Abstract
The inaugural meeting of “New Frontiers in Pediatric Allogeneic Stem Cell Transplantation” organized by the Pediatric Blood and Transplant Consortium (PBMTC) was held at the American Society of Pediatric Hematology and Oncology Annual Meeting. This meeting provided an international platform for physicians and investigators active in the research and utilization of pediatric allogeneic stem cell transplantation (AlloSCT) in children and adolescents with malignant and non-malignant disease, to share information and develop future collaborative strategies. The primary objectives of the conference included: 1) to present advances in AlloSCT in pediatric ALL and novel pre- and post-immunotherapy; 2) to highlight new strategies in alternative allogeneic stem cell donor sources for children and adolescents with non-malignant hematological disorders; 3) to discuss timing of immune reconstitution after AlloSCT and methods of facilitating more rapid recovery of immunity; 4) to identify strategies of utilizing AlloSCT in pediatric myeloproliferative disorders (MPD); 5) to develop diagnostic and therapeutic approaches to hematological complications post pediatric AlloSCT; 6) to enhance the understanding of new novel cellular therapeutic approaches to pediatric malignant and non-malignant hematological disorders; and 7) to discuss optimizing drug therapy in pediatric recipients of AlloSCT. This paper will provide a brief overview of the conference.
Keywords: Pediatric, Allogeneic, Stem cell transplantation, Advances, Malignant, Nonmalignant
INTRODUCTION
The inaugural meeting of “New Frontiers in Pediatric Allogeneic Stem Cell Transplantation” organized by the Pediatric Blood and Transplant Consortium (PBMTC) was held in Miami, Florida on April 24, 2013 at the American Society of Pediatric Hematology and Oncology Annual Meeting. This meeting provided an international platform for physicians and investigators active in the research and utilization of pediatric allogeneic stem cell transplantation (AlloSCT) in children and adolescents with malignant and non-malignant disease, to communicate, share information, and develop future collaborative strategies. The primary objectives of the conference included: 1) to present advances in AlloSCT in pediatric ALL with a focus on minimal residual disease (MRD), eligibility and novel pre- and post-immunotherapy; 2) to highlight new strategies in alternative allogeneic stem cell donor sources for children and adolescents with non-malignant hematological disorders; 3) to discuss timing of immune reconstitution after AlloSCT and methods of facilitating more rapid recovery of immunity; 4) to discuss optimizing drug therapy in pediatric recipients of AlloSCT; 5) to identify strategies of utilizing AlloSCT in pediatric myeloproliferative disorders (MPD); 6) to develop diagnostic and therapeutic approaches to hematological complications post pediatric AlloSCT; and 7) to enhance the understanding of new novel cellular therapeutic approaches to pediatric malignant and non-malignant hematological disorders. A brief overview of the meeting is presented below.
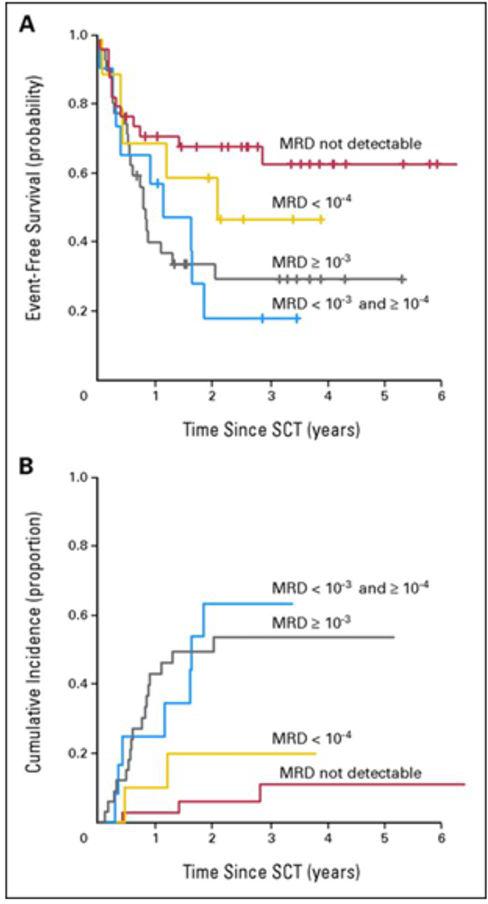
The first plenary session addressed critical issues including MRD, cytogenetics and immunotherapy during AlloSCT in children and adolescents with ALL. First, the importance of MRD pre- and post-AlloSCT in pediatric ALL was reviewed. For all studies, MRD was measured on bone marrow aspirates and performed using 6-color flow cytometry.1 Bader et al. has previously demonstrated that MRD before AlloSCT was shown to predict outcome in children with relapsed ALL who received AlloSCT in ≥ second complete remission (CR2).2 Probability of event-free survival (pEFS) and cumulative incidence of relapse (CIR) in patients with MRD ≥ 10−4 leukemic cells was 0.27 and 0.57 compared with 0.60 and 0.13 in patients with MRD less than 10−4 leukemic cells (EFS, P = .004; CIR, P < .001).2 Intermediate-risk patients with MRD≥ 10−4 leukemic cells had a pEFS of 0.20 and CIR of 0.73, whereas patients with MRD < 10−4 leukemic cells had a pEFS of 0.68 and CIR of 0.09 (EFS, P = .020; CIR, P < .001).2 High-risk patients ≥CR3 who received transplantation with an MRD load < 10−4 leukemic cells showed a pEFS and CRI of 0.53 and 0.18, respectively.2 In contrast, pEFS and CRI were 0.30 and 0.50 in patients who received transplantation with an MRD load of ≥ 10−4 leukemic cells.2 Multivariate Cox regression analysis revealed MRD as the only independent parameter predictive for EFS (P = .006)2 (Figure 1). Additionally, Leung et al. demonstrated in children with very-high-risk ALL or AML who received an AlloSCT and that higher MRD levels at the time of AlloSCT predicted a poorer survival and that MRD was an independent prognostic factor in a multivariate analysis.3
Figure 1. 1a. Event-free survival probability (pEFS) and 1b. cumulative incidence of subsequent relapse (CIR) in patients with first (n = 77) or second (n = 14) relapse of acute lymphoblastic leukemia by minimal residual disease (MRD) status before allogeneic stem-cell transplantation (SCT).
MRD > 10−3 leukemic cells: n = 33; censored, n = 11; deaths, n = 5; relapses, n = 17;1a. pEFS (4 years) = 0.31 ± 0.09; 1b. CIR (4 years) = 0.54 ± 0.09. MRD < 10−3 and ≥ 10−4 leukemic cells: n = 12; censored, n = 3; deaths, n = 2; relapses, n = 7; 1a. pEFS (3.5 years) = 0.19 ± 0.12; 1b. CIR (3.5 years) = 0.64 ± 0.16. MRD < 10−4 leukemic cells: n = 10; censored, n = 5; deaths, n = 3; relapses, n = 2; 1a. pEFS (3.5 years) = 0.48 ± 0.16; 1b. CIR (3.5 years) = 0.20 ± 0.14. MRD not detectable: n = 36; censored, n = 24; deaths, n = 9; relapses, n = 3; 1a. pEFS (4 years) = 0.64 ± 0.09; 1b. CIR (4 years) = 0.11 ± 0.06. 1a. EFS, log-rank, P = .036. 1b. CIR, Gray, P < .001.
From Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol 2009; 27(3): 377-84.
There are a few subtypes of ALL where strong consideration should be made for allotransplant in CR1 in whom the outcomes with chemotherapy alone are dismal.
Historically these have included BCR/ABL, hypodiploid ALL, and MLL rearrangements. However, with the recent results from the Children's Oncology Group (COG) using tyrosine kinase inhibitors (TKIs) versus AlloSCT in patients with Philadelphia chromosome-positive (Ph+) ALL provide interesting insights into the role of AlloSCT in this disease.4 Updates from COG study AALL0031 revealed no significant differences (P = .93) in the estimates of EFS for patients with Ph+ ALL enrolled on cohort 5 receiving imatinib (EFS, 84% ± 7%) versus matched sibling donor (MSD) AlloSCT (EFS, 77% ± 12%) versus matched unrelated donor (MUD) AlloSCT (EFS, 83% ± 15%).4 Hypodiploid ALL is another high risk feature in childhood ALL which has been an indication for AlloSCT in the past.5, 6 Importantly, a high rate of TP53 mutations has been identified in patients with low hypodiploid ALL (32 to 39 chromosomes). Approximately 45% of these patients were subsequently found to have germline events, thus firmly establishing this subtype of childhood ALL within the Li-Fraumeni syndrome. Slow response to induction therapy has been the single most important risk factor for relapse in children and adolescents with ALL and are an indication for AlloSCT in the past.6 Many centers pursue AlloSCT early for patients with frank induction failure (day 29 BM with >25% lymphoblasts) after reinduction strategies.6
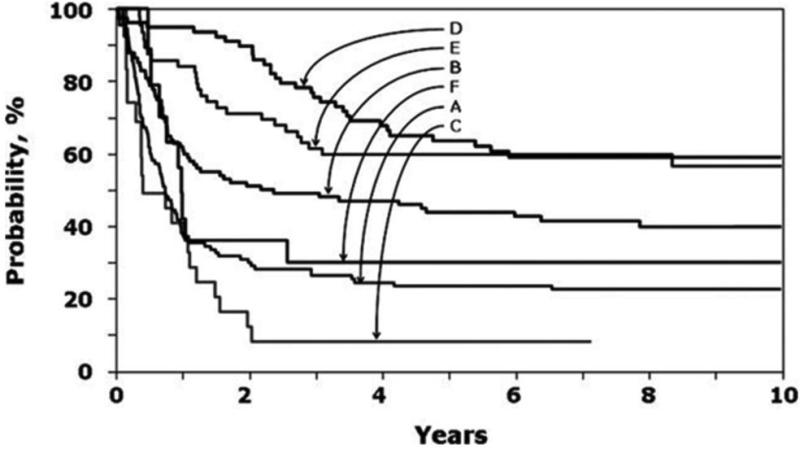
Novel approaches to prevent relapse were discussed and included pre-transplant conditioning, post-transplant immunosuppression, donor lymphocyte infusion (DLI), and novel agents including immunotherapeutic approaches. Myeloablative transplants for ALL which include total body irradiation (TBI) of 1200 cGY or greater have an improved survival benefit compared to non-TBI containing regimens.7 The leukemia-free survival (LFS) is approximately 15-33% higher in patients who received TBI-containing regimens compared to those who received non-TBI containing regimens (Figure 2).8-10 Other strategies to prevent relapse include utilizing lower doses of cyclosporine11 and a rapid taper of cyclosporine and/or DLI for increasing mixed chimerism post-transplant.12
Figure 2. Probability of leukemia-free survival.
A indicates chemotherapy recipients after an early first relapse; B, transplant recipients with TBI regimens after an early first relapse; C, transplant recipients with non-TBI regimens after an early first relapse; D, chemotherapy recipients after late first relapse; E, transplant recipients with TBI regimens after late first relapse; and F, transplant recipients with non-TBI regimens after late first relapse.
From Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood 2006; 107(12): 4961-7.
An overview of upcoming novel agents and immunotherapeutic approaches to reduce MRD pre-transplant was performed, which included moxetumomab pasudotox (anti CD22-immunotoxin) for childhood ALL which is currently in Phase I-II trials at the NCI.13, 14 Anti-CD19 bi-specific T cell engager Blinatumomab has shown promising results in a phase II adult trial in MRD eradication and is currently under investigation in pediatric trials.15, 16 Other novel therapies include genetically engineered cells to target ALL –chimeric antigen receptor transduced T cells, which are also currently in early phase trials including CD19 and CD22 chimeric antigen receptors and thus far, the early results are encouraging.17
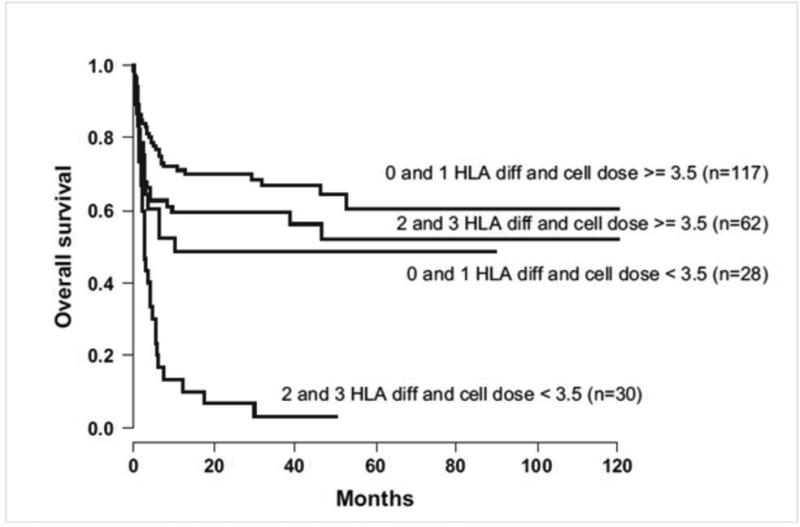
The second session entitled “Alternative Allogeneic Donor Stem Sources in Children with Non-Malignant Hematological Diseases” discussed alternative allografts including matched unrelated donor, umbilical cord blood (UCB), and haploidentical transplantation. The donor chosen for allogeneic transplantation is dependent on a multitude of factors including degree of mismatch, the cell dose if cord is utilized, and timing of transplant. The types of diseases that are transplanted include hemoglobinopathies, immune deficiencies, bone marrow failure syndromes and metabolic diseases. Cairo et al. reported on the experience of using unrelated UCB for non-malignant diseases (NMD).18 The majority of the preparative regimens were busulfan based with 30% utilizing fludarabine and 84% utilizing an anti-T cell therapy. The role of HLA mismatching was abrogated by increasing cell dose of the UCB. Patients given a higher cell dose >3.5 107/kg even with 0-3 HLA disparities had a higher probability of engraftment compared to those given lower cell dose with the same disparities (Figure 3). Horan et al.19 analyzed data from 663 unrelated marrow and peripheral blood stem cell transplants performed from 1995 to 2007 for treatment of NMD. Transplantation from a donor mismatched at the HLA-A, -B, -C, or -DRB1, but not -DQB1 or -DPB1, loci was associated with higher mortality in multivariate analyses (P = .002).19 HLA mismatches were not associated with acute or chronic GVHD, but were strongly associated with graft failure. The authors concluded that patients with NMD should receive transplants from allele matched (8/8) donors if possible.19 However, improvements in BMT supportive care, minimizing preparative regimen intensity and toxicity, and novel post transplant immunosuppressive regimens are making alternative donor transplants a safer option. Finally, antibodies directed against donor-disparate HLA antigens increase graft failure risk which is particularly important in this population of patients with non-malignant disorders.20, 21
Figure 3. OS in 268 patients with nonmalignant disorders in UCBT according to cell dose infused (×107/kg) and HLA differences.
From Cairo MS, Rocha V, Gluckman E, Hale G, Wagner J. Alternative allogeneic donor sources for transplantation for childhood diseases: unrelated cord blood and haploidentical family donors. Biol Blood Marrow Transplant 2008; 14(1 Suppl 1): 44-53.
Alternative allografts in patients with hemoglobinopathies was discussed and Pietro Sodani reviewed their institutional experience with haploidentical stem cell transplantation and CD34 selection using the CliniMACS® device (Miltenyi Biotec, Cambridge, MA) in patients with thalassemia and sickle cell disease. Sodani et al.22 added hydroxyurea, azathioprine and fludarabine to their traditional busulfan and cyclophosphamide preparative regimen for children with class 3 thalassemia. This resulted in a survival of 93% and a decreased rejection from 30% to 8 %.22 To extend transplantation to all patients who do not have a well matched unrelated donor, haploidentical transplant has been performed in children with thalassemia.23 Twenty-two patients underwent transplantation with a 90% survival at 5 years, however, they experienced a 27% rejection rate. This study demonstrated that this approach is feasible and has been recently applied to children with sickle cell disease.24
Clinical trials in Fanconi anemia have focused on obtaining adequate engraftment with less collateral damage. Wagner et al. reported on a fludarabine-based regimen in Fanconi anemia.25 He found the day-100 mortality rate was significantly higher after non-fludarabine-containing regimens compared to fludarabine-containing regimens (65% vs 24%, respectively; P < .001).25 Corresponding the 3-year adjusted overall survival rates were 52% versus 13% (P < .001).25 In addition, mortality was higher in recipients who were older (> 10 years), CMV seropositive, and who received > 20 blood product transfusions before AlloSCT.25
Immune Reconstitution after AlloSCT in Pediatric Recipients was reviewed by allogeneic donor source during the third plenary session. The quantity and quality of immune reconstitution is correlated with long-term clinical outcomes: survival, presence of GVHD, infections and relapse rate. Bartelink et al. compared immune reconstitution by various graft sources in pediatric recipients.26 The nature and speed of T cell, B cell, and natural killer (NK) cell reconstitution were highly dependent on the cell source. In the first 90 days after HSCT, faster B cell and NK cell reconstitution and delayed T cell reconstitution were shown in unrelated cord blood recipients compared with matched sibling BM and unrelated BM recipients.26 A more rapid and sustained CD4 count reflected by higher CD4 + area under the curve (AUC) correlated with reduced mortality. Renard et al. reported on the post-transplant lymphocyte subset recovery of children treated with unrelated UCB transplant (UCBT) or unrelated bone marrow transplant (UBMT) for malignant and NMD.27 NK cells recovered first in a similar time frame for both graft sources. B cells recovered quicker in unrelated UCBT, compared to UBMT.
Outcomes after AlloSCT have steadily improved over the past several decades, and much of this improvement is due to refinement of strategies to prevent and treat infectious complications.28, 29 Effective prophylaxis requires information regarding individual patient risk of developing specific opportunistic infections, as well as the availability of chemoprophylaxis agents that can either prevent development or progression of infection.30-35 The profound deficit in lymphoid immunity imposed by AlloSCT increases the risk of both episodic viral infections and reactivation of latent viruses. Clinically important latent viruses include mostly herpes viruses (CMV, EBV, HHV6, HSV, and VZV), with CMV being the most commonly seen after AlloSCT. AlloSCT recipients are also vulnerable to invasive fungal infections (IFI) both during the pre-engraftment neutropenic period (allogeneic/autologous) and post-engraftment due to T cell lymphopenia (allogeneic).36
Revaccination strategies post-AlloSCT in pediatric recipients were also addressed in this session. In 2009, the CDC and Center for International Blood and Marrow Transplant Research published guidelines with substantial new recommendations.37 These included inactivated vaccines to start as early as 6 months post-HSCT, administering inactivated vaccines despite presence of GVHD, pneumococcal vaccine to start as early as 3 months post-HCT and varicella vaccine changed from contraindicated to optional (Table 1).37
Table 1.
Current CDC/ACIP Recommendations for Immunization Following BMT
| Vaccine | Initiate Vaccine | Number of Doses | Interval Between Doses | Evidence Rating |
|---|---|---|---|---|
| Pneumococcal conjugate (PCV) | 3-6 months | 3-4 | 4 weeks | B1 |
| PPSV23 | After PCV series | 1 | 1 month after PCV series | BII |
| Tetanus, diphtheria, acellular pertussis | 6-12 months | 3 | 4 weeks | BII |
| Haemophilus influenza conjugate | 6-12 months | 3 | 4 weeks | BII |
| Meningococal conjugate (MCV4) | 6-12 months | 2 | 8 weeks | BII |
| IPV | 6-12 months | 3 | 4 weeks | BII |
| Hepatitis B | 6-12 months | 3 | 4 weeks | BII |
| Hepatitis A | 12 months (optional) | 2 | 6 months | CIII |
| Influenza seasonal | 4-6 months | 1 or 2 | Yearly | AIII |
| MMR | 24 months, no active GVHD, no immunosuppression | 2 | 4 weeks | BII |
| VZV | >24 months, no active GVHD, no immunosuppression (optional) | 2 | 3 months | CIII |
| HPV | Age appropriate (optional) | 3 | Follow CDC | CIII |
Note: CDC/ACIP = Centers for Disease Control/Advisory Committee on Immunization Practices; PPSV = pneumococcal polysaccharide vaccine; IPV = inactivated polio vaccine; MMR = measles, mumps and rubella; GVHD = graft-versus-host disease; VZV = varicella-zoster virus; HPV = human papilloma virus.
Optimizing drug therapy in Pediatric AlloSCT was discussed during plenary session four with a focus on pharmacokinetics/pharmacodynamics in pediatric recipients during AlloSCT. Practice patterns of dosing of IV busulfan were reviewed. In 729 children who underwent therapeutic drug monitoring at Seattle Cancer Care Alliance Busulfan Pharmacokinetics Laboratory, the initial busulfan dose achieved the target exposure in only 24.3% of children.38 Pediatric AlloSCT recipients receiving IV busulfan Q12H achieved a target AUC 60% of the time.39 Pediatric AlloSCT recipient results indicates that daily IV busulfan (N=221) has similar pharmacokinetics as every12 hour (N=20) and every 6 hour (N=162) administration.40 Multi-institutional pharmacokinetic studies are needed to improve dosing in pediatric AlloSCT patients.
Therapeutic drug monitoring of mycophenolate mofetil (MMF) in pediatric AlloSCT recipients was discussed. Mycophenolic acid (MPA) exposure is low in AlloSCT relative to organ transplant recipients possibly attributed to loss of enterohepatic recycling due to gut decontamination, destruction of absorptive capacity, enhanced glucuronidation or altered drug transport. This results in high interpatient variability in MPA exposure. IV dosing achieves higher unbound MPA exposures than oral. Total MPA trough ≥1 mcg/mL was associated with better engraftment and less AGVHD.41, 42 Unbound AUC 0-12 > 300 ng·hr/mL was associated with less aGVHD (cumulative AUC of 600 ng·hr/mL in 24 hr period). Proposed MPA target concentrations were achieved in 50% or less of nonmyeloablative AlloSCT recipients receiving MMF 2g/day.
During plenary Session 5 the role of allografting in children with MPD was discussed. The role of AlloSCT in JMML was presented and specifically the results of a recent North American protocol which evaluated tipifarnib, afarnesyltransferase inhibitor in a phase 2 window prior to AlloSCT. The results thus far show a trend toward inferior survival in those patients who received tipifarnib compared to the controls. Currently, there is a new North American protocol underway which is randomizing the conditioning regimen prior to transplantation comparing busulfan and fludarabine vs busulfan, cyclophosphamide and melphalan (COG ASCT1221).
CML in children was discussed addressing the question: with the advent of TKIs should pediatric patients undergo AlloSCT? Millot et al.43 conducted a clinical trial in children with newly diagnosed CML in chronic phase (CP) who received daily imatinib 260 mg/m2. Progression-free survival (PFS), responses, and tolerance were evaluated. With a median follow-up of 31 months, the estimated PFS at 36 months was 98% (95% CI, 85% to 100%). A complete hematologic response was achieved in 98% of the patients. The rates of complete cytogenetic response (CCyR) and major molecular response (MMR) were 61% and 31% at 12 months, respectively. During follow-up, CCyR and MMR were achieved in 36 children (77%) and 25 children (57%), respectively. Overall, 30% of the patients discontinued imatinib, mainly because of unsatisfactory response. The most common adverse events were neutropenia and musculoskeletal events. So the question is which option should we offer patients, long-term cure with AlloSCT or lifelong TKI? A reasonable approach is first to enroll children and adolescents in open CML protocols. If the patient is in CP – starting with hydroxyurea and a TKI and following closely BCR/ABL by cytogenetics and PCR is a reasonable first approach.44 However, if a patient is in accelerated phase or blast crisis, develops resistance to TKI and/or fails to achieve cytogenetic remission (CyR), then proceeding to AlloSCT is a reasonable choice. As children and adolescents are treated for longer periods of time with TKIs, it has become clear that toxicities may make long-term TKI therapy less attractive compared to AlloSCT. Reduced intensity conditioning (RIC) and AlloSCT have significantly less transplant-related morbidity compared to myeloablative conditioning (MAC),36, 45 whereas prolonged TKIs may cause growth failure, hepatic, and cardiac complications. Therefore, randomized international trials are urgently needed to evaluate the best therapies for pediatric CML.
The role of allografting in myelodysplastic syndrome (MDS) in children was also discussed. MDS is a rare disorder affecting ~4/million children/yr and represents a heterogeneous group of a clonal hematopoietic stem cell disorders. The disease is characterized by cytopenias and ineffective and dysplastic hematopoiesis which has a varying propensity to leukemic transformation. MDS can be caused by chemo- or radiation therapy. Strahm et al.46 reviewed the AlloSCT experience in children with MDS. Thirty-nine children were transplanted from MSD, whereas 58 were given the allograft from a MUD (n=57) or alternative family donor (n=1). With a median follow-up of 3.9 years, the 5-year probability of overall survival is 63%, while the 5-year cumulative incidence of transplantation-related mortality (TRM) and relapse is 21% each. Age at AlloSCT >12 years, interval between diagnosis and AlloSCT >4 months, and occurrence of AGVHD or extensive CGVHD were associated with increased TRM. The majority of patients with tMDS were conditioned with Total Body Irradiation based regimens with cyclophosphamide. The risk of relapse increased with more advanced disease.
Hematological complications after allografting in pediatric recipients were discussed in Plenary Session 6. Autoimmune hemolytic anemia (AIHA) can occur in 2-20% post-AlloSCT. Sanz et al.47 reviewed 272 patients and found the 3-year cumulative incidence of AIHA was 4.44%. Horn et al.48 reported a high incidence (19.5%) of AIHA in 41 patients with severe combined immunedeficiency (SCID) who underwent a T cell-depleted haploidentical transplant. Other than infections, AIHA was the most common post-transplant complication in this patient cohort. Delayed reconstitution of T cell immunity, due to T cell depletion, immunosuppressive conditioning and cyclosporine, as well as paucity of regulatory T cells, were possible causes for the occurrence of AIHA. The use of rituximab has been reported in case reports as effective therapy. Other treatments include steroids, intravenous immune globulin (IVIG), splenectomy, and other immunosuppressant drugs. For patients who are resistant to these therapies, recent publications have suggested the use of bortezomib.49, 50 However, future prospective trials are needed to further evaluate this treatment in this setting.
Thrombotic microangiopathy (TMA) post AlloSCT was also discussed. This is an under-recognized entity and occurs in approximately 20% of AlloSCT patients.51 TMA is associated with high dose chemotherapy, radiation, GVHD, AlloSCT, calcineurin inhibitors and infection. There have been reports of a higher incidence of AIHA after UCB transplant compared to other allo donor sources. 52Mortality can be 60-90% in severe disease. Patients with TMA after HSCT are 4 times more likely to develop chronic kidney disease (CKD). AlloSCT patients with prolonged TMA are also at risk of developing pulmonary hypertension. Targeted screening utilizing echocardiography for AlloSCT recipients with hypoxemia, cardiorespiratory failure and/or TMA, aids in detecting pulmonary hypertension. In fact, hypertension can be an early marker of TMA. Treatment involves withdrawal of causative agents: calcineurin inhibitors, treatment of infections, treatment of GVHD, control of hypertension, avoidance of nephrotoxic agents, rituximab, therapeutic plasma exchange (TPE), and eculizumab.53 Previous studies have reported poor response rates to TPE for TMA however, recently Jodele et al. has recently published a case series involving ten patients suggesting that early initiation of TPE might be beneficial even in patients with multiorgan failure due to TA-TMA.54 New diagnostic biomarkers are needed and research is currently ongoing.
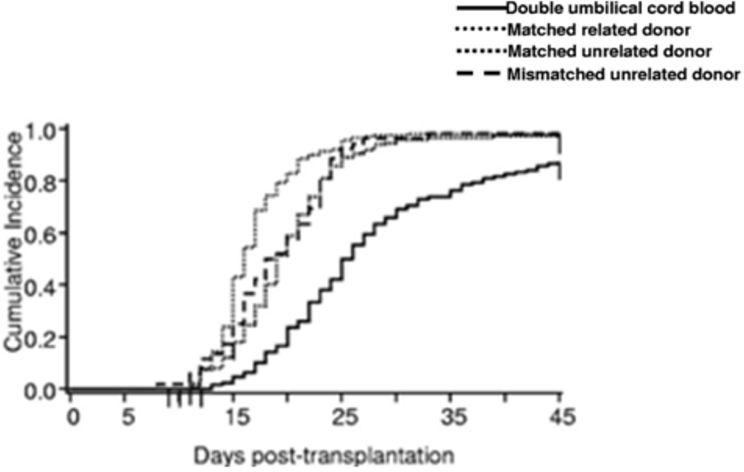
Finally, in the evening plenary session seven, UCB graft engineering was discussed. In cord blood transplantation the continued challenges are related to delayed neutrophil and platelet engraftment55 as well as a higher incidence of primary graft failure compared to other sources allogeneic stem cells (Figure 4). New strategies to enhance hematopoietic recovery will potentially reduce the risk of TRM. To overcome the cell dose barrier in UCBT multiple strategies are being pursued, which include double cord blood transplant, infusion of a haploidentical graft simultaneously with a cord and ex vivo cord blood expansion methods. One method of ex vivo expansion manipulates signaling pathways known to influence hematopoietic stem cell fate using engineered Notch ligands to induce endogenous Notch signaling of CD34+ CB cells. Hematopoietic stem cells are isolated and cultured in the presence of an engineered notch ligand form produced as clinical grade product. The goal of this work is to generate progenitor cells capable of providing rapid short term myeloid engraftment, in order to overcome the delay in hematopoietic recovery in recipients of UCBT. In a current ongoing trial using the engineered product, the median time to engraftment is significantly reduced in patients receiving the manipulated graft product compared to controls double UCB (11 days vs 25 days, p ≤ 0.0001).56
Figure 4. Clinical outcomes.
Neutophil engraftment after double UCB, MRD, MUD and MMUD transplantation.
From Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010; 116(22): 4693-9.
Other strategies to improve engraftment and reduce primary graft failure were discussed including third party facilitator cellular therapy: CB Mesenchymal Precursor Cells (Mesoblast®), CB HSCs (Stem Ex®), and Human Placental Derived Stem Cells (HPDSCs®). Mesenchymal precursor cells may provide cellular and extracellular components of the stem cell “niche” absent in current liquid culture protocols, eliminating the need for positive selection and potentially improving expansion and engraftment. De Lima et al. reported engraftment results in 31 adults with hematologic cancers who received transplants of 2 cord-blood units, 1 of which contained cord blood that was expanded ex vivo in co-cultures with allogeneic mesenchymal stromal cells. In patients in whom engraftment occurred, the median time to neutrophil engraftment was 15 days in the recipients of expanded cord blood, as compared with 24 days in controls who received unmanipulated cord blood only (P<0.001).57 There is currently a phase 3 randomized multicenter trial underway to evaluate the efficacy and safety of UCB cells expanded ex vivo with mesenchymal precursor cells for hematopoietic recovery in patients with hematologic malignancies after MAC.
Finally, utilizing NK cells to enhance the immune system and exploit their antitumor potential was highlighted. NK cells kill tumor targets through multiple pathways including granule exocytosis and a death receptor pathway. Donor-versus-recipient NK cell alloreactivity derives from a mismatch between donor NK clones, carrying specific inhibitory receptors for self MHC class I molecules, and MHC class I ligands on recipient cells. When faced with mismatched allogeneic targets, these donor NK clones sense the missing expression of self HLA class I alleles and mediate killing. Transplantation from NK alloreactive haploidentical donors has been shown to reduce AML relapse and improve engraftment with a low incidence GVHD.58 Ex vivo expansion of NK cells can also be performed and then re-infused into patients post allotransplantation. Liu et al.59 used a strategy of adoptive transfer of NK cells combined with tumor-specific monoclonal antibodies (mAb) against malignancies. Large numbers of activated NK (aNK) cells were grown ex vivo from peripheral blood mononuclear cells of children with high-risk neuroblastoma using artificial antigen-presenting cells. Multiple clinical trials utilizing NK cells in both the allogeneic transplant setting and the non-transplant setting are currently under investigation.
In summary, this conference on “New Frontiers in Pediatric Allogeneic Stem Cell Transplantation” provided a thorough review of the past, present and future strategies and developments in this burgeoning field, and we look forward to this becoming an annual event. A follow-up manuscript will provide a more in depth review of each of the seven plenary sessions at this historical inaugural symposium.
Acknowledgments
Financial Support: Supported by grants from the NCI (#1R13CA177155-01), Pediatric Cancer Research Foundation, Children's Cancer Fund.
Footnotes
Conflict of Interest: All authors declare no conflicts of interest.
References
- 1.Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll WL, et al. The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase III COG/PBMTC trial. Blood. 2014 doi: 10.1182/blood-2013-10-534297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader P, Kreyenberg H, Henze GH, Eckert C, Reising M, Willasch A, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377–84. doi: 10.1200/JCO.2008.17.6065. [DOI] [PubMed] [Google Scholar]
- 3.Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K, et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood. 2012;120(2):468–72. doi: 10.1182/blood-2012-02-409813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, et al. Improved Early Event-Free Survival With Imatinib in Philadelphia Chromosome–Positive Acute Lymphoblastic Leukemia: A Children's Oncology Group Study. Journal of Clinical Oncology. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110(4):1112–5. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochberg J, Khaled S, Forman SJ, Cairo MS. Criteria for and outcomes of allogeneic haematopoietic stem cell transplant in children, adolescents and young adults with acute lymphoblastic leukaemia in first complete remission. British Journal of Haematology. 2013;161(1):27–42. doi: 10.1111/bjh.12239. [DOI] [PubMed] [Google Scholar]
- 7.Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. ASBMT Position Statement. Biol Blood Marrow Transplant 2012. 18(7):979–81. doi: 10.1016/j.bbmt.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543–8. doi: 10.1038/sj.bmt.1704198. [DOI] [PubMed] [Google Scholar]
- 9.Davies SM, Ramsay NK, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY, et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol. 2000;18(2):340–7. doi: 10.1200/JCO.2000.18.2.340. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Raetz E, Zhang MJ, Muehlenbein C, Devidas M, Abshire T, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood. 2006;107(12):4961–7. doi: 10.1182/blood-2005-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locatelli F, Zecca M, Rondelli R, Bonetti F, Dini G, Prete A, et al. Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood. 2000;95(5):1572–9. [PubMed] [Google Scholar]
- 12.Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22(9):1696–705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 13.Barth M, Raetz E, Cairo MS. The future role of monoclonal antibody therapy in childhood acute leukaemias. Br J Haematol. 2012;159(1):3–17. doi: 10.1111/bjh.12002. [DOI] [PubMed] [Google Scholar]
- 14.Kreitman RJ, Pastan I. Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res. 2011;17(20):6398–405. doi: 10.1158/1078-0432.CCR-11-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185–7. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 16.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–8. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 17.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairo MS, Rocha V, Gluckman E, Hale G, Wagner J. Alternative allogeneic donor sources for transplantation for childhood diseases: unrelated cord blood and haploidentical family donors. Biol Blood Marrow Transplant. 2008;14(1 Suppl 1):44–53. doi: 10.1016/j.bbmt.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Horan J, Wang T, Haagenson M, Spellman SR, Dehn J, Eapen M, et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood. 2012;120(14):2918–24. doi: 10.1182/blood-2012-03-417758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciurea SO, Thall PF, Wang X, Wang SA, Hu Y, Cano P, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957–64. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spellman S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–8. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sodani P, Gaziev D, Polchi P, Erer B, Giardini C, Angelucci E, et al. New approach for bone marrow transplantation in patients with class 3 thalassemia aged younger than 17 years. Blood. 2004;104(4):1201–3. doi: 10.1182/blood-2003-08-2800. [DOI] [PubMed] [Google Scholar]
- 23.Sodani P, Isgro A, Gaziev J, Polchi P, Paciaroni K, Marziali M, et al. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood. 2010;115(6):1296–302. doi: 10.1182/blood-2009-05-218982. [DOI] [PubMed] [Google Scholar]
- 24.Freed J, Talano J, Small T, Ricci A, Cairo MS. Allogeneic cellular and autologous stem cell therapy for sickle cell disease: 'whom, when and how'. Bone Marrow Transplant. 2012;47(12):1489–98. doi: 10.1038/bmt.2011.245. [DOI] [PubMed] [Google Scholar]
- 25.Wagner JE, Eapen M, MacMillan ML, Harris RE, Pasquini R, Boulad F, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109(5):2256–62. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartelink IH, Belitser SV, Knibbe CA, Danhof M, de Pagter AJ, Egberts TC, et al. Immune reconstitution kinetics as an early predictor for mortality using various hematopoietic stem cell sources in children. Biol Blood Marrow Transplant. 2013;19(2):305–13. doi: 10.1016/j.bbmt.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Renard C, Barlogis V, Mialou V, Galambrun C, Bernoux D, Goutagny MP, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. Br J Haematol. 2011;152(3):322–30. doi: 10.1111/j.1365-2141.2010.08409.x. [DOI] [PubMed] [Google Scholar]
- 28.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan A, Wang C, Srivastava DK, Burnette K, Shenep JL, Leung W, et al. Timeline, epidemiology, and risk factors for bacterial, fungal, and viral infections in children and adolescents after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(1):94–101. doi: 10.1016/j.bbmt.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boeckh M, Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–9. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelhard D, Akova M, Boeckh MJ, Freifeld A, Sepkowitz K, Viscoli C, et al. Bacterial infection prevention after hematopoietic cell transplantation. Bone Marrow Transplant. 2009;44(8):467–70. doi: 10.1038/bmt.2009.257. [DOI] [PubMed] [Google Scholar]
- 32.Gafter-Gvili A, Fraser A, Paul M, Vidal L, Lawrie TA, van de Wetering MD, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:CD004386. doi: 10.1002/14651858.CD004386.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imran H, Tleyjeh IM, Arndt CA, Baddour LM, Erwin PJ, Tsigrelis C, et al. Fluoroquinolone prophylaxis in patients with neutropenia: a meta-analysis of randomized placebo-controlled trials. Eur J Clin Microbiol Infect Dis. 2008;27(1):53–63. doi: 10.1007/s10096-007-0397-y. [DOI] [PubMed] [Google Scholar]
- 34.Roman E, Osunkwo I, Militano O, Cooney E, van de Ven C, Cairo MS. Liposomal amphotericin B prophylaxis of invasive mold infections in children post allogeneic stem cell transplantation. Pediatr Blood Cancer. 2008;50(2):325–30. doi: 10.1002/pbc.21239. [DOI] [PubMed] [Google Scholar]
- 35.Shereck EB, Cooney E, van de Ven C, Della-Lotta P, Cairo MS. A pilot phase II study of alternate day ganciclovir and foscarnet in preventing cytomegalovirus (CMV) infections in at-risk pediatric and adolescent allogeneic stem cell transplant recipients. Pediatr Blood Cancer. 2007;49(3):306–12. doi: 10.1002/pbc.21043. [DOI] [PubMed] [Google Scholar]
- 36.Satwani P, Jin Z, Duffy D, Morris E, Bhatia M, Garvin JH, et al. Transplantation-related mortality, graft failure, and survival after reduced-toxicity conditioning and allogeneic hematopoietic stem cell transplantation in 100 consecutive pediatric recipients. Biol Blood Marrow Transplant. 2013;19(4):552–61. doi: 10.1016/j.bbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J, et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant. 2009;44(8):521–6. doi: 10.1038/bmt.2009.263. [DOI] [PubMed] [Google Scholar]
- 38.McCune JS, Baker KS, Blough DK, Gamis A, Bemer MJ, Kelton-Rehkopf MC, et al. Variation in prescribing patterns and therapeutic drug monitoring of intravenous busulfan in pediatric hematopoietic cell transplant recipients. J Clin Pharmacol. 2013;53(3):264–75. doi: 10.1177/0091270012447196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Gall JB, Milone MC, Waxman IM, Shaw LM, Harrison L, Duffy D, et al. The pharmacokinetics and safety of twice daily i.v. BU during conditioning in pediatric allo-SCT recipients. Bone Marrow Transplant. 2013;48(1):19–25. doi: 10.1038/bmt.2012.105. [DOI] [PubMed] [Google Scholar]
- 40.Bartelink IH, Boelens JJ, Bredius RG, Egberts AC, Wang C, Bierings MB, et al. Body weight-dependent pharmacokinetics of busulfan in paediatric haematopoietic stem cell transplantation patients: towards individualized dosing. Clin Pharmacokinet. 2012;51(5):331–45. doi: 10.2165/11598180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010;16(3):333–43. doi: 10.1016/j.bbmt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Osunkwo I, Bessmertny O, Harrison L, Cheung YK, Van de Ven C, del Toro G, et al. A pilot study of tacrolimus and mycophenolate mofetil graft-versus-host disease prophylaxis in childhood and adolescent allogeneic stem cell transplant recipients. Biol Blood Marrow Transplant. 2004;10(4):246–58. doi: 10.1016/j.bbmt.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Millot F, Baruchel A, Guilhot J, Petit A, Leblanc T, Bertrand Y, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29(20):2827–32. doi: 10.1200/JCO.2010.32.7114. [DOI] [PubMed] [Google Scholar]
- 44.Suttorp M, Yaniv I, Schultz KR. Controversies in the treatment of CML in children and adolescents: TKIs versus BMT? Biol Blood Marrow Transplant. 2011;17(1 Suppl):S115–22. doi: 10.1016/j.bbmt.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Geyer MB, Jacobson JS, Freedman J, George D, Moore V, van de Ven C, et al. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br J Haematol. 2011;155(2):218–34. doi: 10.1111/j.1365-2141.2011.08822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strahm B, Nollke P, Zecca M, Korthof ET, Bierings M, Furlan I, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25(3):455–62. doi: 10.1038/leu.2010.297. [DOI] [PubMed] [Google Scholar]
- 47.Sanz J, Arriaga F, Montesinos P, Orti G, Lorenzo I, Cantero S, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39(9):555–61. doi: 10.1038/sj.bmt.1705641. [DOI] [PubMed] [Google Scholar]
- 48.Horn B, Viele M, Mentzer W, Mogck N, DeSantes K, Cowan M. Autoimmune hemolytic anemia in patients with SCID after T cell-depleted BM and PBSC transplantation. Bone Marrow Transplant. 1999;24(9):1009–13. doi: 10.1038/sj.bmt.1702011. [DOI] [PubMed] [Google Scholar]
- 49.Danchaivijitr P, Yared J, Rapoport AP. Successful treatment of IgG and complement-mediated autoimmune hemolytic anemia with bortezomib and low-dose cyclophosphamide. Am J Hematol. 2011;86(3):331–2. doi: 10.1002/ajh.21950. [DOI] [PubMed] [Google Scholar]
- 50.Rovira J, Cid J, Gutierrez-Garcia G, Pereira A, Fernandez-Aviles F, Rosinol L, et al. Fatal immune hemolytic anemia following allogeneic stem cell transplantation: report of 2 cases and review of literature. Transfus Med Rev. 2013;27(3):166–70. doi: 10.1016/j.tmrv.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 51.Laskin BL, Goebel J, Davies SM, Jodele S. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118(6):1452–62. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 52.Daikeler T, Labopin M, Ruggeri A, Crotta A, Abinun M, Hussein AA, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2013;121(6):1059–1064. doi: 10.1182/blood-2012-07-445965. [DOI] [PubMed] [Google Scholar]
- 53.Jodele S, Bleesing JJ, Mehta PA, Filipovich AH, Laskin BL, Goebel J, et al. Successful early intervention for hyperacute transplant-associated thrombotic microangiopathy following pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2012;16(2):E39–42. doi: 10.1111/j.1399-3046.2010.01408.x. [DOI] [PubMed] [Google Scholar]
- 54.Jodele S, Laskin BL, Goebel J, Khoury JC, Pinkard SL, Carey PM, et al. Does early initiation of therapeutic plasma exchange improve outcome in pediatric stem cell transplant-associated thrombotic microangiopathy? Transfusion. 2013;53(3):661–7. doi: 10.1111/j.1537-2995.2012.03776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16(2):232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruggeri L, Mancusi A, Perruccio K, Burchielli E, Martelli MF, Velardi A. Natural killer cell alloreactivity for leukemia therapy. J Immunother. 2005;28(3):175–82. doi: 10.1097/01.cji.0000161395.88959.1f. [DOI] [PubMed] [Google Scholar]
- 59.Liu Y, Wu HW, Sheard MA, Sposto R, Somanchi SS, Cooper LJ, et al. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res. 2013;19(8):2132–43. doi: 10.1158/1078-0432.CCR-12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]