Abstract
Background
Clinical trials of human epidermal growth factor receptor 2 (HER2)-targeted agents added to standard treatment have been efficacious for HER2-positive (HER2+) advanced breast cancer. To our knowledge, no meta-analysis has evaluated HER2-targeted therapy including trastuzumab emtansine (T-DM1) and pertuzumab for HER2-positive breast caner and ranked the targeted treatments. We performed a network meta-analysis of both direct and indirect comparisons to evaluate the effect of adding HER2-targeted agents to standard treatment and examined side effects.
Methods
We performed a Bayesian-framework network meta-analysis of randomized controlled trials to compare 6 HER2-targeted treatment regimens and 1 naïve standard treatment (NST, without any-targeted drugs) in targeted treatment of HER2+ breast cancer in adults. These treatment regimens were T-DM1, LC (lapatinib), HC (trastuzumab), PEC (pertuzumab), LHC (lapatinib and trastuzumab), and PEHC (pertuzumab and trastuzumab). The main outcomes were overall survival and response rates. We also examined side effects of rash, LVEF (left ventricular ejection fraction), fatigue, and gastrointestinal disorders, and performed subgroup analysis for the different treatment regimens in metastatic or advanced breast cancer.
Results
We identified 25 articles of 21 trials, with data for 11,276 participants. T-DM1 and PEHC were more efficient drug regimens with regard to overall survival as compared with LHC, LC, HC and PEC. The incidence of treatment-related rash occurs more frequently in the patients who received LC treatment regimen than PEHC and T-DM1 and HC. In subgroup analysis, T-DM1 was associated with increased overall survival as compared with LC and HC. PEHC was associated with increased overall response as compared with LC, HC, and NST.
Conclusions
Overall, the regimen of T-DM1 as well as pertuzumab in combination with trastuzumab and docetaxel is efficacious with fewer side effects as compared with other regimens, especially for advanced HER2+ breast cancer.
Impact
This study suggests that both T-DM1 and PEHC therapy are potentially and equally useful treatments for HER2+ breast cancer.
Introduction
Breast cancer, with more than 1 million new cases confirmed per year in the world[1], is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide. In 2008, breast cancer accounted for 23% (1.38 million) of all new cancer cases and 14% (458,400) of all cancer deaths [1–3]. Amplification of the human epidermal growth factor receptor 2 (HER2) gene occurs in 10%-35% of human breast cancers, and correlates with a more aggressive phenotype and poorer prognosis [4–7]. With regard to the management of HER2-positive breast cancer, trastuzumab [8–11], pertuzumab [12, 13], lapatinib [14, 15] are approved as standard care for inhibiting HER2 activity in the treatment of HER2-positive breast cancer [16], increasing the incidence of progression-free survival (PFS), overall survival (OS) and overall response rate (ORR) compared with chemotherapy alone. The TDM4450g trial reported that trastuzumab emtansine provides a better median PFS, by an increase of 5 months, compared to trastuzumab in combination with docetaxel in HER2-positive metastatic breast cancer [17]. The targeting of HER2 with more than one agent is better than use of a single agent in a first/second-line setting [9, 12, 18–20]. According to the CLEOPATRA study, HER2-positive breast cancer patients received a regimen of combining pertuzumab with trastuzumab and docetaxel, and demonstrated a significantly improvement in overall survival compared with individuals who received a regimen of trastuzumab in combination with placebo and docetaxel [12, 21]. In addition to the CLEOPATRA study, by far there have been only two randomized clinical trials of combination treatments including more than one of the above HER2-targeted drugs to treat HER2-positive breast cancer patients[22, 23]. Furthermore, no randomized clinical trial has compared a lapatinib-containing regimen directly with pertuzumab-containing or T-DM1-containing regimen, so there is a need for indirect meta-analysis to evaluate these different HER2-targeted therapies. One of prior meta-analyses did not stress on the HER2-targeted therapy [24]. The other meta-analysis did not include the HER2-targeted agents of T-DM1 and pertuzumab in the study [25].
Network meta-analysis is one of standard methods for systematic review and meta-analysis [26–37]. Such analysis more comprehensively synthesizes direct and indirect evidence to evaluate data, compared to traditional meta-analysis, which just employs direct data to demonstrate results [38, 39]. More importantly, we can isolate a relatively better treatment among several similar therapies with this network meta-analysis.
To identify relatively a better HER2-targeted treatment regimen among trastuzumab, pertuzumab, T-DM1, lapatinib in combination with standard treatment (chemotherapy or hormone therapy or endocrine therapy without HER2-targeted drugs) in HER2-positive breast cancer, we performed a comprehensive systematic network meta-analysis of HER2-targeted agents combined with standard treatment for HER2+ breast cancer and evaluated the relative merits of the different regimens. We compared overall survival rate (OSR) and overall response rate (ORR) as well as side effects for these treatments.
Materials and Methods
Definition of HER2-targeted therapy
Suitable HER2-targeted agents were identified through the following registries (http://www.clinicaltrials.gov;http://www.cancer.gov/search/clinical_trials/search_clinicaltrialsadvanced.aspx;http://www.who.int/trialsearch). HER2-targeted combination treatment was defined as single- or multi-targeted HER2 agents (e.g. trastuzumab, pertuzumab, lapatinib, and trastuzumab-MCC-DM1) combined with standard treatment (chemotherapy or hormone therapy, e.g. like taxane, anastrozole, letrozole, anthracycline without the addition of any HER2-targeted agents).
Trial criteria
Prospective clinical phase II or III randomized controlled trials (RCTs) of treatment were entered into this network analysis to compare the efficacy and safety of different HER2-targeted treatment regimens in which HER2-targeted agents were combined with standard treatment in any-line HER2+ breast cancer without consideration whether the ages of these patients were or were not over 70 years of age. Trials that compared the efficacy of different HER2+ therapy dosing schedules, compared the efficacy of different administration order (sequentially or concurrently) or administration approach (orally or intravenously), evaluated HER2-targeted vaccines or reported only quality-of-life measures or pharmacokinetic outcomes were excluded.
Search strategy
We searched for clinical trials published in English in MEDLINE via PubMed and ClinicalTrials.gov (http://www.clinicaltrials.gov) with the key words “trastuzumab”, “Herceptin”, “lapatinib”, “Tykerb”, “pertuzumab”, “Omnitarg” and the exploded MeSH term “breast neoplasms” and search line [(breast or mammary) and (cancer* and tumour* and tumor* or neoplas* or carcinoma)] [25]. The last search was on March 1, 2014. The PubMed search was restricted to RCTs and humans. We searched for additional studies in the reference lists of most primary studies and original reviews comparing regimens for HER2+ breast cancer.
Two reviewers (Qiuyan Yu and Zhenli Zhu) independently assessed the titles and abstracts of retrieved articles to determine trial inclusion. Any discrepancies were resolved by a third researcher (Ke Li). The full-text manuscripts of potential trials were reviewed.
Data extraction and quality assessment
Two reviewers (Qiuyan Yu and Zhenli Zhu) extracted data on the trial design, patient eligibility, baseline patient characteristics, dosing regimens, line of treatment, method of HER2+ identification, duration of follow-up and risk of bias of trials. Disagreements in data extraction were resolved by discussion or by a third researcher. If trial results were reported in multiple publications, we extracted the most recently reported endpoints. The risk of bias of trials was assessed as recommended by the Cochrane Collaboration [40].
The main outcomes were OSR (overall survival rate) and ORR (overall response rate) according to the Response Evaluation Criteria in Solid Tumors (RECIST) or the WHO criteria. Secondary outcomes were the side effects rash, left ventricle ejection fraction (LVEF, 10–50%), fatigue, and gastrointestinal disorders (diarrhea, nausea, vomiting) according to the National Cancer Institute (NCI) Common Toxicity Criteria version 2 or Common Terminology Criteria for Adverse Events (CTCAE) version 3. For OSR, ORR and side effects, we extracted or derived the number of patients with outcome of interest (numerator) and total number of patients in the treatment arm (denominator). OSR was defined as the proportion of survivors from the time of randomization to the recently reported endpoint for any casualty. The intent-to-treat analysis was used for the evaluation of the OSR, ORR and side-effects.
Treatment categories
We investigated the following 6 regimens: T-DM1 (trastuzumab emtansine), LC (lapatinib), HC (trastuzumab), PEC (pertuzumab), LHC (lapatinib and trastuzumab), and PEHC (pertuzumab and trastuzumab). Other therapies such as anthracycline, taxane, capecitabine, anastrozole, letrozole, vinorelbine without the addition of any-targeted agents were grouped as naïve standard treatments (NST) [24].
Statistical analyses
OSR and ORR were analyzed by the total number of randomly assigned participants as the denominator [41]. For OSR, if we could not extract survival data, but death data was reported, we used this data to inversely calculate survival. For side effects, if only percentages were reported, we estimated the nearest whole number of events instead of the actual number of incidents [42].
For every direct comparison of HER2-targeted treatment regimens, we first synthesized data, which was extracted from the randomized clinical trials with the same HER2-targeted treatment comparisons, to compare the efficacy and safety of these trials with each other with a random-effects model [41–44]. Both odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for dichotomous outcomes. The I2 statistic as well as forest plots were computed to determine whether there was statistical heterogeneity, with low heterogeneity defined as an I2 value <25%, and high heterogeneity defined as an I2 value >50% [45].
A network meta-analysis was employed to synthesize direct and indirect treatment regimens to assess the therapeutic effect between all the HER2-targeted combination treatments, and was used to rank these results graphically [38, 39, 46]. The Markov Chain Monte Carlo method was used to estimate these regimens [41, 42, 46]. And judge whether the residual deviance was similar with the number of data points with the approach of the posterior mean [42, 47]. For every treatment regimen, we calculated the probability of its efficacy and safety and ranked treatments by rank-grams [39].
In estimating the consistency between direct and indirect evidence, the data from both sources was checked by the Bucher method to determine whether it was so similar enough that we could integrate the direct and the indirect evidence together [42, 47–49]. That is, we calculated the difference between direct and indirect evidence in all closed loops in the network. Once one closed loop was identified with a 95% CI excluding 0, we could confirm clearly the existing differences between the direct and indirect evidence [41, 50].
We performed a sensitivity analysis using a fixed-effects model by repeating the main computations. As well, we performed a subgroup analysis using a random-effects model for advanced HER2+ breast cancer.
Analysis involved use of STATA 12.0 (pair-wise meta-analysis and I2 calculation), R 3.0.2 (estimation of consistency between direct and indirect evidence, rank-grams, and SUCRA graphs), and WinBUGS 1.4.2 (multiple-treatments of meta-analysis models).
Results
Eligible trials
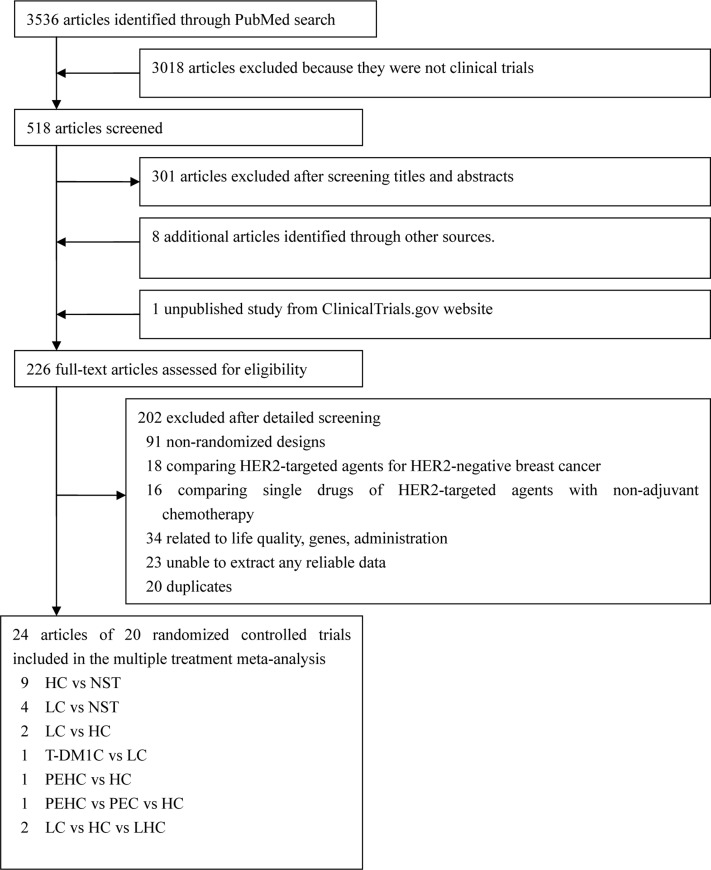
Abstract and titles of 575 articles were identified for first review employing the search strategies (S1 Table) as described in Fig 1. After objectively reading the titles and abstracts, 343 articles were excluded because they were not randomized controlled trials, and 121 unrelated articles were excluded, 8 additional articles identified through other sources and 1 unpublished study from ClinicalTrials.gov website were included, resulting in 120 full-texts evaluating the effect of HER2-targeted drugs in treatment of breast cancer. Of these publications, 69 articles were excluded because 13 of them provided deficient or inaccessible information and the rest were excluded because they compared HER2-targeted agents for HER2-negative or triple-negative breast cancer, and 19 articles were excluded because they focused on single drug comparison without combination with standard chemotherapy or concentrated on different administration order or approach. 7 articles were excluded because they reported end-points about life-quality, genes and cost-efficacy. In the end, 25 articles of 21 trials were included in our network meta-analysis (Fig 1) and analyzed for the following HER2-targeted regimens: T-DM1, LC, HC, PEC, PEHC, LHC and NST (S2 Table). Most trials (n = 18 [85%]) were two-group studies and the remainder were three-group trials [12, 14, 15, 17, 18, 22, 23, 51–67]. Overall, 11,276 patients were randomly assigned to 1 of the 6 HER2-targeted treatments or to naïve standard treatment (NST). The mean sample size was 250 patients per group (range 26–1097 patients). The mean duration of studies was 31 months (2 studies, <10 months; 15, 10–50 months, and 4, >50 months). Supplementary unpublished information was obtained from other reviews and trial investigators. All included studies recruited patients described as grade 2-plus with positive on immunohistochemistry (IHC2+), IHC3+, or positive on fluorescence in situ histochemistry for HER2.
Fig 1. Flow of articles in the study.
21 randomized trials corresponding to 43 groups because 3 were three-group studies.
The number of adverse events was inconsistently reported. More reports counted the events of gastrointestinal disorders as grades 3-plus rather than grades 1–4. So, we used the number of gastrointestinal disorder events as grades 3-plus [42]. However, for the side effects of rash and fatigue, we used the number of all grades to evaluate safety. The number of LVEF cases was imputed as the proportion between 10% and 50%. We found 5,927 cases of overall survival, 2,759 of overall response, 1,334 of rash, 1,918 of fatigue, 397 of diarrhea, 288 of nausea, 214 of vomiting, and 654 of abnormal LVEF (10%-50%).
The overall quality of studies was rated moderate (S1 and S2 Figs). Some studies did not record details about concealment. We judged studies which reported concealment by a central office or an interactive third-party telephone via an interactive voice response system or web-based randomizations via an interactive web-based response system as having no bias.
Traditional meta-analysis
We performed a series of conventional meta-analyses to summarize the same classes of treatment regimens. OSR was better with T-DM1 than LC or with PEHC than HC (S3 Table). ORR was better with PEHC than PEC and HC or with T-DM1 than LC. We found no significant heterogeneity with I2 >50%. In side-effect analysis, we found significant heterogeneity in the meta-analysis of rash for the comparisons LC versus NST, LVEF (10%-50%) for HC versus NST, fatigue for HC versus NST, and diarrhea for LC versus HC (S3 Table). For the meta-analysis of diarrhea, we found I2 > 75% for the comparisons LC versus HC, which represented the difference of treatment-related diarrhea between LC and LC have statistical significance.
Network meta-analysis
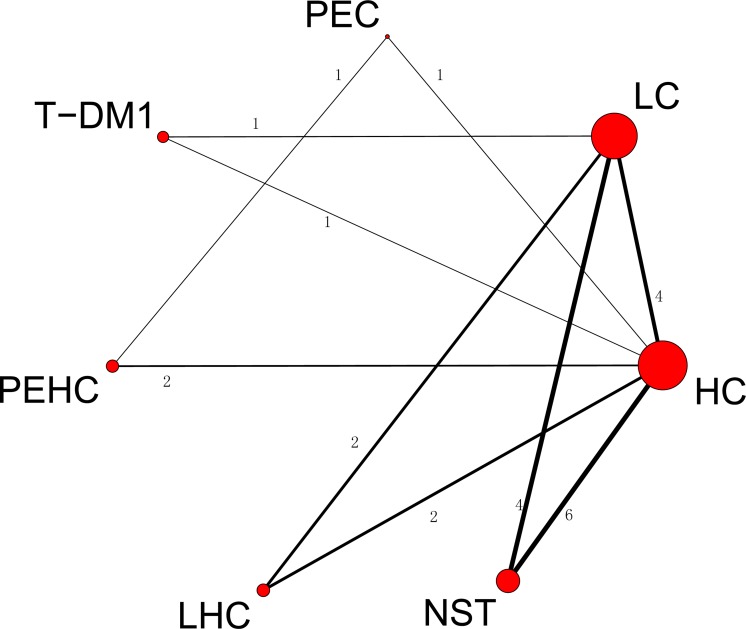
The ORR and OSR of the 7 treatment regimens for are in Fig 2 and S3 Fig, respectively. The size of nodes related to treatment with T-DM1, LC, HC, NST, LHC, PEHC and PEC corresponded to the number of randomized participants (sample size) that studied the treatments. Treatments that were directly compared are linked with a line and the number of trials. For example, the direct comparison between HC versus NST treatments is linked with a line noting 6 trials. The thickness of the line corresponds to the numbers of trials that studied this comparison. Of the 21 pair-wise comparisons between the 7 treatment regimens, 17 trials directly studied efficacy for OSR and ORR and 20 trials for safety.
Fig 2. Network of comparisons for overall response rate.
The size of the nodes corresponds to the number of treatment study trials. Direct comparison treatments are linked with a line noting the number of trials. The thickness of the line corresponds to the number of trials that studied this comparison. Node: T-DM1; LC, lapatinib; HC, trastuzumab; NST, naïve standard treatments; PEHC, pertuzumab and trastuzumab; PEC, pertuzumab; LHC, lapatinib and trastuzumab.
In head-to-head comparisons, for OSR, the T-DM1 regimen was more effective than LC (OR 0.60 [95% CI 0.39, 0.94]) (Table 1). For ORR, the T-DM1 regimen was more effective than LC (OR 0.59 [0.40, 0.85]) and PEC (OR 0.45 [0.18, 0.91]), and the PEHC regimen was more effective than LC (OR 2.06 [1.29, 3.16]), HC (OR 1.85 [1.23, 2.67]), or PEC (OR 3.04 [1.48, 5.60]) (Table 2). Their cumulative SUCRA ranking curves for overall survival rate and overall response rate were shown in S5 Fig.
Table 1. Network meta-analysis comparison of the efficacy of the 5 HER2-targeted treatment regimens for overall survival rate (OSR).
| T-DM1 | 0.61 (0.39,0.94) * | 0.60 (0.33,1.07) | 0.44 (0.24,0.73) * | 0.88 (0.40,1.76) |
| LC | 0.99 (0.71,1.35) | 0.71 (0.52,0.94) * | 1.43 (0.76,2.65) | |
| HC | 0.73 (0.59,0.89) * | 1.44 (0.90,2.28) | ||
| OSR | NST | 2.01 (1.17,3.39) * | ||
| PEHC | ||||
| T-DM1 | 0.61 (0.39,0.94) * | 0.60 (0.33,1.07) | 0.44 (0.24,0.73)* | 0.88 (0.40,1.76) |
| LC | 0.99 (0.71,1.35) | 0.71 (0.52,0.94) * | 1.43 (0.76,2.65) | |
| HC | 0.73 (0.59,0.89) * | 1.44 (0.90,2.28) | ||
| OSR | NST | 2.01 (1.17,3.39) * | ||
| PEHC |
* The difference between A and B has statistical significance with its confidence interval without 1.
Table 2. Network meta-analysis comparison of the efficacy of the the 7 HER2-targeted treatment regimens for overall response rate (ORR).
| T-DM1 | 0.59 (0.40,0.85) * | 0.66 (0.42,1.02) | 0.27 (0.17,0.43) * | 0.45 (0.18,0.91) * | 1.23 (0.66,2.11) | 1.03 (0.53,1.76) |
| LC | 1.12 (0.97,1.42) | 0.45 (0.36,0.58) * | 0.75 (0.35,1.38) | 2.06 (1.29,3.16) * | 1.72 (0.99,2.81) | |
| HC | 0.41 (0.33,0.52) * | 0.67 (0.33,1.23) | 1.85 (1.23,2.66) * | 1.54 (0.89,2.42) | ||
| NST | 1.64 (0.77,3.01) | 4.53 (2.79,6.94) * | 3.80 (1.13,6.40) * | |||
| ORR | PEC | 3.04 (1.48,5.60) * | 2.59 (1.25, 5.26) * | |||
| PEHC | 0.86 (0.45, 1.55) | |||||
| LHC |
* The difference between A and B has statistical significance with its confidence interval without 1
The results for safety for head-to-head comparisons are in S4 Table. The LHC regimen showed greater association with rash than the T-DM1, HC, PEHC and NST regimens, and the PEHC regimen was less associated with rash than the LC regimen. The LC regimen was less associated with diarrhea than the NST and HC regimens. We found no significant effects for LVEF, fatigue, nausea and vomiting, perhaps because of the small sample size. Most loops were consistent. Only for rash for LC versus HC versus NST showed low inconsistency (S4C Fig), which implies that the direct estimate of the summary effect should differ from the indirect estimate according to the loop plots.
S6 Table represented 5 regimens ordered by their probability of being the best in terms of OSR as well as all HER2-targeted treatment regimens ordered by their probability of being the best in terms of ORR, showing the separate contributions of percentage to the overall scores of efficacy are shown.
We performed a sensitivity analysis and found that random- and fixed-effects models produced the similar results (S5, S7 and S8 Tables). Many meta-analyses have focused on advanced or metastasized breast cancer, so we performed a subgroup analysis of HER2+ advanced breast cancer with the T-DM1, LC, HC, NST, PEHC regimens (S9 Table). For OSR, the T-DM1 regimen was more effective than LC, HC and NST. For ORR, the T-DM1 regimen was more effective than LC and NST, and the PEHC regimen was more effective than LC, HC and NST. Rash was more associated with LC than T-DM1 and more associated with LC than HC, and NST. The regimens of T-DM1 and PEHC seemed to be better for HER2-targeted therapy for advanced or metastatic breast cancer with HER2-overexpression (S10 Table).
Discussion
In our network meta-analysis, treatment with T-DM1 or pertuzumab in combination with trastuzumab and docetaxel, outperformed other treatment regimens, and the treatment effects were indistinguishable in magnitude. We found no difference in outcomes among the LC, HC, and LHC regimens for treatment of HER2+ breast cancer. In terms of side effects, rash was more associated with the LHC regimen than PEHC, HC, and T-DM1 regimens, and less associated with the PEHC than LC regimen. The PEC regimen did not differ from other 6 regimens perhaps because of limited sample size. Adding pertuzumab to standard treatment was previously found to be associated with a significant risk of rash [68]. Furthermore, risk of abnormal LVEF was greater with adding trastuzumab to standard treatment than naïve standard treatment [69]. Overall, no two HER2-targeted treatment regimens were significantly different with regard to the remaining side effects.
With 7 treatment regimens for HER2+ breast cancer, direct comparisons are limited clearly by the small number of studies evaluating a specific pair of treatment regimens. Network meta-analysis resolved this problem using indirect comparisons and distinguishes the most effective and safest treatment. Nonetheless, we found no meaningful data for the side effects of fatigue, diarrhea, vomiting and nausea.
A mixed model is as the most appropriate for multiple-treatment meta-analysis [38, 48]. Except for a heterogeneity >75% for the LC versus HC regimens for diarrhea, we found little heterogeneity among all peer-comparisons. The categories of HER2-targeted treatment might be the first attempt to be used to compare direct and indirect data with the network approaches for HER2-targeted treatment. Some network or conventional analyses have been published for advanced breast cancer, but they either just focused on targeted therapy without stressing on the HER2-targeted therapy or did not use meta-analysis for multiple-treatments [24, 25].
Our evidence supports adding HER2-targeted agents to standard treatments for HER2+ advanced or metastatic breast cancer, but the optimal duration of ErbB-family receptor inhibitors added to standard treatment is not clear. In our network meta-analysis and pair-wise meta-analysis, OSR and ORR was associated more with the T-DM1 than LC regimen [70], more with HC and LC regimens than naive standard treatment (NST) [71], and more with the LHC regimen than LC and HC regimens, although linked to rash and gastrointestinal disorders. In the subgroup analysis, because of the limited of sample sizes, we included T-DM1, LC, HC, and PEHC as well as NST for analysis of OSR and all 7 treatment regimens for analysis of ORR. The best treatment was T-DM1 for OSR and PEHC for ORR. In our network analysis, we found T-DM1 and PEHC regimens were potentially and equally as ideal HER2-targeted treatment regimens for treating HER2+ breast cancer.
Our study has some disadvantages. First, the network could not be expanded to unpublished trials and extracted published data rather than individual patient information [42, 72]. Secondly, we chose the suitable trials without consideration of whether the trial was first-line or not because of lack of sufficient trials. Although the accuracy of the results might be misleading to some extent, we performed a subgroup analysis for first/second-line HER2+ advanced or metastatic breast cancer and got more reliable results. In addition, some trials were open-label and the concealment was not detailed clearly; thus, the validity of the results might be underestimated[41]. Third, in any meta-analysis, selection bias and publication bias cannot be avoided. In subgroup analysis, we did not estimate the effect on early HER2+ breast cancer because only 4 trials were available on this topic, which is insufficient for constructing a closed loop. Finally, our study did not stress on high- and low-dosing HER2-targeted agents combined with standard treatments. If we detail such treatment regimens like that, these regimens can not be connected with each other in a closed loop. In the future, with the increasing number of clinical trials for HER2+ breast cancer, we hope to provide details for HER2-targeted agents added to concrete standard treatments to provide meaningful data to clinicians and researchers in this field.
Conclusions
In conclusion, our study supports that treatment with T-DM1 as well as pertuzumab in combination with trastuzumab and docetaxel, currently is the best targeted drug regimen for HER2+ breast cancer in terms of overall survival rate (OSR) and overall response rate (ORR) and with moderate toxicity, especially for HER2+ advanced or metastatic breast cancer. Naive standard treatment (NST) without the HER2-targeted agents showed poor efficacy, but the least toxicity. The suggested hierarchies for the 7 treatment regimens should provide information for clinicians to choose a suitable targeted therapy for HER2+ breast cancer. We hope that more and more randomized trials, consisting of more than one HER2-targeted agent in combination with chemotherapy/hormone therapy in HER2-positive breast cancer such as the comparable randomized trials that T-DM1+/- pertuzumab versus standard treatment with or without T-MD1+/- pertuzumab, can be increased to represent various and efficacious treatment regimens.
Supporting Information
(TIF)
Review authors’ judgments (low, unclear, high) for each risk of bias item presented as percentages across 19 included studies, one study was from the ClinicalTrials.gov website without bias description.
(TIF)
The size of the nodes corresponds to the number of trials that studied the treatment. The directly comparable treatments are linked with a line. The thickness of the line corresponds to the number of trials that studied this comparison. T-DM1C, T-DM1; LC, lapatinib; HC, trastuzumab; NST, naïve standard treatments; PEHC, pertuzumab and trastuzumab; PEC, pertuzumab; LHC, lapatinib and trastuzumab.
(TIF)
Inconsistency estimated as the difference between direct and indirect estimate (called inconsistent factor; IF) and the corresponding 95% confidence interval (95% CI) for the IF in each closed loop. The forest plots show all closed triangular loops (loops formed by 3 treatments) in each outcome network. Inconsistent loops present inconsistent loops per network (maximum 9% of the loops), which can be attributed to chance.
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1. Chu D, Lu J. Novel therapies in breast cancer: What is new from ASCO 2008. J Hematol Oncol 2008:1:16 10.1186/1756-8722-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA: a cancer journal for clinicians. 2007;57(1):43–66. [DOI] [PubMed] [Google Scholar]
- 4. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. [DOI] [PubMed] [Google Scholar]
- 5. Vogel UF. Confirmation of a low HER2 positivity rate of breast carcinomas—limitations of immunohistochemistry and in situ hybridization. Diagnostic pathology. 2010;5:50 10.1186/1746-1596-5-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zahnow CA. ErbB receptors and their ligands in the breast. Expert reviews in molecular medicine. 2006;8(23):1–21. [DOI] [PubMed] [Google Scholar]
- 7. Zhang H, Berezov A, Wang Q, Zhang G, Drebin J, Murali R, et al. ErbB receptors: from oncogenes to targeted cancer therapies. The Journal of clinical investigation. 2007;117(8):2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(3):264–271. [DOI] [PubMed] [Google Scholar]
- 9. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. The New England journal of medicine. 2012;366(2):109–119. 10.1056/NEJMoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baselga J, Manikhas A, Cortes J, Llombart A, Roman L, Semiglazov VF, et al. Phase III trial of nonpegylated liposomal doxorubicin in combination with trastuzumab and paclitaxel in HER2-positive metastatic breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25(3):592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burstein HJ, Keshaviah A, Baron AD, Hart RD, Lambert-Falls R, Marcom PK, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110(5):965–972. [DOI] [PubMed] [Google Scholar]
- 12. Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. The Lancet Oncology. 2013;14(6):461–471. 10.1016/S1470-2045(13)70130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCormack PL. Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer. Drugs. 2013;73(13):1491–1502. 10.1007/s40265-013-0109-0 [DOI] [PubMed] [Google Scholar]
- 14. Guan Z, Xu B, DeSilvio ML, Shen Z, Arpornwirat W, Tong Z, et al. Randomized trial of lapatinib versus placebo added to paclitaxel in the treatment of human epidermal growth factor receptor 2-overexpressing metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(16):1947–1953. [DOI] [PubMed] [Google Scholar]
- 15. Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. The Lancet Oncology. 2012;13(2):135–144. 10.1016/S1470-2045(11)70397-7 [DOI] [PubMed] [Google Scholar]
- 16. Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, et al. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11(7):753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(9):1157–1163. [DOI] [PubMed] [Google Scholar]
- 18. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. The Lancet Oncology. 2012;13(1):25–32. 10.1016/S1470-2045(11)70336-9 [DOI] [PubMed] [Google Scholar]
- 19. Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, et al. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(14):1719–1725. [DOI] [PubMed] [Google Scholar]
- 20. Miller KD, Dieras V, Harbeck N, Andre F, Mahtani RL, Gianni L, et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2-positive, locally advanced, or metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(14):1437–1444. [DOI] [PubMed] [Google Scholar]
- 21. Cortes J, Baselga J, Im YH, Im SA, Pivot X, Ross G, et al. Health-related quality-of-life assessment in CLEOPATRA, a phase III study combining pertuzumab with trastuzumab and docetaxel in metastatic breast cancer. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(10):2630–2635. 10.1093/annonc/mdt274 [DOI] [PubMed] [Google Scholar]
- 22. Azim HA Jr, Agbor-Tarh D, Bradbury I, Dinh P, Baselga J, Di Cosimo S, et al. Pattern of rash, diarrhea, and hepatic toxicities secondary to lapatinib and their association with age and response to neoadjuvant therapy: analysis from the NeoALTTO trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(36):4504–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robidoux A, Tang G, Rastogi P, Geyer CE Jr, Azar CA, Atkins JN, et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): an open-label, randomised phase 3 trial. The Lancet Oncology. 2013;14(12):1183–1192. 10.1016/S1470-2045(13)70411-X [DOI] [PubMed] [Google Scholar]
- 24. Mauri D, Polyzos NP, Salanti G, Pavlidis N, Ioannidis JP. Multiple-treatments meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. Journal of the National Cancer Institute. 2008;100(24):1780–1791. 10.1093/jnci/djn414 [DOI] [PubMed] [Google Scholar]
- 25. Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2011;22(6):1308–1317. 10.1093/annonc/mdq593 [DOI] [PubMed] [Google Scholar]
- 26. Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. Jama. 2003;289(19):2534–2544. [DOI] [PubMed] [Google Scholar]
- 27. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369(9557):201–207. [DOI] [PubMed] [Google Scholar]
- 28. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. The Lancet Oncology. 2011;12(1):65–82. 10.1016/S1470-2045(10)70260-6 [DOI] [PubMed] [Google Scholar]
- 29. Lam SK, Owen A. Combined resynchronisation and implantable defibrillator therapy in left ventricular dysfunction: Bayesian network meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2007;335(7626):925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owen A. Antithrombotic treatment for the primary prevention of stroke in patients with non valvular atrial fibrillation: a reappraisal of the evidence and network meta analysis. International journal of cardiology. 2010;142(3):218–223. 10.1016/j.ijcard.2009.11.045 [DOI] [PubMed] [Google Scholar]
- 31. Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schomig A, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ (Clinical research ed). 2008;337:a1331 10.1136/bmj.a1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stettler C, Wandel S, Allemann S, Kastrati A, Morice MC, Schomig A, et al. Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet. 2007;370(9591):937–948. [DOI] [PubMed] [Google Scholar]
- 33. Strassmann R, Bausch B, Spaar A, Kleijnen J, Braendli O, Puhan MA. Smoking cessation interventions in COPD: a network meta-analysis of randomised trials. The European respiratory journal. 2009;34(3):634–640. 10.1183/09031936.00167708 [DOI] [PubMed] [Google Scholar]
- 34. Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. European heart journal. 2008;29(9):1086–1092. 10.1093/eurheartj/ehn106 [DOI] [PubMed] [Google Scholar]
- 35. Trikalinos TA, Alsheikh-Ali AA, Tatsioni A, Nallamothu BK, Kent DM. Percutaneous coronary interventions for non-acute coronary artery disease: a quantitative 20-year synopsis and a network meta-analysis. Lancet. 2009;373(9667):911–918. 10.1016/S0140-6736(09)60319-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van der Valk R, Webers CA, Lumley T, Hendrikse F, Prins MH, Schouten JS. A network meta-analysis combined direct and indirect comparisons between glaucoma drugs to rank effectiveness in lowering intraocular pressure. Journal of clinical epidemiology. 2009;62(12):1279–1283. 10.1016/j.jclinepi.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 37. Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ (Clinical research ed). 2010;341:c4675 10.1136/bmj.c4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in medicine. 2004;23(20):3105–3124. [DOI] [PubMed] [Google Scholar]
- 39. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of clinical epidemiology. 2011;64(2):163–171. 10.1016/j.jclinepi.2010.03.016 [DOI] [PubMed] [Google Scholar]
- 40. Higgins J, Green S. Cochrane handbook for systematic reviews of intervention,version 5.0.0 1st ed The Cochrane Collaboration Hoboken: Wiley-Backwell;2008. [Google Scholar]
- 41. Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746–758. 10.1016/S0140-6736(09)60046-5 [DOI] [PubMed] [Google Scholar]
- 42. Zhu Z, Zhang J, Liu Y, Chen M, Guo P, Li K. Efficacy and toxicity of external-beam radiation therapy for localised prostate cancer: a network meta-analysis. British journal of cancer. 2014;110(10):2396–2404. 10.1038/bjc.2014.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28(2):105–114. [DOI] [PubMed] [Google Scholar]
- 44. Borenstein M, Hedge L, Higgins J, Rothstein HR. Introduction to Meta-analysis. 1st ed United Kingdom: Wiley-Backwell; 2011. [Google Scholar]
- 45. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. PharmacoEconomics. 2006;24(1):1–19. [DOI] [PubMed] [Google Scholar]
- 47. Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. Royal Statist Soc B. 2002;64(4):583–639. [Google Scholar]
- 48. Caldwell DM, Welton NJ, Ades AE. Mixed treatment comparison analysis provides internally coherent treatment effect estimates based on overviews of reviews and can reveal inconsistency. Journal of clinical epidemiology. 2010;63(8):875–882. 10.1016/j.jclinepi.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 49. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. Journal of clinical epidemiology. 1997;50(6):683–691. [DOI] [PubMed] [Google Scholar]
- 50. Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. Journal of clinical epidemiology. 2009;62(8):857–864. 10.1016/j.jclinepi.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 51. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England journal of medicine. 2001;344(11):783–792. [DOI] [PubMed] [Google Scholar]
- 52. Eiermann W, International Herceptin Study G. Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: pivotal trial data. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2001;12 Suppl 1:S57–62. [PubMed] [Google Scholar]
- 53. Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(19):4265–4274. [DOI] [PubMed] [Google Scholar]
- 54. Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, et al. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast cancer research and treatment. 2007;101(3):355–365. [DOI] [PubMed] [Google Scholar]
- 55. von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(12):1999–2006. [DOI] [PubMed] [Google Scholar]
- 56. Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(33):5529–5537. [DOI] [PubMed] [Google Scholar]
- 57. Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer—results of the eLEcTRA trial. Breast (Edinburgh, Scotland). 2012;21(1):27–33. 10.1016/j.breast.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 58. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. The New England journal of medicine. 2012;367(19):1783–1791. 10.1056/NEJMoa1209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cameron D, Casey M, Oliva C, Newstat B, Imwalle B, Geyer CE. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. The oncologist. 2010;15(9):924–934. 10.1634/theoncologist.2009-0181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. The New England journal of medicine. 2006;355(26):2733–2743. [DOI] [PubMed] [Google Scholar]
- 61. Schwartzberg LS, Franco SX, Florance A, O'Rourke L, Maltzman J, Johnston S. Lapatinib plus letrozole as first-line therapy for HER-2+ hormone receptor-positive metastatic breast cancer. The oncologist. 2010;15(2):122–129. 10.1634/theoncologist.2009-0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johnston S, Pippen J Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(33):5538–5546. [DOI] [PubMed] [Google Scholar]
- 63. Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(34):5544–5552. 10.1200/JCO.2008.16.2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. The New England journal of medicine. 2012;366(2):109–119. 10.1056/NEJMoa1113216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perez EA, Suman VJ, Davidson NE, Gralow JR, Kaufman PA, Visscher DW, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(34):4491–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. The New England journal of medicine. 2011;365(14):1273–1283. 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. The New England journal of medicine. 2006;354(8):809–920. [DOI] [PubMed] [Google Scholar]
- 68. Drucker AM, Wu S, Dang CT, Lacouture ME. Risk of rash with the anti-HER2 dimerization antibody pertuzumab: a meta-analysis. Breast cancer research and treatment. 2012;135(2):347–354. 10.1007/s10549-012-2157-7 [DOI] [PubMed] [Google Scholar]
- 69. Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer treatment reviews. 2011;37(4):312–320. 10.1016/j.ctrv.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 70. Niculescu-Duvaz I. Trastuzumab emtansine, an antibody-drug conjugate for the treatment of HER2+ metastatic breast cancer. Curr Opin Mol Ther. 2010;12(3):350–360. [PubMed] [Google Scholar]
- 71. Botrel TE, Paladini L, Clark OA. Lapatinib plus chemotherapy or endocrine therapy (CET) versus CET alone in the treatment of HER-2-overexpressing locally advanced or metastatic breast cancer: systematic review and meta-analysis. Core evidence. 2013;8:69–78. 10.2147/CE.S50474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Trikalinos TA, Ioannidis JP. Predictive modeling and heterogeneity of baseline risk in meta-analysis of individual patient data. Journal of clinical epidemiology. 2001;54(3):245–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Review authors’ judgments (low, unclear, high) for each risk of bias item presented as percentages across 19 included studies, one study was from the ClinicalTrials.gov website without bias description.
(TIF)
The size of the nodes corresponds to the number of trials that studied the treatment. The directly comparable treatments are linked with a line. The thickness of the line corresponds to the number of trials that studied this comparison. T-DM1C, T-DM1; LC, lapatinib; HC, trastuzumab; NST, naïve standard treatments; PEHC, pertuzumab and trastuzumab; PEC, pertuzumab; LHC, lapatinib and trastuzumab.
(TIF)
Inconsistency estimated as the difference between direct and indirect estimate (called inconsistent factor; IF) and the corresponding 95% confidence interval (95% CI) for the IF in each closed loop. The forest plots show all closed triangular loops (loops formed by 3 treatments) in each outcome network. Inconsistent loops present inconsistent loops per network (maximum 9% of the loops), which can be attributed to chance.
(TIF)
(TIF)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.