Abstract
OBJECTIVES
This study sought to determine the use of intravenous fluids in the early care of patients with acute decompensated heart failure (HF) who are treated with loop diuretics.
BACKGROUND
Intravenous fluids are routinely provided to many hospitalized patients.
METHODS
We conducted a retrospective cohort study of patients admitted with HF to 346 hospitals from 2009 to 2010. We assessed the use of intravenous fluids during the first 2 days of hospitalization. We determined the frequency of adverse in-hospital outcomes. We assessed variation in the use of intravenous fluids across hospitals and patient groups.
RESULTS
Among 131,430 hospitalizations for HF, 13,806 (11%) were in patients treated with intravenous fluids during the first 2 days. The median volume of administered fluid was 1,000 ml (interquartile range: 1,000 to 2,000 ml), and the most commonly used fluids were normal saline (80%) and half-normal saline (12%). Demographic characteristics and comorbidities were similar in hospitalizations in which patients did and did not receive fluids. Patients who were treated with intravenous fluids had higher rates of subsequent critical care admission (5.7% vs. 3.8%; p < 0.0001), intubation (1.4% vs. 1.0%; p = 0.0012), renal replacement therapy (0.6% vs. 0.3%; p < 0.0001), and hospital death (3.3% vs. 1.8%; p < 0.0001) compared with those who received only diuretics. The proportion of hospitalizations that used fluid treatment varied widely across hospitals (range: 0% to 71%; median: 12.5%).
CONCLUSIONS
Many patients who are hospitalized with HF and receive diuretics also receive intravenous fluids during their early inpatient care, and the proportion varies among hospitals. Such practice is associated with worse outcomes and warrants further investigation. (J Am Coll Cardiol HF 2015;3:127–33) © 2015 by the American College of Cardiology Foundation.
Keywords: diuretics, heart failure, intravenous fluids
Many signs and symptoms of heart failure (HF) are the result of volume overload (1). Diuretic therapy, which reduces excess volume, is the most common treatment applied to improve symptoms and cardiovascular function (2,3). For patients treated with diuretics, the administration of intravenous fluids is counterintuitive. Although some studies have investigated the benefits of co-administration of small volumes of hypertonic saline (4,5), the guidelines generally suggest fluid restriction for patients with HF and do not generally recommend intravenous fluid therapy (6–8). However, intravenous fluids are routinely administered to hospitalized patients (9,10), and little is known about the frequency with which this occurs in patients with HF who are treated with diuretics. If this practice were common, it could indicate conflicting treatment patterns.
We investigated the frequency and pattern of early treatment with intravenous fluids among inpatients with HF who received loop diuretic therapy in a national sample of hospitals. We focused on early treatment to avoid treatments that are in response to changes in the clinical condition of the hospitalized patient, such as use of fluids in response to intensive diuretic therapy. We also examined the association between the early administration of intravenous fluids and in-hospital events, including subsequent critical care admission, subsequent endotracheal intubation, subsequent renal replacement therapy, and in-hospital death. Further, we examined the variability in hospital rates of intravenous fluid administration in patients who concomitantly received loop diuretics.
METHODS
DATA SOURCE AND STUDY SAMPLE
We conducted a retrospective cohort study using a database created by Premier, Inc. (Charlotte, North Carolina) that roughly represents 20% of annual acute care hospitalizations in the United States. In addition to information available in the standard hospital discharge file, the database contains a date-stamped log of all billed items at the patient level, including diagnostic tests, medications, and therapeutic services (11).
We included hospitalizations from 2009 and 2010 for patients age 18 years or older with a principal discharge diagnosis of HF, as defined by International Classification of Diseases-Ninth Revision-Clinical Modification (ICD-9-CM) codes 402.01, 402.11, 402.91, 404.01, 404.11, 404.91, or 428.xx, who were treated with loop diuretic therapy in the first 2 days of hospitalization. Hospitalizations were excluded if the patients were hospitalized for <2 days, had a pediatric attending physician, or were transferred in. We focused on patients who were stable and excluded those who may have received intravenous fluids for another reason such as invasive cardiovascular procedures in the first 2 days; those with a secondary discharge diagnosis of sepsis, bleeding, or anaphylaxis; and those who received vasopressor or inotrope therapies. We excluded patients who had ICD-9-CM codes for stage 5 chronic kidney disease or end-stage renal disease because the use of diuretics and fluids is largely driven by kidney function status in such patients (Online Appendix 1). We also excluded hospitalizations during which patients had a critical care admission in the first 2 days, underwent endotracheal intubation in the first 2 days, or received renal replacement therapy in the first 2 days because we were interested in studying in-hospital outcomes of new critical care admission, new intubation, and new dialysis after the first 2 days.
INTRAVENOUS FLUID USE, LOOP DIURETIC USE, AND CLASSIFICATION OF TREATMENT INTENSITY
We defined intravenous fluid use as any use ≥500 ml of normal saline, half-normal saline solution, Ringer’s/lactated Ringer’s, or a combination of these solutions during the first 2 days of hospitalization, using 34 different administrative codes for intravenous fluids. We selected the volume cutoff to ensure that we captured fluid use that was administered for fluid management rather than for administration of other medications such as antibiotics or intravenous antiarrhythmics (12). We did not include codes related to administration of dextrose 5% solution because dextrose 5% would minimally enhance the intravascular volume.
We defined loop diuretic use as any use of furosemide, bumetanide, torsemide, or a combination during the first 2 days. In line with published studies, we converted the doses of bumetanide and torsemide to bioequivalent doses of furosemides: 40 mg intravenous furosemide = 80 mg oral furosemide = 20 mg (oral or intravenous) torsemide = 1 mg (oral or intravenous) bumetanide (13–15).
OUTCOME MEASURES
Our primary outcome was the proportion of hospitalizations in which patients were treated with intravenous fluids in the first 2 days of hospitalization. We noted the dose of each treatment over the first 2 days.
Secondary outcomes included length of stay, in-hospital death, and receipt of subsequent (after day 2) critical care admission, intubation, and renal replacement therapy by treatment group (i.e., fluid treatment or no fluid treatment). We also determined the risk-standardized utilization rates of intravenous fluids across hospitals.
STATISTICAL ANALYSES
We calculated summary statistics for categorical variables using frequencies and percentages. We used a chi-square test to determine differences between treatment groups for unadjusted outcomes and hierarchical logistic regression to calculate adjusted odds ratios for all outcomes and to calculate hospital-level fluid utilization rates. We calculated odds ratios and 95% confidence intervals (CIs) of the fluid treatment versus no fluid treatment group for each outcome. Each patient could have been hospitalized more than once during the study period, although the majority had a single hospitalization. We used all hospitalizations when calculating odds ratios for renal replacement therapy, admission to an intensive care unit, and late intubation, and 1 randomly selected hospitalization per patient when calculating odds ratios for in-hospital mortality. We adjusted for clinically pre-specified patient characteristics and comorbidities (Table 1). A p value of <0.05 was considered statistically significant; however, no adjustment for multiple comparisons was made. Interpretation of the results should take this into consideration.
TABLE 1.
Patient Characteristics (N = 131,430)
| Overall (n = 131,430) |
Patients Treated With IV Fluids* (n = 13,806) |
Patients Not Treated With IV Fluids* (n = 117,624) |
|
|---|---|---|---|
| Age, yrs | |||
| 18–54 | 14,127 (11) | 1,439 (10) | 12,688 (11) |
| 55–64 | 17,156 (13) | 1,805 (13) | 15,351 (13) |
| 65–74 | 25,454 (19) | 2,712 (20) | 22,742 (19) |
| 75–84 | 38,189 (29) | 3,950 (29) | 34,239 (29) |
| 85+ | 36,504 (28) | 3,900 (28) | 32,604 (28) |
|
| |||
| Sex | |||
| Female | 69,408 (53) | 7,521 (54) | 61,887 (53) |
| Male | 62,021 (47) | 6,285 (46) | 55,736 (47) |
|
| |||
| Race | |||
| White | 83,262 (63) | 8,599 (62) | 74,663 (63) |
| Black | 24,680 (19) | 2,199 (16) | 22,481 (19) |
| Hispanic | 5,967 (5) | 731 (5) | 5,236 (4) |
| Other | 17,521 (13) | 2,277 (16) | 15,244 (13) |
|
| |||
| Comorbidities | |||
| Obesity | 22,237 (17) | 2,258 (16) | 19,979 (17) |
| Diabetes with and without complications | 59,041 (45) | 6,181 (45) | 52,860 (45) |
| Hypertension | 92,522 (70) | 9,518 (69) | 83,004 (71) |
| Anemia | 40,572 (31) | 4,748 (34) | 35,824 (30) |
| Renal failure | 50,089 (38) | 5,274 (38) | 44,815 (38) |
| Liver disease | 3,077 (2) | 385 (3) | 2,692 (2) |
| Cancer | 1,395 (1) | 203 (1) | 1,192 (1) |
| Peripheral vascular disease | 16,793 (13) | 1,870 (14) | 14,923 (13) |
Values are n (%).
All patients were treated with loop diuretics.
IV = intravenous.
K.M.S. and S.L. conducted the analyses with SAS version 9.2 (SAS Institute, Inc., Cary, North Carolina). The Yale University Human Investigation Committee (New Haven, Connecticut) reviewed the study protocol and determined that it was not considered Human Subjects Research as defined by the Office of Human Research Protections.
RESULTS
COHORT SELECTION AND BASIC CHARACTERISTICS
We identified 250,256 hospitalizations with HF in which patients were 18 years or older and had a nonpediatric attending physician in the 2009 to 2010 database. After applying the exclusions, there were 131,430 hospitalizations in the final cohort (Figure 1). The majority of patients were older than 75 years (57%), female (53%), and white (63%) (Table 1). The most common comorbidity was hypertension (70%), followed by diabetes (45%). The group that received intravenous fluids had demographic characteristics that were similar to those of the group that did not receive intravenous fluids (see Table 1).
FIGURE 1. Flow Diagram of Patient Selection.
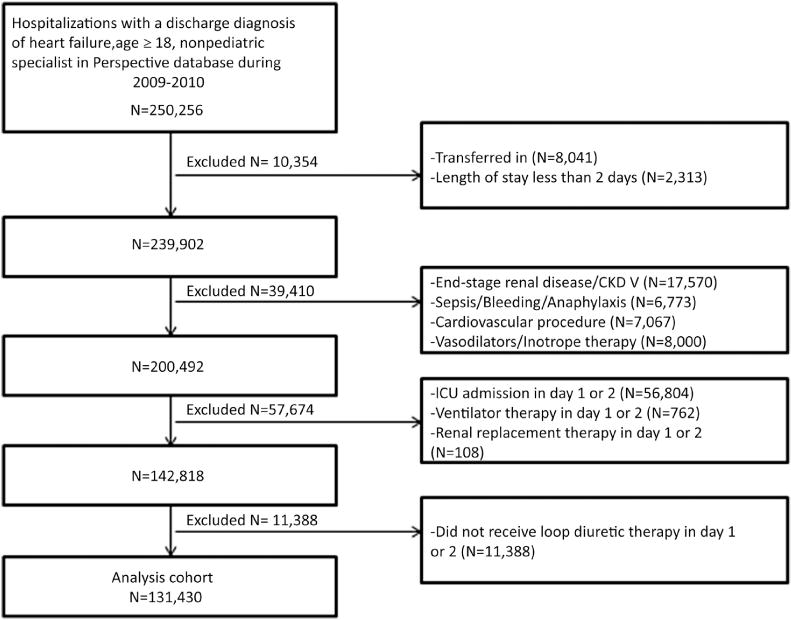
CKD V = stage V chronic kidney disease; ICU = intensive care unit.
TREATMENT WITH INTRAVENOUS FLUIDS AND LOOP DIURETICS
Patients received intravenous fluid therapy in 11% of hospitalizations, of which 80% involved normal saline solution as the only fluid treatment and 91% involved furosemide as the only loop diuretic (Table 2). For hospitalizations in which patients received intravenous fluids, the median total intravenous fluid volume was 1,000 ml (interquartile range [IQR]: 1,000 to 2,000 ml) during the first 2 days, and the median total diuretic dose in intravenous furosemide-equivalent doses was 120 mg (IQR: 80 to 200 mg) over the first 2 days. The median total loop diuretic dose in intravenous furosemide-equivalent doses for those who did not receive intravenous fluids was 140 mg (IQR: 80 to 210 mg) during the first 2 days.
TABLE 2.
Loop Diuretic and Fluid Utilization Among Hospitalizations in Which Patients Received Intravenous Fluids (N = 13,806)
| Type of Therapy | Utilization |
|---|---|
| Diuretic therapy | |
|
| |
| Furosemide | 12,534 (91) |
| Bumetanide | 488 (3) |
| Torsemide | 85 (1) |
| Mixed* | 699 (5) |
|
| |
| Fluid therapy | |
|
| |
| Normal saline solution | 11,097 (80) |
| Half-normal saline solution | 1,591 (12) |
| Ringer’s/lactated Ringer’s | 226 (2) |
| Mixed† | 892 (6) |
Values are n (%).
Defined as any combination of loop diuretics.
Defined as any combination of intravenous fluids.
OUTCOMES
Median hospital length of stay was 4 days (IQR: 3 to 7 days) among hospitalizations in which patients received intravenous fluids and 4 days (IQR: 2 to 6 days) among hospitalizations in which patients did not receive intravenous fluids. Hospitalizations in which patients received intravenous fluids were associated with higher rates of subsequent critical care admission (5.7% vs. 3.8%; p < 0.0001), late intubation (1.4% vs. 1.0%; p = 0.0012), renal replacement therapy (0.6% vs. 0.3%; p < 0.0001), and in-hospital mortality (3.3% vs. 1.8%; p < 0.0001) compared with hospitalizations in which the patient did not receive intravenous fluids.
After adjusting for demographics and comorbidities, hospitalized patients who received intravenous fluids were, after day 2, 1.57 (95% CI: 1.45 to 1.71) times more likely to be admitted to critical care, 1.46 (95% CI: 1.25 to 1.71) times more likely to undergo intubation, 2.04 (95% CI: 1.62 to 2.55) times more likely to receive renal replacement therapy, and 2.02 (95% CI: 1.82 to 2.24) times more likely to experience in-hospital death compared with hospitalized patients who did not receive intravenous fluids (Table 3).
TABLE 3.
Risk-Adjusted Hospitalization Outcomes by Therapy
| Critical Care Admission* | Late Intubation | Renal Replacement Therapy | In-Hospital Death | |
|---|---|---|---|---|
| No treatment with intravenous fluids† | – | – | – | – |
| Treatment with intravenous fluids† | 1.57 (1.45–1.71) |
1.46 (1.25–1.71) |
2.04 (1.62–2.55) |
2.02 (1.82–2.24) |
Values are odds ratio (95% confidence interval).
Includes admission to step-down units.
All patients were treated with loop diuretics.
TREATMENT WITH INTRAVENOUS FLUIDS ACROSS HOSPITALS
Across hospitals with at least 25 HF hospitalizations during the study period, the risk-standardized fluid utilization rate ranged from 0% to 71.1%, with a median of 12.5% (Figure 2).
FIGURE 2. Intravenous Fluid Administration Rate by Hospital (N = 346).
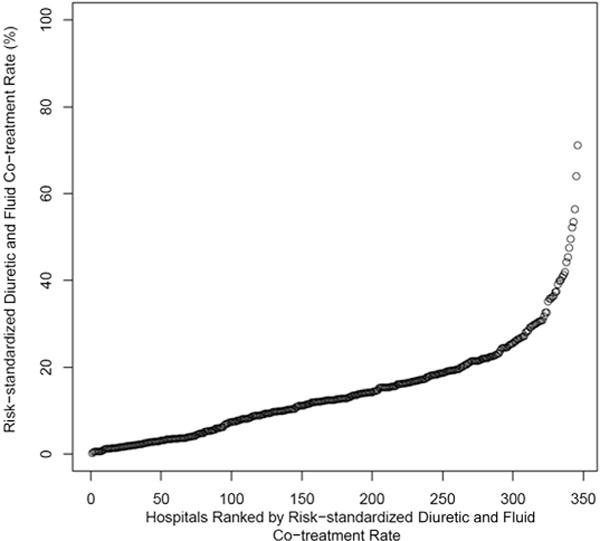
Each circle represents a hospital.
DISCUSSION
Among hospitalizations for acute decompensated HF in which patients were treated with loop diuretics in the first 2 days, we found that 11% were also given volumes of intravenous fluids (median: 1,000 ml) that were neither negligible nor used primarily for administration of other therapies. The remarkable variation among hospitals suggests that factors beyond differences in patient populations influence co-administration of fluids and diuretics.
Our study is the first to investigate the use of intravenous fluids among patients hospitalized with decompensated HF. Because fluid overload is a major contributor to acute decompensation in patients with HF (8), the administration of intravenous fluids in addition to loop diuretic therapy is an unanticipated observation. There are several possible explanations for the considerable rate of intravenous fluid administration in acutely decompensated inpatients with HF. First, some patients may have received fluids because of hemodynamic instability. We excluded patients most likely to have hemodynamic instability (i.e., those with discharge diagnoses of bleeding, sepsis, or anaphylaxis), as well as those who underwent invasive cardiovascular procedures and who received vasopressor or inotropic treatment in the first 2 days. Nevertheless, it is still likely that some unstable patients remained in our study cohort. Second, fluids could have been administered to some patients with acute decompensated right ventricular failure. However, this is not likely to reflect a large proportion of the study patients who received treatment with intravenous fluids. Usually, patients with acute right ventricular failure need inotrope or vasopressor therapy and, accordingly, we excluded those who received inotropes or vasopressors during the first 2 days (16). Third, fluids may have been administered to counter the detrimental effects of excessive loop diuretic therapy, but we did not observe a difference in the loop diuretic dose between the 2 groups. Further, our examination of use of intravenous fluids during the first 2 days of hospitalization minimized the possibility of including fluid therapy that was administered in response to excessive diuresis. Another possibility is the inadvertent administration of intravenous fluids.
Finally, it is possible that the concomitant use of diuretics and fluids was a purposeful treatment strategy. Although several small studies have shown hemodynamic and clinical improvement in patients receiving co-treatment with loop diuretics and small volumes of intravenous hypertonic saline (4,5,17,18), the amounts of fluid were very small (100 to 150 ml twice daily), and the small volume of hypertonic fluid introduced a notable amount of salt for the treated patients, unlike the fluids that we studied. Because of using administrative data, we are, however, unable to specify the reasons for use of intravenous fluids in each of the patients in our cohort.
We observed higher rates of late critical care admission, late intubation, late renal replacement therapy, and in-hospital death for patients who received intravenous fluids compared with those who did not. One possibility, consistent with expert opinion, is that fluid overload may directly worsen the clinical outcomes of patients with HF (6–8,19), who are commonly volume overloaded. However, it is also possible that the administration of fluids is a marker of greater clinical severity rather than a cause of worse outcomes. Although we had used rigorous inclusion and exclusion criteria to minimize the possibility of having unstable patients in our cohort, our findings should not be considered causal at this stage.
STUDY LIMITATIONS
Our results need to be interpreted in the context of the study limitations. Our study identified that intravenous fluids are administered in 11% of hospitalizations for patients with acute decompensated HF who are treated with loop diuretics. We believe that the rates of intravenous fluid use would have been even higher had we not excluded hospitalizations in patients who were more likely to be unstable (e.g. those receiving inotropes). Although we were unable to distinguish between the types of HF (i.e., HF with preserved versus reduced ejection fraction), previous studies have shown the high specificity of the codes we used for principal diagnosis of HF among hospitalized patients (20–22). Another limitation of our study was lack of access to kidney function biomarkers, left ventricular function indices, and hemodynamic parameters such as heart rate or blood pressure. Availability of these indices could have been helpful for further mechanistic evaluations and prognostication. Despite not having access to such variables, however, we minimized the chance of including hemodynamically unstable patients by excluding those who received vasopressor or inotrope therapy, those who underwent invasive cardiovascular procedures, and those with discharge diagnoses of sepsis, bleeding, or anaphylaxis. Even if we overestimated the problem because of lack of clinical information that could explain the use of intravenous fluids in some patients, it is important in its magnitude. It should be mentioned that our final cohort, built using these inclusions and exclusions, had similar characteristics compared with patients in existing HF registries, such as the ADHERE (Acute Decompensated HEart failure REgistry). (13). Another limitation of our study is the use of billing to determine fluid administration rather than direct observation. It is possible that in some cases, the fluid was dispensed but not administered or partially used. Even in the unlikely event that this attribution error commonly occurred, the rates of fluid administration to patients with HF are sufficiently high to indicate a need for further examination.
Finally, as stated earlier, the associations that we observed should not be considered causal. Although it is possible that inadvertent use of fluids may lead to worse outcomes, there are other potential explanations that, despite our exclusion and inclusion criteria, cannot be eliminated. Until we have further data from future prospective investigations, decisions about the use of intravenous fluids in patients with decompensated HF remain a challenge and should be made on a case-by-case basis with respect to factors such as HF status and renal function. Whereas practitioners may deliberately choose to administer intravenous fluids for patients with HF who may need such therapy, it appears prudent for hospitals to implement strategies that would help minimize the possibility of inadvertent intravenous fluid therapy. These would include review of standard emergency department order sets that could routinely call for intravenous fluids, as well as use of automated reminders that could help minimize unnecessary administration of fluids to patients with decompensated HF.
CONCLUSIONS
We found that the administration of fluids to patients admitted with HF is not uncommon and varies substantially across hospitals, with potential adverse consequences. This practice may occur inadvertently for many patients, warrants further investigation, and may be an opportunity for improvement.
Supplementary Material
Acknowledgments
This study was supported by grant DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut and by grant UL1 RR024139-06S1 from the National Center for Advancing Translational Sciences in Bethesda, Maryland. This study was also funded, in part, by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute in Bethesda, Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. Dr. Bikdeli is now a PGY-2 Internal Medicine Resident at Yale University and Yale-New Haven Hospital. Dr. Mody is a PGY-3 Internal Medicine Resident at the University of Texas Southwestern Medical Center. Dr. Dharmarajan is an Assistant Professor of Medicine at Yale University School of Medicine. Dr. Krumholz is the recipient of research agreements from Medtronic and from Johnson & Johnson, through Yale University, to develop methods of clinical trial data sharing; and he chairs a cardiac scientific advisory board for United Healthcare. Dr. Dharmarajan is supported by grant HL007854 from the National Heart, Lung, and Blood Institute; and is supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale University by the John A. Hartford Foundation and the American Federation for Aging Research. Dr. Horwitz is supported by the National Institute on Aging (K08 AG038336) and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program.
ABBREVIATIONS AND ACRONYMS
- HF
heart failure
- ICD-9-CM
International Classification of Diseases-Ninth Revision-Clinical Modification
Footnotes
During the time the work was conducted, Drs. Bikdeli and Mody were post-doctoral associates, Dr. Dharmarajan was a post-doctoral fellow, and Dr. Partovian was an instructor in the Section of Cardiovascular Medicine, Department of Internal Medicine, Yale University School of Medicine in New Haven, Connecticut.
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For a list of cardiovascular procedures and a list of International Classification of Diseases-Ninth Revision-Clinical Modification codes indicating stage V or end-stage renal disease, please see the online version of this paper.
References
- 1.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–85. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Digitalis Investigators Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 4.Issa VS, Andrade L, Ayub-Ferreira SM, et al. Hypertonic saline solution for prevention of renal dysfunction in patients with decompensated heart failure. Int J Cardiol. 2013;5:34–40. doi: 10.1016/j.ijcard.2011.11.087. [DOI] [PubMed] [Google Scholar]
- 5.Paterna S, Di Pasquale P, Parrinello G, et al. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure: a double-blind study. J Am Coll Cardiol. 2005;45:1997–2003. doi: 10.1016/j.jacc.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–96. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 9.Hilton AK, Pellegrino VA, Scheinkestel CD. Avoiding common problems associated with intravenous fluid therapy. Med J Aust. 2008;189:509–13. doi: 10.5694/j.1326-5377.2008.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 10.Prowle JR, Bellomo R. Fluid administration and the kidney. Curr Opin Crit Care. 2010;16:332–6. doi: 10.1097/MCC.0b013e32833be90b. [DOI] [PubMed] [Google Scholar]
- 11.Premier Research Services. Premier healthcare alliance. Available at: http://www.premierinc.com/prs. Accessed November 14, 2014.
- 12.GlobalRPH. The clinician’s ultimate reference. Available at: http://www.globalrph.com. Accessed December 4, 2014.
- 13.Peacock WF, Costanzo MR, De Marco T, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–9. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 14.Shankar SS, Brater DC. Loop diuretics: from the Na-K-2Cl transporter to clinical use. Am J Physiol Renal Physiol. 2003;284:F11–21. doi: 10.1152/ajprenal.00119.2002. [DOI] [PubMed] [Google Scholar]
- 15.Mielniczuk LM, Tsang SW, Desai AS, et al. The association between high-dose diuretics and clinical stability in ambulatory chronic heart failure patients. J Card Fail. 2008;14:388–93. doi: 10.1016/j.cardfail.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Piazza G, Goldhaber SZ. The acutely decompensated right ventricle: pathways for diagnosis and management. Chest. 2005;128:1836–52. doi: 10.1378/chest.128.3.1836. [DOI] [PubMed] [Google Scholar]
- 17.Licata G, Di Pasquale P, Parrinello G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution infusion in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long-term effects. Am Heart J. 2003;145:459–66. doi: 10.1067/mhj.2003.166. [DOI] [PubMed] [Google Scholar]
- 18.Drazner MH, Palmer BF. Hypertonic saline: a novel therapy for advanced heart failure? Am Heart J. 2003;145:377–9. doi: 10.1067/mhj.2003.165. [DOI] [PubMed] [Google Scholar]
- 19.Yeates KE, Singer M, Morton AR. Salt and water: a simple approach to hyponatremia. CMAJ. 2004;170:365–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–41. doi: 10.1111/j.1475-6773.2007.00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–5. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 22.Kumler T, Gislason GH, Kirk V, et al. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658–60. doi: 10.1016/j.ejheart.2008.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


