiratory status and a decrease in methemoglobin levels to 1.9%. Cimetidine was also administered due to literature reports suggesting it may provide a benefit to patients with dapsone induced methemoglobinemia [10].
Methylene blue was repeated the next day due to a rise in methemoglobin levels. Temozolomide was held, although the patient continued radiation therapy. Four days after starting methylene blue, the patient developed a hemolytic anemia evidenced by an increase in serum lactate dehydrogenase (LDH) levels, elevated indirect bilirubin, markedly decreased haptoglobin, and increasing reticulocytes. Two separate transfusions of packed red cells were given 2 days apart for the drop in hemoglobin from 12.8 to 7.5 g/dl. The peripheral blood smear was examined and did not reveal classic bite cells or blister cells. It was suspected that the patient was experiencing a hemolytic reaction to methylene blue due to possibly occult G6PD deficiency. No Heinz Bodies were observed on a Heinz Body preparation. However, the patient had received packed red cells the previous day so accuracy of the result is questionable. The patient recovered without complication and was discharged in stable condition to complete radiation therapy as an outpatient. Although G6PD testing could not be repeated because the patient was lost to follow up, the patient's methemoglobinemia and hemolysis accurately demonstrate two potential complications of dapsone therapy.
Discussion
Dapsone is increasingly being used for prophylaxis against P. jiroveci pneumonia in patients who are immunocompromised due to radiation, high dose steroids, and temozolomide therapy. Rarely, as in our patient, the use of dapsone may lead to acquired methemoglobinemia. Dapsone is metabolized and bioactivated in the liver to a potent, oxidizing hydroxylamine [11]. This dapsone hydroxylamine can then rapidly deplete erythrocyte gluatathione levels, and directly increase the rate of oxidation of hemoglobin to methemoglobin [8, 9, 11, 12]. Methemoglobin is unable to bind oxygen and shifts the oxygen saturation curve to the left, resulting in a decrease in oxygen delivery to tissues which results in cyanosis, dyspnea and tachycardia. Ash-Bernal et al. describe a characteristic “chocolate-brown” color to arterial blood in these patients [13]. There is also a frequent discrepancy between non-invasive pulse oximetry readings and oxygen saturation from arterial blood gas studies, with noninvasive readings indicating hypoxia. This is known as a “saturation gap” and is a useful clue to increase the suspicion for this diagnosis [13]. The level of hypoxia as measured by non-invasive pulse oximetry can plateau at 80–85% and will fail to reveal worsening hypoxemia [9]. It is important to recognize the inaccuracy of pulse oximetry in this setting and to use co-oximetry and arterial blood gas measurements as guides for management. As methemoglobin levels rise, patients become more confused and experience severe cardiovascular and respiratory symptoms with an increased risk of mortality due to heart failure at methemoglobin levels above 70% [9].
Methemoglobinemia can be caused by genetic enzyme deficiencies, nitrate exposure, local anesthetics (benzo-caine) and antibiotics such as dapsone, a sulfone antibiotic. In large series, 5.8–20% of patients taking dapsone developed an increase in methemoglobinemia levels [13, 14]. Patients most at risk for dapsone-induced methemoglobinemia include anemic patients and the elderly, both of whom are frequently seen in neuro-oncology practice. A rare etiology of acquired methemoglobinemia are patients with G6PD deficiency because they lack the biochemical pathways to efficiently reduce methemoglobin to oxyhemoglobin [15]. Persons with African, Asian, or Mediterranean heritage are most commonly affected [16]. Patients with the G6PD Mediterranean variant are at increased risk of severe hemolysis following oxidant injury to hemoglobin [17].
Prophylactic therapy to prevent potentially life-threatening opportunistic infections such as P. jiroveci in immunocompromised patients with gliomas is of critical importance. Methemoglobinemia is a well-known and serious toxicity of dapsone and should be considered in patients who present with symptoms of hypoxemia. The classic symptoms of methemoglobenemia such as shortness of breath and confusion are common to other diagnoses in glioma patients including pulmonary embolism, pneumonia, and chemotherapy-induced anemia. Recognition of this potential problem is also important because standard pulse oximetry and arterial blood gas measurements do not detect methemoglobin levels. The “saturation gap” in this case prompted further investigation of an alternative etiology of this patient's hypoxemia. Patients receiving dapsone who present with increased confusion and shortness of breath should have co-oximetry to evaluate for methemoglobinemia [18].
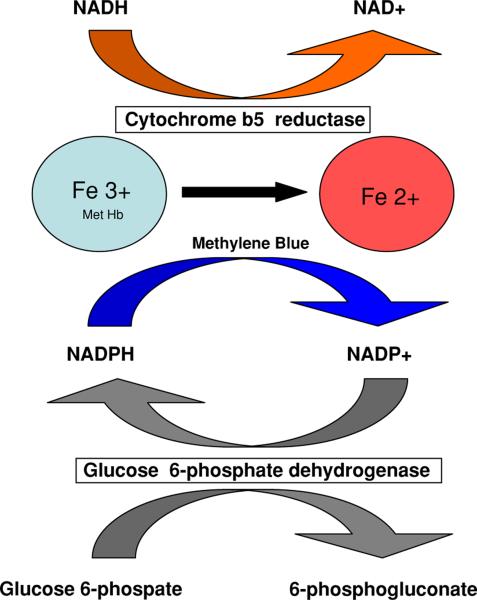
Treatment of methemoglobinemia begins with discontinuing the offending agent which will often reverse the process. Pharmacological treatment consists primarily of methylene blue, which provides an artificial electron carrier for reduction of methemoglobin via an alternate, NADPH-dependent pathway [19]. Usual dosage is 1–2 mg/kg IV and may be repeated in 1h. The NADPH that is required for this reaction is generated in erythrocytes from the pentose phosphate shunt, by the action of glucose-6-phosphate-dehydrogenase (G6PD). Methylene blue is able to accept the electron from NADPH, and donate it the ferric iron, facilitating the reduction of methemoglobin to oxyhemoglobin. [7, 8, 20] (see Fig. 1). In patients with G6PD deficiency methylene blue, which is itself an oxidative agent, may induce a more severe hemolytic reaction in the absence of sufficient levels of NADPH. Because of our patient's history of Mediterranean descent, this became a concern as severe hemolysis, requiring transfusion, followed methylene blue administration.
Fig. 1.
Redox pathways for methemoglobin. The major physiologic pathway for reduction of methemoglobin to hemoglobin is through cytochrome b5 reductase. NADH required for this reaction is supplied by intermediates of glycolysis. Defects in the cytochrome b5 pathway are seen in congential methemoglobinemia. In an alternative pathway, exogenously delivered electron acceptors such as methylene blue can reduce methemoglobin. Electrons from this pathway come from NADPH that is generated in the pentose phosphate shunt through the action of glucose-6-phosphate dehydrogenase (G6PDH)
Conclusion
Primary brain tumor patients have multiple risk factors for P. jiroveci and may require prophylaxis with TMP-SMZ, dapsone, atovaqone, or pentamidine. Although dapsone is generally safe and efficacious, methemoglobinemia should be considered for glioma patients who present with worsening confusion or cardiopulmonary system dys-function. A high index of suspicion should be given to high-risk patients due to ethnic heritage, anemia, or advanced age. Co-oximetry should be performed to assist in the diagnosis. Given the toxicities of TMP-SMZ and dapsone, further work is needed to determine if glioma patients with CD4+ counts less than 300/mm3 require empiric prophylaxis.
Contributor Information
Julie G. Walker, Department of Neuro-Oncology, The University of Texas - M. D. Anderson Cancer Center, Houston, TX, USA
Tapan Kadia, Department of Leukemia, The University of Texas - M. D. Anderson Cancer Center, Houston, TX, USA.
Latrondria Brown, Department of Neuro-Oncology, The University of Texas - M. D. Anderson Cancer Center, Houston, TX, USA.
Harinder S. Juneja, Department of Internal Medicine, University of Texas Health Science Center, Houston, TX, USA
John F. de Groot, Department of Neuro-Oncology, The University of Texas - M. D. Anderson Cancer Center, Houston, TX, USA
References
- 1.Hughes MA, Parisi M, Grossman S, et al. Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. doi:10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 2.Su YB, Sohn S, Krown SE, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. doi:10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 3.Grossman SA, Desideri S, Ye X, Szajna K, Chamberlain M, Lesser G. Iatrogenic immunosuppression in patients with newly diagnosed high-grade gliomas. J Clin Oncol, 2007 ASCO annual meeting proceedings. 2007;25 Abstract 2012. [Google Scholar]
- 4.Yu SK, Chalmers AJ. Patients receiving standard-dose temozolomide therapy are at risk of Pneumocystis carinii pneumonia. Clin Oncol (R Coll Radiol) 2007;19:631–632. doi: 10.1016/j.clon.2007.06.003. doi:10.1016/j.clon.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Hughes WT, Rivera GK, Schell MJ, et al. Successful intermittent chemoprophylaxis for Pneumocystis carinii pneumonitis. N Engl J Med. 1987;316:1627–1632. doi: 10.1056/NEJM198706253162604. [DOI] [PubMed] [Google Scholar]
- 6.Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A controlled trial of trimethoprimsulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776–782. doi: 10.1056/NEJM199009203231202. [DOI] [PubMed] [Google Scholar]
- 7.Mansouri A, Lurie AA. Concise review: methemoglobinemia. Am J Hematol. 1993;42:7–12. doi: 10.1002/ajh.2830420104. doi:10.1002/ajh.2830420104. [DOI] [PubMed] [Google Scholar]
- 8.Umbreit J. Methemoglobin-it's not just blue: a concise review. Am J Hematol. 2007;82:134–144. doi: 10.1002/ajh.20738. doi:10.1002/ajh.20738. [DOI] [PubMed] [Google Scholar]
- 9.Turner MD, Karlis V, Glickman RS. The recognition, physiology, and treatment of medication-induced methemoglobinemia: a case report. Anesth Prog. 2007;54:115–117. doi: 10.2344/0003-3006(2007)54[115:TRPATO]2.0.CO;2. doi: 10.2344/0003-3006(2007)54[115:TRPATO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman MD, Rhodes LE, Scott AK, et al. The use of cimetidine to reduce dapsone-dependent methaemoglobinaemia in dermatitis herpetiformis patients. Br J Clin Pharmacol. 1992;34:244–249. doi: 10.1111/j.1365-2125.1992.tb04131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly TP, Woster PM, Svensson CK. Methemoglobin formation by hydroxylamine metabolites of sulfamethoxazole and dapsone: implications for differences in adverse drug reactions. J Pharmacol Exp Ther. 1999;288:951–959. [PubMed] [Google Scholar]
- 12.Abidi MH, Kozlowski JR, Ibrahim RB, et al. The sulfone syndrome secondary to dapsone prophylaxis in a patient undergoing unrelated hematopoietic stem cell transplantation. Hematol Oncol. 2006;24:164–165. doi: 10.1002/hon.780. doi:10.1002/hon.780. [DOI] [PubMed] [Google Scholar]
- 13.Ash-Bernal R, Wise R, Wright SM. Acquired methemoglobinemia: a retrospective series of 138 cases at 2 teaching hospitals. Medicine (Baltimore) 2004;83:265–273. doi: 10.1097/01.md.0000141096.00377.3f. doi:10.1097/01.md. 0000141096.00377.3f. [DOI] [PubMed] [Google Scholar]
- 14.Williams S, MacDonald P, Hoyer JD, et al. Methemoglobinemia in children with acute lymphoblastic leukemia (ALL) receiving dapsone for pneumocystis carinii pneumonia (PCP) prophylaxis: a correlation with cytochrome b5 reductase (Cb5R) enzyme levels. Pediatr Blood Cancer. 2005;44:55–62. doi: 10.1002/pbc.20164. doi:10.1002/ pbc.20164. [DOI] [PubMed] [Google Scholar]
- 15.Walenta S, Dotsch J, Bourrat-Flock B, et al. Size-dependent oxygenation and energy status in multicellular tumor spheroids. Adv Exp Med Biol. 1990;277:889–893. doi: 10.1007/978-1-4684-8181-5_102. [DOI] [PubMed] [Google Scholar]
- 16.Vulliamy T, Mason P, Luzzatto L. The molecular basis of glucose-6-phosphate dehydrogenase deficiency. Trends Genet. 1992;8:138–143. doi: 10.1016/0168-9525(92)90372-B. [DOI] [PubMed] [Google Scholar]
- 17.Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 1991;324:169–174. doi: 10.1056/NEJM199101173240306. [DOI] [PubMed] [Google Scholar]
- 18.Dunford LM, Roy DM, Hahn TE, et al. Dapsone-induced methemoglobinemia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:241–242. doi: 10.1016/j.bbmt.2005.10.008. doi:10.1016/j.bbmt.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Kane GC, Hoehn SM, Behrenbeck TR, et al. Benzocaine-induced methemoglobinemia based on the Mayo Clinic experience from 28 478 transesophageal echocardiograms: incidence, outcomes, and predisposing factors. Arch Intern Med. 2007;167:1977–1982. doi: 10.1001/archinte.167.18.1977. doi:10.1001/archinte.167.18.1977. [DOI] [PubMed] [Google Scholar]
- 20.Goluboff N, Wheaton R. Methylene blue induced cyanosis and acute hemolytic anemia complicating the treatment of methemoglobinemia. J Pediatr. 1961;58:86–89. doi: 10.1016/s0022-3476(61)80064-4. doi:10.1016/S0022-3476 (61)80064-4. [DOI] [PubMed] [Google Scholar]