Abstract
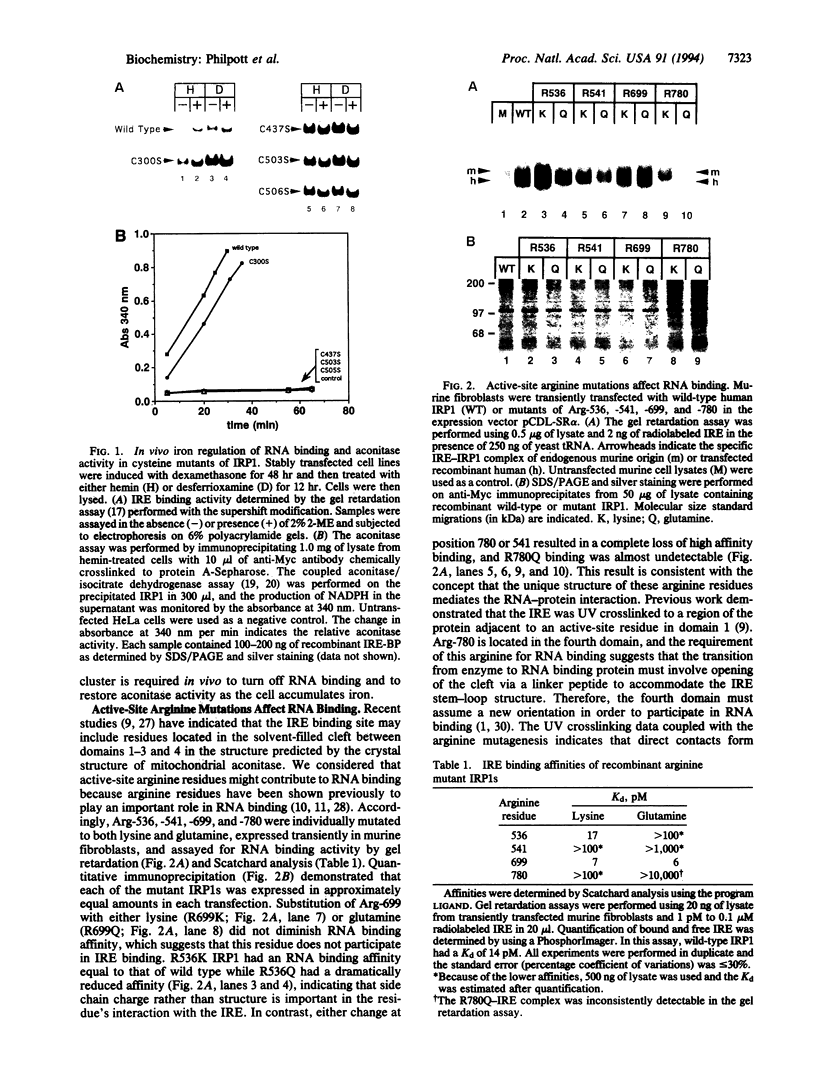
The iron-responsive element binding protein/cytosolic aconitase functions as either an RNA binding protein that regulates the uptake, sequestration, and utilization of iron or an enzyme that interconverts citrate and isocitrate. These mutually exclusive functions are regulated by changes in cellular iron levels. By site-directed mutagenesis we show that (i) ligation of a [4Fe-4S] cluster is necessary to inactivate RNA binding and activate enzyme function in vivo, (ii) three of four arginine residues of the aconitase active site participate in RNA binding, and (iii) aconitase activity is not required for iron-mediated regulation of RNA binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilion J. P., Rouault T. A., Massinople C. M., Klausner R. D., Burgess W. H. The iron-responsive element-binding protein: localization of the RNA-binding site to the aconitase active-site cleft. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):574–578. doi: 10.1073/pnas.91.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K., Klausner R. D., Harford J. B. Two genetic loci participate in the regulation by iron of the gene for the human transferrin receptor. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1787–1791. doi: 10.1073/pnas.85.6.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell G. J., Illangesekare M., Yarus M. Three small ribooligonucleotides with specific arginine sites. Biochemistry. 1993 Jun 1;32(21):5497–5502. doi: 10.1021/bi00072a002. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993 Sep;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug abuse in business. Nature. 1970 Jul 25;227(5256):331–332. doi: 10.1038/227331b0. [DOI] [PubMed] [Google Scholar]
- Emery-Goodman A., Hirling H., Scarpellino L., Henderson B., Kühn L. C. Iron regulatory factor expressed from recombinant baculovirus: conversion between the RNA-binding apoprotein and Fe-S cluster containing aconitase. Nucleic Acids Res. 1993 Mar 25;21(6):1457–1461. doi: 10.1093/nar/21.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. R., Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991 Oct 15;266(29):19328–19333. [PubMed] [Google Scholar]
- Guo Q., Lambowitz A. M. A tyrosyl-tRNA synthetase binds specifically to the group I intron catalytic core. Genes Dev. 1992 Aug;6(8):1357–1372. doi: 10.1101/gad.6.8.1357. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H., Henderson B. R., Kühn L. C. Mutational analysis of the [4Fe-4S]-cluster converting iron regulatory factor from its RNA-binding form to cytoplasmic aconitase. EMBO J. 1994 Jan 15;13(2):453–461. doi: 10.1002/j.1460-2075.1994.tb06280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Evans C. T., Malloy C., Srere P. A. Metabolic studies on citrate synthase mutants of yeast. A change in phenotype following transformation with an inactive enzyme. J Biol Chem. 1989 Jul 5;264(19):11204–11210. [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A. A double life: cytosolic aconitase as a regulatory RNA binding protein. Mol Biol Cell. 1993 Jan;4(1):1–5. doi: 10.1091/mbc.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Lauble H., Kennedy M. C., Beinert H., Stout C. D. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry. 1992 Mar 17;31(10):2735–2748. doi: 10.1021/bi00125a014. [DOI] [PubMed] [Google Scholar]
- MORRISON J. F. The activation of aconitase by ferrous ions and reducing agents. Biochem J. 1954 Dec;58(4):685–692. doi: 10.1042/bj0580685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S., White J. H. A steroid-inducible promoter for the controlled overexpression of cloned genes in eukaryotic cells. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5603–5607. doi: 10.1073/pnas.90.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- O'Halloran T. V. Transition metals in control of gene expression. Science. 1993 Aug 6;261(5122):715–725. doi: 10.1126/science.8342038. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 1988 Apr;7(4):913–918. doi: 10.1002/j.1460-2075.1988.tb02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. C., Haile D., Rouault T. A., Klausner R. D. Modification of a free Fe-S cluster cysteine residue in the active iron-responsive element-binding protein prevents RNA binding. J Biol Chem. 1993 Aug 25;268(24):17655–17658. [PubMed] [Google Scholar]
- Puglisi J. D., Chen L., Frankel A. D., Williamson J. R. Role of RNA structure in arginine recognition of TAR RNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3680–3684. doi: 10.1073/pnas.90.8.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A. 1989 May;86(10):3639–3643. doi: 10.1073/pnas.86.10.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. The structure of aconitase. Proteins. 1989;5(4):289–312. doi: 10.1002/prot.340050406. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Tan R., Chen L., Buettner J. A., Hudson D., Frankel A. D. RNA recognition by an isolated alpha helix. Cell. 1993 Jun 4;73(5):1031–1040. doi: 10.1016/0092-8674(93)90280-4. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Kennedy M. C., Beinert H., Zalkin H. Mutational analysis of active site residues in pig heart aconitase. J Biol Chem. 1992 Apr 15;267(11):7895–7903. [PubMed] [Google Scholar]