Abstract
The phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α) is activated in response to various stresses such as viral infection, nutrient deprivation, and stress to the endoplasmic reticulum. Severe stress to the endoplasmic reticulum, for instance, induces an apoptotic pathway, while mild stress, on the contrary, leads to a pro-survival pathway. Little has been known about the elaborate role of eIF2α phosphorylation in the development of bone-forming osteoblasts and bone-resorbing osteoclasts. Using salubrinal and guanabenz as inhibitors of the de-phosphorylation of eIF2α, we have recently reported that the phosphorylation of eIF2α significantly alters fates of both osteoblasts and osteoclasts. Based on our recent findings, we review in this research highlight the potential mechanisms of the enhancement of osteoblastogenesis and the suppression of osteoclastogenesis through the elevated level of phosphorylated eIF2α.
Keywords: osteoclast, eIF2α, Salubrinal, Guanabenz, principal component analysis
Mechanism of the enhancement of osteoblastogenesis by the inhibition of de-phosphorylation of eIF2α
Bone remodeling is a combined process of bone formation by osteoblasts and bone resorption by osteoclasts. We first examined the involvement of eIF2α in regulation of osteoblasts. Stress to the endoplasmic reticulum leads to the elevated phosphorylation level of eIF2α and suppresses general translation initiation except for some stress-responsive genes, including activating transcription factor 4 (ATF4) [1, 2]. ATF4 is an important transcription factor for differentiation of mature osteoblasts[3], prompting a question: Does the inhibition of de-phosphorylation of eIF2α stimulate development of osteoblasts? Salubrinal and guanabenz are synthetic chemical agents known to specifically de-phosphorylate eIF2α by inhibiting protein phosphatase 1 (PP1) [4, 5]. They are also known as suppressors of stress to the endoplasmic reticulum.
In response to salubrinal and guanabenz, the level of phosphorylation of eIF2α was elevated in MC3T3 E1 osteoblast-like cells. These agents also increased the level of ATF4 as well as osteocalcin, which is known as a marker for osteoblastogenesis[6, 7]. Furthermore, the process of mineralization is enhanced. Thus, the inhibition of de-phosphorylation of eIF2a by salubrinal and guanabenz enhances development and mineralization of osteoblasts.
Mechanism of the suppression of osteoclastogenesis by the inhibition of de-phosphorylation of eIF2α
We next investigated the involvement of eIF2α in regulation of bone-resorbing osteoclasts. In RAW264.7 cells and mouse primary macrophages, treatment with receptor activator of nuclear factor kappa-B (RANKL) stimulate their development to mature osteoclasts. However, the administration of salubrinal and guanabenz decreased the number of tartrate-resistant acid phosphatase (TRAP) positive cells and suppressed osteoclastogenesis[6–9]. As a mechanism for the observed suppression of osteoclastogenesis, it was reported that these synthetic agents reduced the level of RANKL-induced activation of nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1)[6, 7], which is a master transcription factor of osteoclastogenesis[10]. In order to identify transcription factor(s) that downregulated NFATc1, genome-wide microarray analysis was performed.
Principal component analysis (PCA) is a statistical procedure used to reduce the dimensions of a large dataset to help identify axes that best explain the variance of the data [11]. PCA can be used to analyze genome-wide microarray data and identify principal axes and genes that highly contribute to those axes. PCA predicted a set of stimulatory and inhibitory transcription factor candidates underlying salubrinal- and guanabenz-driven suppression of osteoclastogenesis. Among these, two AP-1 transcription factors (c-Fos and JunB) were included.
As predicted, expression levels of c-Fos and JunB were upregulated by RANKL, and their upregulation was suppressed by salubrinal and guanabenz in mouse primary macrophage and RAW264.7 cells [9]. In RAW264.7 cells, a partial silencing of c-Fos by RNA interference attenuated RANKL-driven expression of NFATc1, TRAP, and cathepsin K. A partial silencing of JunB reduced NFATc1 and TRAP, but not cathepsin K. To further analyze regulatory linkages among NFATc1, c-Fos and JunB, a partial silencing of NFATc1 was conducted. Twelve hours after RANKL treatment in RAW264.7 cells, treatment with NFATc1 siRNA did not alter expression of c-Fos and JunB. In 24 h, however, the level of c-Fos was significantly reduced without affecting the level of JunB[9]. Collectively, the result suggests a potential feedback loop between NFATc1 and c-Fos.
Conclusion
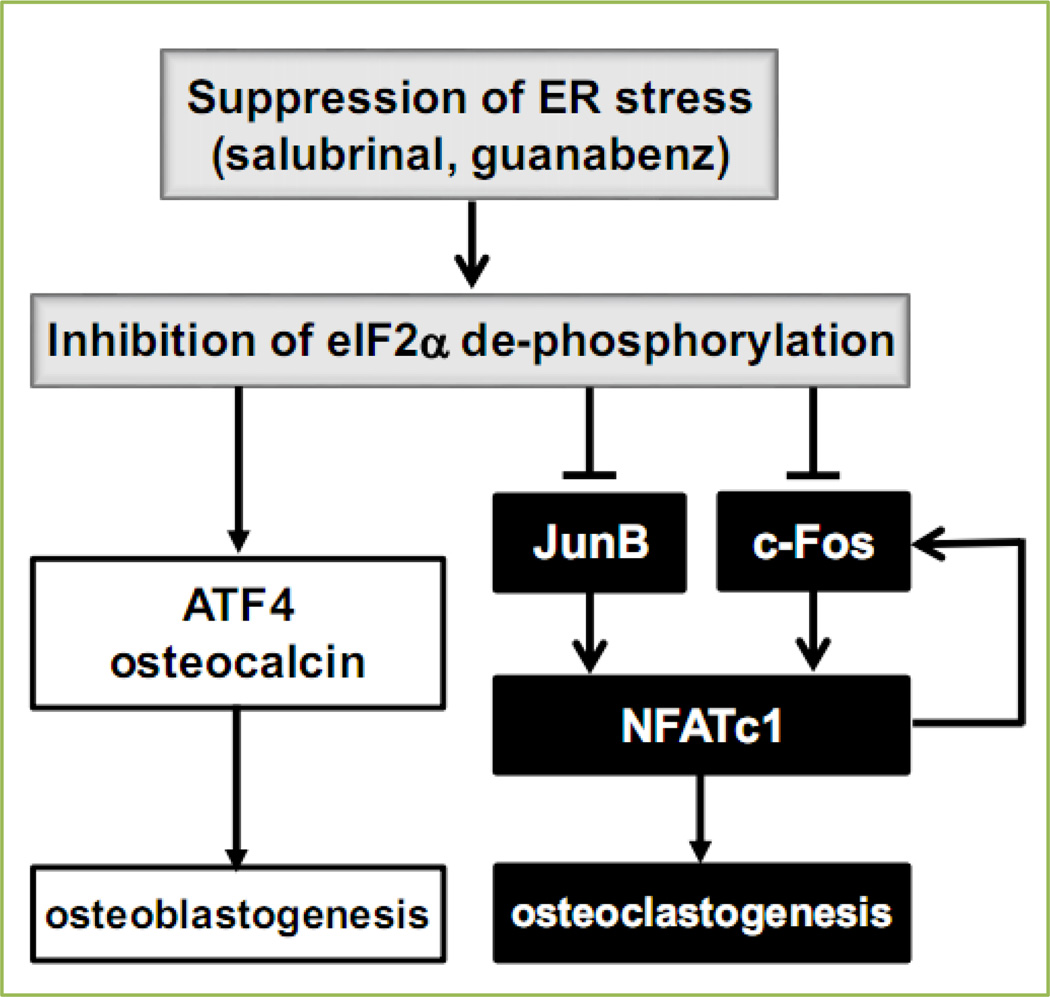
Inhibition of de-phosphorylation of eIF2α promotes differentiation and mineralization of osteoblasts through the upregulation of ATF4. The same inhibition in osteoclasts, on the other hand, suppresses osteoclastogenesis by downregulating RANKL-driven NFATc1. A genome-wide expression analysis demonstrates that the regulation of NFATc1 is mediated by c-Fos and JunB (Fig. 1). In summary, the current study demonstrates that inhibitors of de-phosphorylation of eIF2α such as salubrinal and guanabenz may provide a novel therapeutic possibility for bone diseases including osteoporosis.
Figure 1.
Proposed model for the enhancement of osteoblastogenesis and the suppression of osteoclastogenesis in response to salubrinal and guanabenz.
Acknowledgment
This work was supported by grant DOD W81XWH-11-1-0716.
Footnotes
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- 1.Baird TD, Wek RC. Eukaryotic initiation factor 2 phosphorylation and translational control in metabolism. AdvNutr. 2012;3:307–321. doi: 10.3945/an.112.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly N, Gorman AM, Gupta S, Samail A. The eIF2α kinases: their structures and functions. Cell Mol Life Sci. 2013;70:3493–3511. doi: 10.1007/s00018-012-1252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. Cell. 2004;117:387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 4.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2 alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 5.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 6.He L, Lee J, Jang JH, Sakchaisri K, Hwang J, Cha-Molstad HJ, et al. Osteoporosis regulation by salubrinal through eIF2a mediated differentiation of osteoclast and osteoblast. Cell Signal. 2013;25:552–560. doi: 10.1016/j.cellsig.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamamura K, Tanjung N, Yokota H. Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J Bone Miner Metab. 2013;31:618–628. doi: 10.1007/s00774-013-0450-0. [DOI] [PubMed] [Google Scholar]
- 8.Yokota H, Hamamura K, Chen A, Dodge TR, Tanjung N, Abedinpoor A, et al. Effects of salubrinal on development of osteoclasts and osteoblasts from bone marrow derived cells. BMC Musculoskelet Disord. 2013;14:197. doi: 10.1186/1471-2474-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamamura K, Chen A, Tanjung N, Takigawa S, Sudo A, Yokota H. In vitro and in silico analysis of an inhibitory mechanism of osteoclastogenesis by salubrinal and guanabenz. Cell Signal. 2015;27:353–362. doi: 10.1016/j.cellsig.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 11.Raychaudhuri S, Stuart JM, Altman RB. Principal components analysis to summarize microarray experiments: application to sporulation time series. In Pacific Symposium on Biocomputing. Pac Symp Biocomput. 2000:455–466. doi: 10.1142/9789814447331_0043. [DOI] [PMC free article] [PubMed] [Google Scholar]