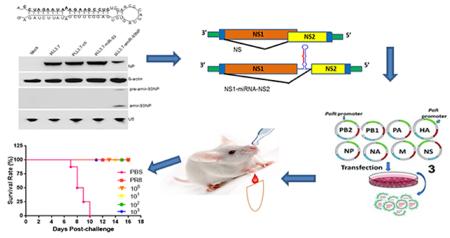
Abstract
Vaccination with live attenuated vaccines (LAVs) is an effective way for prevention of infectious disease. While several methods are employed to create them, efficacy and safety are still a challenge. In this study, we evaluated the feasibility of creating a self-attenuated RNA virus expressing a functional species-specific artificial microRNA. Using influenza virus as a model, we produced an attenuated virus carrying a mammalian-specific miR-93 expression cassette that expresses a viral nucleoprotein (NP)-specific artificial microRNA from an insertion site within the non-structural (NS) gene segment. The resulting engineered live-attenuated influenza virus, PR8-amiR-93NP, produced mature and functional artificial microRNA against NP in mammalian cells, but not in avian cells. Furthermore, PR8-amiR-93NP was attenuated by 104 fold in mice compared with its wild-type counterpart. Importantly, intranasal immunization with PR8-amiR-93NP conferred cross-protective immunity against heterologous influenza virus strains. In short, this method provides a safe and effective platform for creation of live attenuated RNA viral vaccines.
Keywords: Live attenuated vaccine, artificial microRNA, influenza virus, safety, cross-protection
Graphical abstract
1. Introduction
Classical LAVs were produced experimentally by repeated passaging of a virus in cultured cells, but this method is not always reliable, and safety issues occurred in some cases, for example, when there was a reversion to wild-type virulence. With advances in molecular virology, several novel methods, such as altering replication fidelity[1], deoptimizing codons[2], have been employed in creation of live attenuated vaccines with better-controlled replication and pathogenesis. MicroRNAs (miRNAs) are non-coding endogenous RNAs that direct post-translational regulation of gene expression by interacting with messenger RNAs and targeting them for degradation. miRNA-based gene silencing is also a promising approach to controlling viral replication and may be used to improve the safety of attenuated live vaccine. Recent studies showed many miRNAs are species-and tissue-specific [3-5]. These characters of miRNAs can be used to modify the replicative tropism of RNA and DNA viruses [6-9]. A number of studies have inserted miRNA target sequence into some viral genomes for successful RNA inhibition (RNAi)[5, 10, 11]. Although miRNA targeting is a promising approach to the rational design of LAVs, the risk of accumulating mutations in the miRNA target sequence that cause virulence reversion should be kept in mind.
Previously, design of artificial miRNAs (amiRNAs) that produce functional short interfering RNAs was only limited to DNA viruses and retroviruses[11-14]. miRNAs were hypothesized to be problematic in RNA viruses because of the potential degradation of the viral RNA genome during the excision of virus-encoded pre-miRNA. However, a very intriguing study by Varble et al. showed that a miRNA cassette can be successfully inserted into the non-structural (NS) segment of influenza virus[15], and the rescued influenza virus produced functional miRNAs in vitro and in vivo. Results published by Schmid et al showed that replication-incompetent influenza virus could be developed as an RNA viral vector for delivery of amiRNAs[16]. Recent published results by other groups also showed that tick-borne encephalitis virus (TBEV), Sindbis virus (SV), and vesicular stomatitis virus (VSV) can produce functional amiRNAs[17-19]. These results suggest that it is possible to create live attenuated RNA virus vaccine by incorporating an amiRNA cassette into the RNA virus genome.
In this study, we took influenza virus as a test case and designed an artificial miR-93 cassette for insertion into NS gene segment of influenza viral genome, which produces a specific amiRNA for NP gene that would result in a virus that is attenuated in mammalian cells, but could be propagated in chicken eggs at reasonable titers. In animal experiments, vaccination with this novel attenuated influenza virus provides potent and cross immune protection against challenge with lethal influenza viruses.
2. Materials and Methods
2.1. Eggs and cell culture
Embryonated chicken eggs were purchased from Charles River Laboratories, CT. Upon been received, the eggs were incubated at 37.5°C for up to 9 days for use in virus propagation. MDCK cells (ATCC, #CCL-34) were cultured in MEM (Sigma) supplemented with 10% FBS (Gibco, NY), 50 μg/ml gentamicin, and 1mM sodium pyruvate. HEK293T (ATCC, #CRL-11268), MEF (ATCC, #CRL-2214), MEF Dicer−/−(provided by Dr. Wu), DF1 (ATCC, #CRL-12203), and A549 (ATCC, #CCL-185) cells were cultured in DMEM (Gibco, NY) supplemented with 10% FBS, 1% penicillin, and 1 μg/ml streptomycin (Gibco, NY).
2.2. Artificial microRNA design and expression
The miR-93 cassette with a scrambled control sequence, the miR-93 locus, and amiR-93NP were synthesized by GenScript and cloned into the microRNA-expressing plasmid pLL3.7 [20]. For transfection, 8×105 per well of 293T cells were seeded into 6-well plates. The next day, the cells were transfected with 1 μg plasmid pcDNA-NP with 1 μg pLL3.7, pLL3.7-ctl, pLL3.7-miR-93, or pLL3.7-amir-93NP. At 24 hours after transfection, cells were harvested and lysed. Expression of NP and amiR-93NP were detected by western-blot and northern-blot, respectively.
2.3. Virus design, rescue, and titration
Modified NS gene segments with miR-93 locus and amir-93NP cassette insertions were synthesized by GenScript. Reorganization of the NS gene segment was as described in a previous study [21]. Viruses were rescued using a plasmid-based rescue system [22]. The viruses designed were wild-type PR8 (PR8-wt), PR8-control (PR8-ctl), PR8-miR-93, and PR8-amiR-93NP. Viral stocks were titrated in chicken eggs and expressed as EID50. Briefly, ten-fold serial dilutions of viruses were prepared in PBS. Each egg was inoculated with a 100 μl dilution. Virus from allantoic fluid was tested by hemagglutination (HA) assay, and the titer was calculated according to the Reed and Muench method[23].
2.4. Mammalian cell infection
Cells were seeded in different culture vessels one day prior to infection. For the infection, cells were washed with Dulbecco’s phosphate-buffered saline (DPBS) supplemented with Ca++/Mg++ and infected with influenza virus at specified MOIs diluted in fresh medium without serum. After 1-hour incubation, cells were washed with DPBS again supplemented with Ca++/Mg++before adding culture medium supplemented with 0.3% BSA. Cells were harvested according to assay-dependent requirements. Infection in MDCK cells also required the addition of tosyl phenylalanyl chloromethyl ketone (TPCK)-trypsin (Sigma) to the culture medium.
2.5. Northern blot analysis
RNAs were extracted from different cell lines using the miRNeasy Mini Kit (Qiagen) and stored at −80°C. Probes used for northern blot analyses included probes for U6 (5′-CACGAATTTGCGTGTCATCCTT-3′), miR-93 (5′-CTACCTGCACGAACAGCACTTTG-3′), and amiR-93NP (5′- GAGGCTTCTTTATTCTAGG-3′). Northern blot experiments were performed using the High Sensitive miRNA Northern Blot Assay Kit as per the manufacturer’s protocol (Signosis), and membranes were developed with chemiluminescent HRP substrate (Takara Bio). Images were acquired using the Image Quant LAS400 (GE Healthcare).
2.7. Western blot analysis
Lysed MDCK cell samples were loaded and separated on 10% SDS-PAGE, then transferred onto nitrocellulose membrane using a semi-dry transblot apparatus (Bio-rad). The membrane was blocked in PBS with 1% Tween (PBST) and 5% non-fat milk for 1 hour with an anti-NP monoclonal antibody (Abcam) at 4°C overnight. After washing with PBST, the membrane was incubated with alkaline phosphatase-conjugated goat anti-mouse IgG antibody (Cell Signaling) at room temperature for 1 h. After washing, the membrane was developed with chemiluminescent HRP substrate before imaging. Finally, expression of NP protein was normalized with β-actin (actin).
2.8. Virulence test in vivo
Mice (6–8 week old) were purchased from Jackson Laboratory and divided randomly into groups with four mice in each group. For determination of the MLD50, virus was serially diluted in DPBS, and 50 μl were intranasally inoculated into mice anesthetized by injection with ketamine and xylazine. The MLD50 was calculated according to the method of Reed and Muench[23]. After infection, mice were monitored daily for clinical symptoms, weight loss, and death. All animal experiments were approved by the Institutional Animal Care and Use Committee at Texas Tech University Health Sciences Center and carried out in accordance with the US Public Health Service Guide for the Care and Use of Laboratory Animals (NRC Publication, 8th ed.) and other related federal statutes and regulations of the Animal Welfare Act.
2.9. Humoral immune response and protective immunity
Mice (6–8 week old) were randomly divided into groups and intranasally inoculated with 50 μl of diluted influenza virus. Mouse blood was collected on days 15 and 29, and serum was isolated for analysis by micro-neutralization assay and ELISA for anti-HA responses. For testing the IgG antibody concentration in mouse serum, plates were coated with HA of the PR8 virus, and specific IgG, IgG1, and IgG2a were measured in the sera of immunized mice. After the last bleeding, mice were challenged with 100×LD50 mouse-adapted PR8 (H1N1), A/California/04/2009(H1N1) (CA09), or 104 PFU A/Hong HK/1/68 (H3N2) (HK68) influenza virus. The challenged mice were monitored for clinical symptoms and survival. ELISA and micro-neutralization were performed as we have previously described[24].
2.10. Statistical analysis
Comparisons between vaccinated groups were performed by using a nonparametric one-way ANOVA with the Tukey multiple comparison test and Fisher’s exact test. Survival curves were analyzed by log-rank test. The analyses were performed using GraphPad Prism version 5.0 software for Windows (GraphPad Software). P values < 0.05 were considered to indicate a significant difference.
3. Results
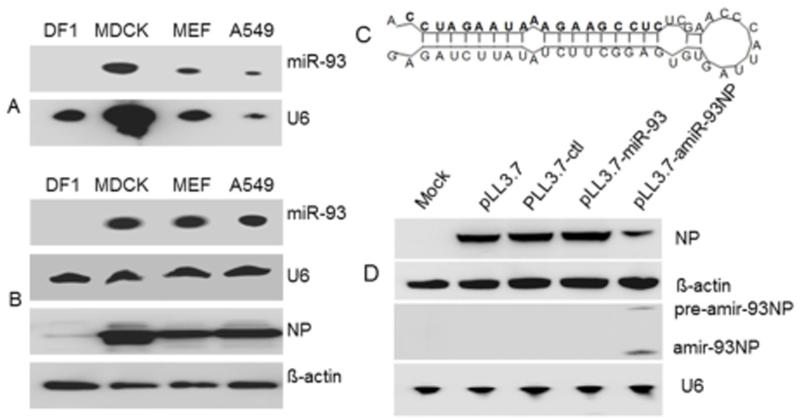
To confirm that a miR-93 backbone would be appropriate for our design, we first analyzed the expression of mature miR-93 in avian cells and mammalian cells by northern-blot. The mammalian cells included Madin-Darby canine kidney (MDCK), mouse epithelial fibroblast (MEF), and A549 human lung epithelial cell lines. The DF1 chicken fibroblast cell line served as a representative avian cell line. We also tested whether miR-93 expression changed after infection with influenza virus. The results showed that miR-93 was not expressed in the uninfected (Fig.1A) or infected (Fig.1B) DF1 cells. By contrast, miR-93 was expressed in uninfected and infected MDCK, MEF, and A549 cells (Fig.1A and B). Upon confirmation of the specificity of miR-93, we designed an artificial miRNA-93NP (amiR-93NP) cassette that would produce an amiRNA targeted against the NP gene of influenza virus. As denoted in bold in Figure 1C, the sequence within the mature miR-93 loop was replaced with the sequence: 5′-AGAUCUUAUAUCUUCGGAGUGUGAUUACCCAACCUCUCCGAAGAAAUAAGAUCC-3′. To test whether the NP-targeted amiRNA could be functionally processed, miR-93, amiR-93NP, and a scrambled miRNA control (ctl) of similar size were individually cloned into pLL3.7 plasmids. Each plasmid was co-transfected along with pcDNA-NP into 293T cells. At 24 hours, the expression of NP protein and amiRNA were analyzed by western-blot and northern-blot, respectively. As shown in Fig.1D, NP protein expression decreased by about 75% in pLL3.7-amiR-93NP-transfected cells compared with pLL3.7-transfected cells. Furthermore, pre-amiR-93NP and amiR-93NP were detected in cell lysates of pLL3.7-amiR-93NP-transfected cells by northern-blot analysis using a probe specific for amiR-93NP. This result indicated that mature and functional amiRNA was produced.
Fig. 1.
Detection miR-93 in non-infected cells and PR8- infected cells and design of artificial miRNA based on miR-93 backbone. (A) Evaluation of natural miR-93 expression in DF1, MDCK, MEF, and A549 cell lines. U6 was used as a control RNA probe. (B) Evaluation of miR-93 expression in cells infected with PR8 influenza virus. NP protein expression was used to verify viral infection, and β-actin was used as loading control. (C) Sequence and secondary structure of amiR-93NP. Mature artificial miR93-NP sequence is highlighted in bold. (D) pcDNA-NP with pLL3.7, pLL3.7-ctl, pLL3.7-miR93, or pLL3.7-amiR93NP was transfected into 293 T cells. Cells were harvested at 24 h post transfection. Cell lysates were prepared for Western-blot analysis to detect NP or β-acting proteins. RNA was prepared for northern blot analyses to detect amiR-93NP expression.
Following this result, we rearranged the NS gene segment of PR8 influenza virus so that NS2 would no longer overlap with the NS1 segment and inserted either the miR-93 or the amiR-93NP cassettes into the newly created intergenic region between NS1 and NS2 (Fig.2A). Influenza viruses with modified NS gene segments were rescued and propagated in embryonated chicken eggs. Since the influenza virus engineered with amiR-93NP couldn’t grow well in MDCK cells, we titrated viruses in chicken eggs, with titers expressed as the 50% egg infective dose (EID50). The modifications in the NS segment resulted in influenza viruses that grew at roughly 10-fold lower titers than the wild-type virus (Table 1). However, there was no significant difference between the titers of viruses engineered with scrambled miRNA, miR-93, or miR-93NP.
Fig. 2.
Engineering of NS gene segment and verification of rescued influenza viruses. (A) Diagram of engineered and original NS gene segments. Green represents 5′ and 3′ noncoding regions; blue represents packaging signal within open reading frame; orange with blue to the left represents NS1 coding sequence; yellow with blue to the right represent NS2 coding sequence.(Top) Organization of original NS gene segment. (Bottom) Organization of modified NS gene segment engineered with ctl, miR-93 or amiR-93NP expression cassettes. (B) RNA was isolated from purified PR8 wild type, PR8-ctl, PR8-miR-93 and PR8-amiR93-NP influenza viruses, and 1 μg was separated on a 4% acrylamide TBE urea gel. Bands were visualized by silver staining. Each RNA segment is labeled to the right of the gel. (C) NS and NP gene segments were amplified by RT-PCR and separated by electrophoresis on agarose gel.
Table 1.
Viral growth in chicken eggs (×108EID50) and 50% lethal dose in mouse (unit: EID50).
| Virus | Titer | MLD50 |
|---|---|---|
| PR8-wt | 5.6±0.56 | 101.76 |
| PR8-ctl | 0.53±0.47 | 103 |
| PR8-miR-93 | 0.65± 0.31 | 103.17 |
| PR8-amiR-93NP | 0.49±0.61 | 105.75 |
Next, viruses were concentrated by ultracentrifugation, and the viral genomes were extracted and detected by silver staining. As shown in Fig.2B, the wild-type NS gene segment migrated at 890bp, while the modified NS gene segments migrated at 1540bp, almost overlapping with the NP gene segment (1565 bp). Next, the NS and NP gene segments were amplified by RT-PCR and separated on an agarose gel by electrophoresis. The modified NS gene segments migrated at almost the same size as the NP gene segment (Fig.2C). The NS gene segments were also verified by sequencing. Taken together, these results showed that miRNA/amiRNA insertions into the influenza virus NS gene segment still allowed for successful rescue of influenza viruses from the PR8 DNA plasmid system.
The rescued wild-type and NS-modified PR8 viruses were then evaluated for NP expression and growth characteristics in A549, MEF, MEF Dicer−/−, or MDCK cells. Cells were infected with influenza viruses at a multiplicity of infection of 1 EID50 (MOI=1). Western-blot analysis showed that NP expression was reduced by approximately 40% in A549 cells infected with PR8-amiR-93NP virus compared with A549 cells infected with wild-type PR8 virus at 8 hrs post-infection (Fig.3A). Expression of pre-amiR-93NP (56nt) and amiR-93NP (19nt) was confirmed by northern-blot. In MEF wild-type cells infected with PR8-amiR-93NP virus, NP expression as normalized with β-actin expression, was reduced by 44% and 92% at 8 and 16 hrs post-infection, respectively, compared with those infected with wild-type virus at the same time points (Fig.3B). By contrast, NP expression at 8 hrs and 16 hrs post-infection almost did not differ in MEF Dicer−/−cells infected with PR8-amiR-93NP virus and wild-type PR8 virus. In MDCK cells, infection with PR8-amiR-93NP virus also resulted in reduced expression of NP compared with infection with wild-type virus (Fig.3C).
Fig. 3.
Replication of wild-type and engineered PR8 viruses. NP protein expression was normalized with β-actin (actin). (A) A549 cells were infected with influenza virus (MOI=1) and samples were harvested at 8 hour post-infection. NP protein and amiRNA against NP gene were analyzed by western-blot and northern-blot, respectively. (B) MEF and MEFdicer−/−cells were infected with different influenza viruses, and then harvested at 8 and 16 hours post-infection. NP and NS1proteins were analyzed by western-blot. (C) MDCK cell line was infected with PR8-wt, or PR8-ctl virus, PR8-miR-93, or PR8-amiR-93NP virus.
The modified viruses were also attenuated in mice as determined by the 50% mouse lethal dose (MLD50). Compared with the wild-type virus, PR8-ctl and PR8-miR-93 virus levels decreased by 20 fold (Table 1). However, the PR8-amiR-93NP virus was substantially more attenuated in mice, and its MLD50 was reduced by a factor of 104 compared with the wild-type virus. These results confirmed that the PR8-amiR-93NP virus was substantially attenuated in mammalian species.
Next, we tested whether the species-attenuated PR8-amiR-93NP virus would stimulate potent immune responses and protection against lethal challenge with wild-type PR8 influenza virus in mice. Mice were intranasally immunized once with different doses of PR8-amiR-93NP, ranging from 1–103 EID50 per mouse, or wild-type PR8 virus at 10 EID50 per mouse. First, humoral responses were evaluated by ELISA and micro-neutralization. As shown in Figure 4A, all doses of PR8-amiR-93NP virus elicited robust total IgG responses that were similar to the response elicited by wild-type PR8 virus on day 15. There was little increase in total IgG and IgG2a levels between days 15 and 29 (Fig.4A and C). Fig.4B shows that by day 15, IgG1 levels became detectable and increased by 3–7 fold by day 29. All groups responded similarly, with the exception that PR8-amiR-93NP vaccinations at 100 and 101 EID50 were statistically different on days 15 and 29 (p<0.05). There was no significant difference between IgG and IgG2a levels among immunized groups. In Table 2, results show that immunization with PR8-amiR-93NP induced potent functional IgG neutralizing PR8 wild-type influenza virus.
Fig. 4.
Humoral immune responses induced by PR8-amiR-93NP and protection against wild PR8 influenza virus in mice. Mice (n=8) were vaccinated with different doses of PR8-amiR-93NP and bled at days 0, 15 and 29 post-vaccination. At 29 days post-vaccination, Mice (n=8) were challenged with 100×LD50.HA-specific antibodies IgG (A), IgG1 (B), and IgG2a (C) were measured by ELISA. (* p>0.05, **p<0.05). (D) Mouse weight change after challenge and (E) survival rate (* p>0.05) are shown.
Table 2.
Micro-Neutralization (MN) titers against PR8 (H1N1) influenza virus in sera from vaccinated mice.
| Groups | Titers at day 15 post- immunization |
Titers at day 29 post- immunization |
|---|---|---|
| PBS | <10 | <10 |
| PR8 | 1920±684 | ≥2560 |
| 100 EID50 | 1282.5±901 | 1460±980 |
| 101 EID50 | 1760±889 | ≥2560 |
| 102 EID50 | 1920±684 | ≥2560 |
| 103 EID50 | 2080±662 | ≥2560 |
Finally, the challenge results show that immunization with a PR8-amiR-93NPdose as low as 1 EID50was completely protective against lethal challenge with influenza PR8 virus, with no morbidity as measured by weight loss (Fig.4D) or mortality observed in these mice (Fig.4E). By contrast, mice that were immunized with PBS exhibited rapid weight loss, and all died by day 10 post-challenge.
Immunization with PR8-miR-93NPvirus also conferred protection against heterologous viral infections. Groups of mice were immunized with 102 EID50 of PR8-miR-93NP and challenged at 29 days after immunization. As shown in Figure5A and 5B, immunization with PR8-miR-93NP or HK68 (H3N2) viruses protected mice against influenza illness caused by challenge with HK68. By contrast, mice administered with PBS lost over 20% of their weight. As this virus is not lethal to mice, all animals survived (Fig.5B). Immunization with PR8-miR-93NP also conferred protection against lethal challenge with mouse-adapted CA09 (H1N1) pandemic influenza virus. All mice immunized with PR8-miR-93NP or CA09 were protected against morbidity (Fig.5C) and mortality (Fig.5D) following lethal challenge with CA09 virus.
Fig. 5.
Evaluation of cross-protection conferred by PR8-amiR-93NP against infection with H3N2 influenza virus and pandemic 2009 H1N1 influenza virus. Mice (n=8) were vaccinated with 102 EID50 PR8-amiR-93NP and challenged with 104 PFU HK68 H3N2 influenza virus or 100 LD50 CA09 H1N1 pandemic influenza virus. (A) Mouse weight change and (B) survival rate for mice challenged with HK68 H3N2 influenza virus; (C) mouse weight change and (D) survival rate for mice challenged with CA09 H1N1 pandemic influenza virus. (**p<0.05).
4. Discussion
Effective delivery is still a challenge for RNAi technology and its successful therapeutic application. It has been recently shown that RNA viruses, including influenza virus, can be modified to produce functional miRNAs[15-17, 19]. Engineering influenza virus with naturally occurring or artificial miRNAs (amiRNAs) is appropriate for small RNA delivery. Low pathogenic influenza virus is confined to the respiratory tract and can produce high transient levels of small RNAs, making it an ideal vector for treatment of respiratory infection and diseases. In this study, we took advantage of the species-specific expression pattern of miRNAs to substantially attenuate influenza virus in mammalian species while still allowing for stimulation of an effective immune response.
As shown in this study, we successfully reorganized the NS gene segment of PR8 influenza virus and inserted an amiRNA expression cassette that produced a functional amiRNA. To produce an attenuated influenza vaccine, we selected the NP gene as the target of the amiRNA. So far, chicken eggs are the best known growth vector for influenza virus vaccine seeds. Thus, miR-93, which is not detected in chicken cells, was employed as backbone to produce an amiRNA. While viral titers in viruses with miRNA elements engineered into the NS segment decreased compared with wild-type virus, we were still able to recover viruses at titers of 5×107 EID50. Of interest, the PR8-amiR-93NP virus produced NP-specific short hairpin RNA (shRNA) that inhibited NP gene expression. Since the target sequence is highly conserved, prior exposure to the engineered virus dramatically decreases the risk when new influenza virus is introduced.
This study introduced an effective method by which influenza viruses can be attenuated for safer use as vaccines. It is possible to improve this strategy to make safer vaccine viruses by expressing multiple amiRNAs to target various conserved motifs within viral genomes. Specifically, it may be possible to simultaneously express a series of amiRNAs targeting different regions of the viral genome in a single amiRNA expression cassette. In this research, the engineered PR8-amiR-93NP virus showed pathogenesis in mice when 106 EID viruses were used for challenge. Others have shown that it is feasible to design an amiRNA cassette to express multiple amiRNAs in a single vector[25]. Thus, to decrease virulence, we will insert multiple amiRNA expression cassettes into the NS gene segment for evaluation as a vaccine candidate in future research. So far, reversion of live attenuated vaccine virus into a virulent virus, and/or reassortment with seasonal influenza viruses are still potential risks. The genomic stability of rescued live influenza viruses carrying amiRNA cassette will need to be evaluated in a future study. We believe that this method would still allow for sufficient growth in fertilized chicken eggs and would result in an attenuated virus with little chance of reverting to a fully virulent virus. Furthermore, this method can also be employed to create novel vaccines against other RNA viruses or to attenuate RNA viruses for use as vectors for therapeutic gene delivery.
Acknowledgements
This work was supported by the US Public Service research grant AI072139 (M.Z.) from the National Institute of Allergy and Infectious Diseases and an internal fund from Texas Tech University Health Sciences Center, Paul L. Foster School of Medicine. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript. We thank Dr. Haoquan Wu for kindly sharing with us some materials such as MEF Dicer−/− cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Vignuzzi M, Stone JK, Arnold JJ, Cameron CE, Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang C, Skiena S, Futcher B, Mueller S, Wimmer E. Deliberate reduction of hemagglutinin and neuraminidase expression of influenza virus leads to an ultraprotective live vaccine in mice. Proc Natl Acad Sci U S A. 2013;110:9481–9486. doi: 10.1073/pnas.1307473110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang R, Wang YQ, Su B. Molecular evolution of a primate-specific microRNA family. Molecular biology and evolution. 2008;25:1493–1502. doi: 10.1093/molbev/msn094. [DOI] [PubMed] [Google Scholar]
- [4].Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH. Diversity of microRNAs in human and chimpanzee brain. Nature genetics. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- [5].Perez JT, Pham AM, Lorini MH, Chua MA, Steel J, tenOever BR. MicroRNA-mediated species-specific attenuation of influenza A virus. Nat Biotechnol. 2009;27:572–576. doi: 10.1038/nbt.1542. [DOI] [PubMed] [Google Scholar]
- [6].Barnes D, Kunitomi M, Vignuzzi M, Saksela K, Andino R. Harnessing endogenous miRNAs to control virus tissue tropism as a strategy for developing attenuated virus vaccines. Cell host & microbe. 2008;4:239–248. doi: 10.1016/j.chom.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, Bell JC. A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther. 2008;16:1437–1443. doi: 10.1038/mt.2008.130. [DOI] [PubMed] [Google Scholar]
- [8].Kelly EJ, Hadac EM, Greiner S, Russell SJ. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat Med. 2008;14:1278–1283. doi: 10.1038/nm.1776. [DOI] [PubMed] [Google Scholar]
- [9].Lee TC, Lin YL, Liao JT, Su CM, Lin CC, Lin WP, Liao CL. Utilizing liver-specific microRNA-122 to modulate replication of dengue virus replicon. Biochemical and biophysical research communications. 2010;396:596–601. doi: 10.1016/j.bbrc.2010.04.080. [DOI] [PubMed] [Google Scholar]
- [10].Ylosmaki E, Martikainen M, Hinkkanen A, Saksela K. Attenuation of Semliki Forest virus neurovirulence by microRNA-mediated detargeting. J Virol. 2013;87:335–344. doi: 10.1128/JVI.01940-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumar M, Follenzi A, Garforth S, Gupta S. Control of HBV replication by antiviral microRNAs transferred by lentiviral vectors for potential cell and gene therapy approaches. Antiviral therapy. 2012;17:519–528. doi: 10.3851/IMP2014. [DOI] [PubMed] [Google Scholar]
- [12].Zhang T, Cheng T, Wei L, Cai Y, Yeo AE, Han J, Yuan YA, Zhang J, Xia N. Efficient inhibition of HIV-1 replication by an artificial polycistronic miRNA construct. Virol J. 2012;9:118. doi: 10.1186/1743-422X-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu Z, Xue Y, Wang B, Du J, Jin Q. Broad-spectrum antiviral activity of RNA interference against four genotypes of Japanese encephalitis virus based on single microRNA polycistrons. PLoS One. 2011;6:e26304. doi: 10.1371/journal.pone.0026304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boden D, Pusch O, Silbermann R, Lee F, Tucker L, Ramratnam B. Enhanced gene silencing of HIV-1 specific siRNA using microRNA designed hairpins. Nucleic Acids Res. 2004;32:1154–1158. doi: 10.1093/nar/gkh278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Varble A, Chua MA, Perez JT, Manicassamy B, Garcia-Sastre A, tenOever BR. Engineered RNA viral synthesis of microRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11519–11524. doi: 10.1073/pnas.1003115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schmid S, Zony LC, tenOever BR. A versatile RNA vector for delivery of coding and noncoding RNAs. Journal of virology. 2014;88:2333–2336. doi: 10.1128/JVI.03267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rouha H, Thurner C, Mandl CW. Functional microRNA generated from a cytoplasmic RNA virus. Nucleic Acids Res. 2010;38:8328–8337. doi: 10.1093/nar/gkq681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shapiro JS, Varble A, Pham AM, Tenoever BR. Noncanonical cytoplasmic processing of viral microRNAs. Rna. 2010;16:2068–2074. doi: 10.1261/rna.2303610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pica N, Langlois RA, Krammer F, Margine I, Palese P. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge. J Virol. 2012;86:10293–10301. doi: 10.1128/JVI.01131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Zhang M, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature genetics. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- [21].Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, Garcia-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Reed LH, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:5. [Google Scholar]
- [24].Li J, Arevalo MT, Chen Y, Posadas O, Smith JA, Zeng M. Intranasal immunization with influenza antigens conjugated with cholera toxin subunit B stimulates broad spectrum immunity against influenza viruses. Human vaccines & immunotherapeutics. 2014;10 doi: 10.4161/hv.28407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen SC, Stern P, Guo Z, Chen J. Expression of multiple artificial microRNAs from a chicken miRNA126-based lentiviral vector. PloS one. 2011;6:e22437. doi: 10.1371/journal.pone.0022437. [DOI] [PMC free article] [PubMed] [Google Scholar]