Summary
Purpose
Traumatic brain injury (TBI) is an important cause of morbidity and mortality in children and early post-traumatic seizures (EPTS) are a contributing factor to ongoing acute damage. Continuous video EEG monitoring (cEEG) was utilized to assess the burden of clinical and electrographic EPTS.
Methods
Eighty-seven consecutive, unselected (mild – severe), acute TBI patients requiring pediatric intensive care unit (PICU) admission at 2 academic centers were prospectively monitored with cEEG per established clinical TBI protocols. Clinical and subclinical seizures and status epilepticus (SE, clinical and subclinical) were assessed for their relation to clinical risk factors and short-term outcome measures.
Key findings
Of all patients, 42.5% (37/87) had seizures. Younger age (p=0.002) and mechanism (abusive head trauma - AHT, p<0.001) were significant risk factors. Subclinical seizures occurred in 16.1% (14/87), 6 of whom had only subclinical seizures. Risk factors for subclinical seizures included: younger age (p<0.001), AHT (p<0.001) and intraaxial bleed (p<0.001). Status Epilepticus (SE) occurred in 18.4% (16/87) with risk factors including: younger age (p<0.001), AHT (p<0.001), and intraaxial bleed (p=0.002). Subclinical SE was detected in 13.8% (12/87) with significant risk factors including: younger age (p<0.001), AHT (p=0.001), and intraaxial bleed (p=0.004). Subclinical seizures were associated with lower discharge KOSCHI score (p=0.002). SE and subclinical SE were associated with increased hospital length of stay (p=0.017 and p=0.041 respectively) and lower hospital discharge KOSCHI (p=0.007 and p=0.040 respectively).
Significance
cEEG monitoring significantly improves detection of seizures/SE and is the only way to detect subclinical seizures/SE. cEEG may be indicated after pediatric TBI, particularly in younger children, AHT cases, and those with intraaxial blood on CT.
Keywords: EEG, Pediatric, Trauma, Subclincal, Seizure
Introduction
Traumatic Brain Injury (TBI) is the number one cause of death and disability in children, annually affecting nearly half a million in the U.S. alone. (Faul M, 2010; Langlois et al., 2005) Early posttraumatic seizures (EPTS) may indicate more severe primary injury or could cause secondary brain injury by increasing metabolic requirements and cerebral blood flow, elevating intracranial pressure, inducing relative cerebral hypoxia/ischemia, exacerbating indiscriminate neurotransmitter release, and elevating temperature. (Mansfield, 1997; Vespa et al., 2007a; Vespa et al., 2007b) PTS may indicate an ongoing cerebral injury process such as intracranial hemorrhage, cerebral edema, or hypoxia, alerting clinicians to prompt additional investigation. Additionally, prolonged PTS should also be considered in the differential diagnosis for both a child deteriorating after a lucid interval or the etiology for persistent, unexplained coma. (Snoek et al., 1984)
An early post-traumatic seizure (EPTS) is classically defined as any PTS occurring <7 days post-injury.(Jennett & Lewin, 1960) In studies reporting clinical seizure occurrence after pediatric TBI of all severities without utilizing continuous electroencephalography (cEEG) monitoring, EPTS incidence ranges from 2.6–20%. (Annegers et al., 1980; Chiaretti et al., 2000; Desai et al., 1983; Hendrick & Harris, 1968; Jennett, 1973; Jennett & Lewin, 1960; Ong et al., 1996; Pagni, 1990; Ratan et al., 1999) More specifically, EPTS incidence ranges from 1–27% in pediatric moderate TBI, and from 22–45% in pediatric severe TBI. Reportedly, 59 – 95% of clinically detected pediatric EPTS occur within the first 24hrs post-injury. (Annegers et al., 1980; Chiaretti et al., 2000; Hendrick & Harris, 1968; Jennett, 1973; Ong et al., 1996; Pagni, 1990) Studies report children are more prone to EPTS than adults, and in some cases over a 2 fold higher incidence. (Annegers et al., 1980; Desai et al., 1983; Jennett & Lewin, 1960; Pagni, 1990) Further, younger children appear more prone to EPTS than older children. (Hendrick & Harris, 1968; Ong et al., 1996; Ratan et al., 1999) To date, cEEG has not been utilized prospectively in pediatric cohorts to characterize EPTS incidence or time of onset post-injury.
In pediatric intensive care unit (PICU) TBI patients many factors can confound the ability to detect seizures clinically including high frequency of altered mental status/coma and the use of sedatives, paralytics, and even anticonvulsants. Therefore, the current practice of relying on clinical markers such as mental status and physical exam to monitor for seizures is insufficient. Continuous video electroencephalographic (cEEG) monitoring has significantly increased the detection rates of seizures and epileptiform abnormalities following moderate-severe adult TBI; 22% had EPTS, over half were nonconvulsive or subclinical. (Vespa et al., 1999b) High rates of epileptiform discharges were also reported in non-seizure groups. (Ronne-Engstrom & Winkler, 2006; Vespa et al., 1999b) Furthermore, cEEG was shown to improve daily management decisions, reduce cost and improve outcome in a mixed retrospective/prospective adult ICU brain injury cohort. (Vespa et al., 1999a)
The use of cEEG has become more widespread in PICU, revealing the relatively high rate of subclinical seizures in critically ill children, and identifying younger age as a risk factor. However, these reports included relatively small numbers of TBI patients, and relied on a single EEG reader or multiple readers without validation between readers. (Abend et al., 2011; Shahwan et al., 2010; Williams et al., 2011)
TBI outcome depends on the severity of primary brain injury, and the efficacy of preventing/ limiting secondary brain injury. EPTS is a potentially treatable cause of secondary brain injury in TBI patients. Both clinical and subtle/subclinical seizures are reported to be associated with TBI morbidity/outcome. (Chiaretti et al., 2000; Desai et al., 1983; Hahn et al., 1988; Ong et al., 1996; Vespa et al., 2007a) The current study is the first prospective multi-center investigation of the incidence and risk factors for EPTS as detected using standardized cEEG monitoring in consecutive children requiring admission to the PICU for acute, unselected (mild – severe) TBI.
Methods
Institutions and subjects
The study was reviewed and approved by respective institutional review boards (UCLA IRB, COMIRB). Consecutive, acute TBI patients of all severities (GCS 3–15) requiring PICU admission at two institutions (Mattel Children’s Hospital - UCLA, Children’s Hospital of Colorado - CHC) were identified. Determination of PICU admission was made by attending emergency physician in consultation with the attending PICU physician. All moderate-severe TBI patients were admitted to the PICU at each institution. No mild TBI patients were admitted to PICU at CHC, while 8 mild TBI patients deemed to require frequent neurological checks required nursing staffing levels only provided in the PICU at UCLA. Of 99 eligible patients, 87 were prospectively consented and enrolled from 10/2008 to 10/2011 (100% consent rate at CHC and 83.3% at UCLA; overall 87.9%); the 12 that did not consent (all from UCLA) were demographically similar to those consented. Five consented patients did not receive monitoring because 2 were admitted >48 hours after the injury and 3 were missed. Patient management followed PICU standards of care. Continuous EEG monitoring was initiated after patient identification. cEEG readings were reported out once or twice daily. Injury severity was defined using post-resuscitation Glasgow Coma Score (GCS); by convention GCS 13–15 was classified as mild, 9–12 as moderate and 3–8 severe (Teasdale & Jennett, Lancet 1974). Subjects with GCS 13–15 and intracranial abnormalities on noncontrast brain CT were classified as moderate, considering recent studies suggesting that GCS is only a partial factor in characterizing TBI severity, and that underlying pathology as detected by acute CT may be valuable in considering risks for long-term sequelae (Maas et al., 2011; Saatman et al., 2008). Such abnormalities included contusions, hematomas or edema but excluded nondisplaced linear skull fractures. Injury mechanism was classified as non-abusive (falls, motor vehicle accidents (MVA) including bicycle vs. motor vehicle, blunt trauma or bicycle only) or abusive head trauma (AHT). AHT was diagnosed clinically after consultation with the neurology and child abuse teams. KOSCHI scores upon PICU and hospital discharge were utilized to classify global outcome following TBI, and obtained by a combination of prospective collaboration with pediatric physiatry (CHC) and retrospective chart review or interview (CHC/UCLA). (Crouchman et al., 2001) Secondary outcomes were analyzed, including PICU and hospital length of stay.
Inclusion and exclusion criteria
All consecutive pediatric patients (ages 1 month - 18 years) admitted to the PICU with a diagnosis of acute TBI were eligible. Eight patients, all at UCLA, were admitted with mild TBI and were monitored in the PICU for frequent neurologic exams throughout the night not available on the pediatric ward. Patients were included without regard to prior history of febrile seizures, family history of epilepsy, prior history of seizures and/or epilepsy.
Continuous EEG protocol
Institutional protocols initiated cEEG monitoring as soon as possible for any patient admitted to the PICU for acute TBI. Patients were monitored for minimum 24 hours unless clinical needs necessitated shorter or longer monitoring (i.e. death, hospital discharge, ongoing seizures etc.). All studies utilized standard international 10–20 system placement of gold-plated or plastic electrodes with standard filter settings and sampling rate. Digital EEG studies were completed on XLTEK at CHC and Stellate Harmonie at UCLA. Both a pediatric epilepsy fellow and board certified pediatric electroencephalographer at each institution reviewed all studies in their entirety during clinical review. Studies were read remotely when clinically indicated.
Seizure Classification
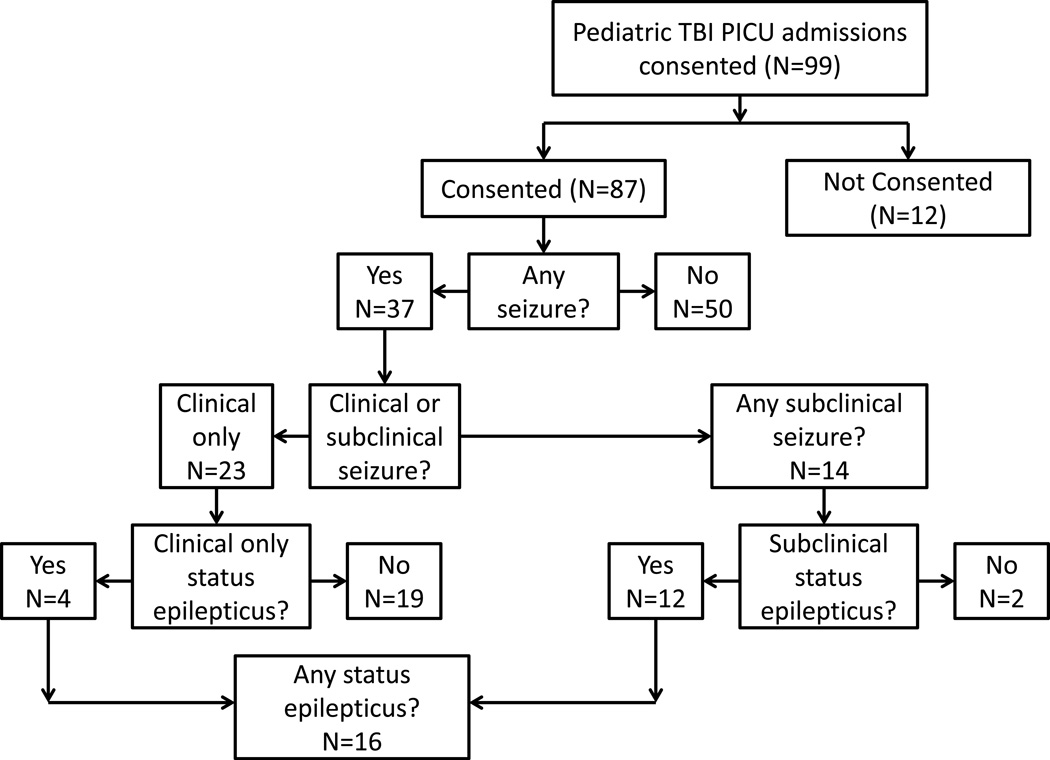
The seizures were classified as: any seizure, subclinical seizures, status epilepticus (SE) and subclinical SE. Seizures (“any seizures”) were counted if they were observed in the field, in the emergency department, in the hospital prior to hookup of the cEEG or after cEEG was started. Clinical events prior to cEEG hookup were reviewed and included as “any seizure” if episodic alteration in mental status, convulsive activity, tonic motor activity or seizure was reported in the medical record. Electrographic criteria for seizure was rhythmic repetitive sharp/spike waves with an electrographic field lasting >10 seconds and showing evolution in frequency, morphology and/or amplitude. Subclinical seizures were defined as either electrographic seizures without clinical correlate or seizures with subtle clinical findings only apparent upon video review of EEG detected seizures. This represents the subset of seizures that would be missed without cEEG monitoring. SE was classified as an ongoing seizure duration of >15 minutes, or repeated seizures occurring at a rate of >3 per hour; similar standards are utilized in adult TBI prospective cEEG data. (Vespa et al., 1999b) Subclinical SE was defined as a combination of subclinical seizures and SE. Interictal/background EEG features will be addressed in future manuscript. The exact breakdown of subjects by seizure classification is outlined in Figure 1.
Figure 1.
Patient flow chart – revised data 2nd submission
Antiepileptic medications
Antiepileptic therapy was recorded as a therapy given on the scene, in the ambulance, in the ER or in the PICU. Specific therapies included: benzodiazepines, fosphenytoin, phenytoin, phenobarbital, levetiracetam or topiramate.
Inter-observer validation
Four reviewers independently appraised forty unique 30-minute unselected pediatric cEEG recordings using the standardized definitions of seizures listed above. A single EEG technologist created the EEG epochs, which were taken from an existing database of over 5000 studies (not including any of the present study’s participants). All identifying information was removed. The age range of the patients in this selection was between 6 months and 18 years and did not contain neonates. Approximately half of the epochs contained one or more seizures commencing at random time within each study. Each reviewer recorded the presence or absence of seizure and the lateralization of the seizure (right or left hemisphere or generalized). Inter-observer validation was calculated as a percent concordance between the different EEG reviewers and using a Kappa coefficient.
Analysis/statistics
All statistical calculations were conducted using R software (version 2.15.2). For the continuous variables of age-at-injury, means were compared between XX and YY using a Welch two-sample test, and, because the distributions were not normal, the analogous robust statistic (Yuen test). (Wilcox, 2012) Categorical variables were compared between centers or between the presence or absence of a particular seizure type, using Pearson’s Chi-squared tests or Fisher Exact tests when cell sizes were small. Significance was set at a p<0.05. Variances were expressed as standard deviations (SD). Conventional linear regression models were examined, in concert with multimodal inference (Burnham & Anderson, 2010) using R package MuMln (Bartoń, 2012) to determine best fitting model subsets by rigorous statistical criteria.
Results
Demographics
87 subjects were enrolled (UCLA 60, CHC 27, Table 1). The average age at injury was 6.2±5.8 years (range: 6 weeks – 17 years). No age or gender differences between centers were identified. Institutional differences were noted for injury characteristics including severity, mechanism of injury, CT findings and seizures (Table 1).
Table 1.
Clinical characteristics of the two observational cohorts
| Combined n=87 (%) |
UCLA n=60 (%) |
CHC n=27 (%) |
p | ||
|---|---|---|---|---|---|
| Gender (M:F) | 56:31 | 39:21 | 17:10 | NS | |
| Age (years) ± SEM | 6.2 ± 5.8 | 6.7 ± 5.9 | 5.02 ± 5.4 | NS | |
| Severity | Mild | 8 (9.2) | 8 (13.3) | 0 | <0.001 |
| Moderate | 47 (54) | 42 (70) | 5 (18.5) | ||
| Severe | 32 (36.8) | 10 (16.7) | 22 (81.5) | ||
| Mechanism of injury | Fall | 37 (42.5) | 35 (58.3) | 2 (7.4) | <0.001 |
| MVA | 20 (33.3) | 10 (16.7) | 10 (37.0) | ||
| AHT | 22 (25.3) | 9 (15) | 13 (48.1) | ||
| Bicycle | 3 (3.4) | 3 (5) | 0 | ||
| Blunt | 5 (5.7) | 3 (5) | 2 (7.4) | ||
| CT findings | Skull fracture | 44 (50.6) | 33 (55) | 11 (40.7) | NS |
| EDH | 15 (17.2) | 15 (25) | 0 | 0.004 | |
| SDH | 39 (44.8) | 19 (31.7) | 20 (74.1) | <0.001 | |
| SAH | 33 (37.9) | 22 (36.7) | 11 (40.7) | NS | |
| Intra-axial bleed | 45 (51.7) | 22(36.7) | 23 (85.2) | <0.001 | |
| Any bleed | 68 (78.2) | 43 (71.7) | 25 (92.6) | 0.047 | |
| Any seizure | 37 (42.5) | 22 (36.7) | 15 (55.5) | NS | |
| Subclinical seizure | 14 (16.1) | 4 (6.7) | 10 (37.0) | <0.001 | |
| Status epilepticus | 16 (18.4) | 4 (6.7) | 12 (44.4) | <0.001 | |
| Subclinical status epilepticus | 12 (13.8) | 3 (5) | 9 (33.3) | <0.001 | |
EEG validation
Four reviewers (3 from UCLA (J.T.L, J.H.M., J.Y.W.) and 1 from CHC (D.H.A) demonstrated 98.8% agreement on the presence/absence of seizures, and 96.3% agreement on the hemisphere of onset of the seizures recorded. Kappa values calculated for the presence of a seizure and for both presence of a seizure and hemispheric location were 0.949 (p<0.001) and 0.878 (p<0.001) respectively (Kappa 95% CI = 0.680 – 1.000).
Any Seizures
Seizures of any type occurred in 43.7% (37/87) for the combined cohort: 38.3% (23/60) at UCLA and 55.5% (15/27) at CHC. Age at injury was a risk factor for seizures (p=0.002) as was presence of fracture on CT (p=0.039). Patients with seizures were younger (mean = 3.8±5.1 years) than those without seizures (mean = 7.7±5.8) (p=0.002). This also held true when patients were stratified into two groups; 61.5% (16/26) of children <1 year had seizures while only 29.5% (18/61) of patients >1 year had seizures (p=0.010). Seizures were most likely to be present in AHT (77.3%, 17/22) and less likely in blunt trauma (20.0%, 1/5), falls (27.0%, 10/37), bicycle accidents (33.3%, 1/3) and MVAs (25.0%, 5/20) (p<0.001). Other findings on CT scan, severity of injury and gender were not related to seizures (Table 2). If patients with seizures only occurring in the field were excluded from the cohort, similar results were found with age and mechanism also statistically significant. When patients with severe injury were evaluated as a subgroup, there was no relationship between the presence of seizures and KOSCHI score, hospital length of stay or PICU length of stay.
Table 2.
Risk factors for seizure types.
| Age | Gender | Severity | Mechanism | Skull fracture |
EDH | SDH | SAH | Intra-axial bleed |
Any bleed |
KOSCHI | Hospital LOS |
PICU LOS |
AED in field |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any seizure | p=0.002 | NS | NS | p<0.001 | p=0.04 | p=0.004 | NS | NS | NS | NS | NS | NS | NS | NS |
| Subclinical seizure | p<0.001 | NS | NS | p<0.001 | NS | NS | p=0.008 | NS | p<0.001 | NS | p=0.002 | NS | NS | p=0.021 |
| Status epilepticus | p<0.001 | NS | NS | p<0.001 | p=0.029 | NS | NS | NS | p=0.002 | NS | p=0.002 | p=0.022 | NS | p<0.001 |
| Subclinical status epilepticus | p<0.001 | NS | NS | p<0.001 | NS | NS | NS | NS | p=0.004 | NS | p=0.001 | p=0.049 | NS | p=0.002 |
Subclinical seizures
Subclinical seizures were detected in 16.1% (14/87) of all patients studied; 42.9% (6/14) of these patients - 7.5% (6/87) of the total population - had only subclinical seizures. Patients with any subclinical seizure had a mean age of 5.6±3.7 months and all patients with only subclinical seizures were less than 1 year old (mean age 3.7±1.8 months). Subclinical seizures occurred more often in younger children, (p<0.001) (Table 2) and those with AHT (p<0.001). Subclinical seizures were found more often in children with subdural hematomas (SDH) (p=0.008), or intraaxial hemorrhage (p<0.001), but not with epidural hematoma (EDH), subarachnoid hemorrhage (SAH) or skull fractures. No relationship was found between subclinical seizures and gender or injury severity. Only 4/33 patients given neuromuscular blocking agents were found to have subclinical seizures. The presence of this medication was not significantly related to the presence or absence of subclinical seizures. Regression analysis evaluating the relationship between subclinical seizures and age at injury, mechanism, intraaxial hemorrhage and severity was not significant. In this initial model, age at injury (p=0.181) and intraaxial hemorrhage (p=0.057) approached significance. However, additional statistical modeling following Bartoń (2012) indicated that when considered together, injury age and intraaxial hemorrhage (omitting mechanism and severity all together) yielded the best fitting predictors. The reduced regression analysis using only those two predictors found that intraaxial hemorrhage was significant (p=0.006) while injury age was not (p=0.113).
Status epilepticus
Status epilepticus was seen in 12.5% (1/8) of patients with mild injuries, 10.6% (5/47) of patients with moderate injuries and 31.3% (10/32) of patients with severe injuries. While not significant, there was a trend toward SE with severe injuries (p=0.075). Age was strongly associated with presence of SE. The mean age of patients who had SE was 1.5±3 years and the mean age of those without SE was 7.2±5.8 years (p<0.001). Mechanism of injury was related to SE; 54.5% of patients with AHT had SE while it occurred in less than 10% of patients with all other mechanisms of injury (p<0.001). In regards to CT imaging, only skull fracture (p=0.029) and intraaxial bleed (p=0.002) were related to SE.
Subclinical status epilepticus
Subclinial status epilepticus was seen in 13.8% (12/87) of all patients studied. Similar to any seizures and SE, patients with subclinical SE were younger (p<0.001), with 10/11 patients under the age of 1 year. Mechanism of injury was related to subclinical SE; 45.5% (10/22) of patients with AHT had subclinical SE while patients with other mechanisms of injury had rates of 17% (11/65) (p<0.001). Subclinical status was more frequently seen in patients with intraaxial blood on CT scan (p=0.004). No relationship was found between subclinical SE and severity of injury, gender, SAH, EDH, SDH or fracture. The presence of a neuromuscular blocking agent was not significantly related to the presence or absence of subclinical SE. Regression analysis evaluating the relationship between subclinical status epilepticus and age at injury, mechanism, intraaxial hemorrhage and severity was not significant. In this initial model, age at injury (p=0.216) and intraaxial hemorrhage (p=0.083) approached significance. However, additional statistical modeling following Bartoń (2012) indicated that when considered together, injury age and intraaxial hemorrhage (omitting mechanism and severity altogether) yielded the best fitting predictors. The reduced regression analysis using only those two predictors found that intraaxial hemorrhage was significant (p=0.006) while injury age was not (p=0.113).
Antiepileptic therapy
Patients who received antiepileptic therapy in the field, on the way to the emergency room or in the emergency room were older (mean = 7.9 ± 6.2 years vs. 4.5 ± 4.8 years, p=0.006), more likely to have severe injuries (p<0.001), any type of bleed on CT scan (p=0.001), subclinical seizures (p=0.021), SE (p<0.001) or subclinical SE (p=0.002) during their hospital course. Further analysis is required to screen for electrical-clinical dissociation effects known to occur with some antiepileptic drugs.
Outcomes
Patients with SE and subclinical SE had on average a longer length of hospital stay than those without (SE 23.6±4.7 [SEM] days vs no SE 11.2±2.1 days, p=0.022 and subclinical SE 22.6±4.7 [SEM] days vs no subclinical SE 12.0±2.1 days, p=0.049); however there was no difference in the length of stay in the PICU. Finally, KOSCHI scores at hospital discharge were not related to any seizures (NS) but were significantly lower in patients with subclinical seizures (p=0.001), SE (p=0.002) and subclinical SE (p=0.001). Univariate regression analysis was performed evaluating the relationship between KOSCHI score and age at injury, mechanism, intraaxial hemorrhage, severity, any seizure and subclinical seizures. The only significant predictor found was severity of injury (p=0.009).
Discussion
The incidence rates of 42.5% (37/87) for EPTS and 16.1% (14/87) for subclinical seizures in this study are higher than those reported in a comparable adult cohort (22.3% and 11.7%, for EPTS and subclinical seizures, respectively).(Vespa et al., 1999b) This suggests a greater risk for EPTS in pediatric patients. The rate was also higher than found in two retrospective pediatric TBI evaluations without standardized cEEG (EPTS = 12.0%).(Chiaretti et al., 2000; Liesemer et al., 2011) The use of cEEG to detect subclinical seizures in the PICU has been published with prevalence rates between 7% and 39%.(Abend et al., 2011; Hahn, 2011; Jette et al., 2006; Saengpattrachai et al., 2006; Shahwan et al., 2010; Williams et al., 2011) Most of the studies had few TBI patients, were retrospective and initiation of the EEG was based on clinical judgment, which may impart bias into the composition of the cohort. This is the first study to prospectively utilize cEEG for all consecutive, unselected, acute TBI patients admitted to the PICU.
This study showed that younger age, AHT mechanism and presence of intraaxial blood were clinical predictors of subclinical seizures or SE. The presence of status epilepticus (clinical or subclinical) was significantly associated with worse outcomes as measured by KOSCHI and hospital length of stay.
Our cohort confirmed past findings that EPTS were more frequent in younger children, specifically <1 year of age. (Chiaretti et al., 2000; Hendrick & Harris, 1968; Liesemer et al., 2011; Ong et al., 1996; Ratan et al., 1999). Status epilepticus was seen most often in children <1.5 years of age. Mechanism of injury has been reported as a risk factor for EPTS (Barlow et al., 2000; Liesemer et al., 2011) and in our cohort EPTS occurred in 77.3% of patients with AHT, more than twice the rate of any other mechanism. One retrospective study of children with AHT found a lower rate (33.0%) of seizures; however, less than half of the children studied had an EEG. (Goldstein et al., 2010) Our rates were higher likely owing to the systematic incorporation of cEEG monitoring in all patients enabling detection of subclinical seizures. Higher TBI severity has been consistently reported to correlate with EPTS risk, (Chiaretti et al., 2000; Hahn et al., 1988; Ratan et al., 1999) but was not found to be significant in our study. This may be a direct consequence of the regular utilization of cEEG detecting more subclinical seizures in mild/moderate TBI. Statistical modeling revealed that injury age and intraaxial hemorrhage (omitting mechanism and severity altogether) were the best fitting predictors of subclinical status epilepticus. Reduced regression analysis further suggested that intraaxial hemorrhage was more important than age at injury for abusive head injury patients. This could have implications for abusive head trauma patients which typically occurs <1–2 years old, and suggest that intra-axial hemorrhage is more important to these patients than their age as a risk factor for clinical or subclinical EPTS.
Physiological changes during subclinical seizures include increases in intracranial pressure and metabolic stress.(Vespa et al., 2007b) Subclinical seizures may also cause longitudinal anatomic changes, such as hippocampal atrophy. (Vespa et al., 2010) Outcomes in patients with subclinical SE have been poor. (Claassen et al., 2006) Therefore, subclinical seizure detection could be important prognostically. Sixteen patients in our study were diagnosed with SE, 5 showing only clinical seizures, 6 showing both clinical and subclinical seizures and 5 showing only subclinical seizures. Without cEEG monitoring the 5 subclinical cases would have been missed and some of the cases with both types may not have been detected until much later. Studies have also shown cEEG to be useful to evaluate paroxysmal events that are not seizures, preventing unnecessary therapy.(Shahwan et al., 2010)
Intradural/intraaxial blood is known to be a precipitant of post-traumatic seizures, and is used in preclinical animal PTS models.(Willmore et al., 1978) In the current study, intraaxial blood was strongly associated with the risk of subclinical seizures, SE and subclinical SE but not of any seizures. Fracture on CT scan was associated with SE. This contrasts with a recent study (Goldstein et al., 2010) of AHT patients without standardized cEEG use where none of these findings were associated with seizures; however, other findings indicative of more severe injury (cerebral edema, infarction, blurring of the grey-white matter junction and midline shift) were related to seizures.
Risk-benefit of prophylaxis for pediatric EPTS
Existing evidence suggests that EPTS may reflect the severity of injury as well as contribute to secondary injury with the potential to worsen long-term outcomes (Barlow et al., 2000; Keenan et al., 2007; Vespa et al., 2010; Vespa et al., 2007a) and increase the risk for subsequent development of post-traumatic epilepsy (PTE). (Annegers & Coan, 2000) However, it has been shown that antiepileptic drugs (AEDs) do not prevent late PTE and are not without their own risk, particularly on the developing brain. (Bittigau et al., 1999; Kaindl et al., 2006; Olney et al., 2004; Temkin et al., 1990; Young et al., 2004). Thus, accurate detection of seizures and SE could help direct therapy. The finding of subclinical seizures in 1 in 6 pediatric TBI patients admitted to the ICU may be key to altering possible clinical interventions as well as accurately determining of the effect of EPTS on global outcomes and late PTE.
Caveats/limitations
To our knowledge, this is the first study to prospectively evaluate EPTS using the gold standard of cEEG in consecutive pediatric TBI patients admitted to the PICU. Differences were noted in the patient populations at 2 major academic centers. While there were similar age and gender representations, there were significant differences in injury severity and mechanism of injury. These differences in severity and mechanism likely account for center differences in CT findings and rates of seizures detected. However, the overall range of injuries was still consistent with epidemiological data from pediatric cohorts of moderate-severe TBI and the variables associated with subclinical seizures were similar at both centers, as well as in the combined analysis. Methodological and statistical analyses were optimized to minimize potential bias. A potential bias identified is not excluding those with a history of epilepsy, reported by some to be a risk factor for post-traumatic seizures. (Ronne-Engstrom & Winkler, 2006)
Electroencephalographer-dependent differences in EEG interpretation represent another potential source of variability. Previous studies of EEG interpretation using multiple readers have shown relatively modest inter-observer correlation. (Abend et al., 2011) Relying on a single EEG interpreter can reduce variability but may pose limitations on the generalizability of findings. In the validation carried out in this study, EEG parameters were set to simply evaluate the presence or absence of a seizure and the hemisphere of onset to maximize opportunity for interobserver agreement and allow broader interpretation of relevant results. Within these parameters, high concordance (>96% for both) was achieved, although the use of more subtle EEG parameters will require further investigation.
Proposed indications for and value of cEEG use after TBI in children
We propose that cEEG monitoring for subclinical EPTS be strongly considered for all children under the age of 2 yo admitted to the PICU for acute TBI. In addition, presence of intraaxial blood or demonstration/suspicion of AHT indicates greater risk for subclinical EPTS and thus signifies high-risk groups for cEEG monitoring. This would allow selective intervention with AEDs in patients having EPTS, without indiscriminately exposing all patients to potential adverse effects. Severity of injury and skull fracture on CT has been shown to be possible risk factors based on other literature and may be considered as well. Based upon the outcome variables used in this study we show that subclinical seizures, SE and subclinical SE, may negatively impact outcome. We interpret this to indicate that rapid detection and treatment of EPTS could be of benefit in the management of pediatric TBI patients.
We emphasize that the long-term effects of EPTS in pediatric patients on later developmental outcomes and risk of PTE remain an important area for ongoing investigation. Without clear demonstration of long-term risks among pediatric patients with EPTS, the decision to intervene with AEDs for EPTS remains primarily based upon related adult data. Those data may not accurately reflect acute or long-term negative effects of AEDs on the developing brain.
Continued follow-up for this cohort will help decipher a major enigma from other PTE studies. Namely, it is known that EPTS is a risk for PTE, and that early prophylaxis can prevent EPTS and associated acute complications, yet has not been shown to diminish the eventual rate of developing late PTE. By accurately identifying pediatric patients with EPTS (clinical or subclinical), a better understanding of the relationship between EPTS and eventual TBI outcome may be achieved.
Acknowledgements
We thank the following people for their roles in supporting this project: Susan Koh MD, Pramote Laoprasert MD, Kelly Knupp MD, Kristen Park MD, Mark Tripputi PhD, Christine Thompson, and EEG technologists: Andrea Duran, Jimmy Nguyen, Bereket Habibda, Pat Oliver, and Sy Turner. Funding was primarily supported by a grant from the Thrasher Research Foundation. Support for D.H.A. was primarily through the National Epifellows Foundation. Additional support from the Child Neurology Foundation/Winokur Family Foundation, Epilepsy Foundation of America, Today’s and Tomorrow’s Children Fund and UCLA Brain Injury Research Center (NS 058489) was also greatly appreciated.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. Dr. Wu serves on the professional advisory board for the Tuberous Sclerosis Alliance; has received honoraria from and serves on the scientific advisory board and speakers' bureau for Novartis Pharmaceutical Inc. and Lundbeck; and has received research support from the Tuberous Sclerosis Alliance, Today's and Tomorrow's Children Fund, Novartis Pharmaceuticals Inc, Department of Defense/Congressionally Directed Medical Research Program, and the NIH (K23 NS051637, P20 NS080199, R01 HD073980, R34 MH089299). Ms. Leung and Mr. Buxey are funded partially by NIH grants for the Brain Injury Research Center (BIRC) and Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network and is a consultant for Karl Storz/GCQ. Dr. Sankar serves on scientific advisory boards for and has received honoraria and funding for travel from UCB Pharma, Lundbeck Pharma, Sunovion, Supernus, Upsher-Smith. Serves on speakers’ bureaus for and has received speaker honoraria from UCB, GlaxoSmithKline, and Lundbeck. Research Support: Pfizer (Lyrica pediatric partial seizures trial); NIH-MH079933 [Co-I]. Received royalties from the publication of Pediatric Neurology, 3rd ed. (Demos Publishing, 2008) and Epilepsy: Mechanisms, Models, and Translational Perspectives (CRC Press, 2011). Dr. Brooks-Kayal has been funded by grants from NINDS (NS051710), Department of Defense CDMRP, Citizens United for Research in Epilepsy (CURE), American Epilepsy Society, and the Colorado Center for Drug Discovery. Her husband has a leadership position in the company SPI Pharma, and she and her husband own stock in and Johnson and Johnson Pharmaceuticals. Dr. Giza is a commissioner on the California State Athletic Commission, a member of a steering committee for the Sarah Jane Brain Project and a member of the Advisory Board for the American Association for Multi-Sensory Environments (AAMSE); has received funding for travel for invited lectures on TBI/concussion; received royalties from Blackwell Publishing for “Neurological Differential Diagnosis”; received honoraria for invited lectures on TBI/concussion; received research support from NINDS/NIH, University of California, and DOD; gives expert testimony, acted as a witness or consultant, or prepared an affidavit for 2–4 legal cases per year.
Footnotes
Disclosure of Conflicts of Interest
The remaining authors have no conflicts of interest.
References
- Abend NS, Gutierrez-Colina A, Zhao H, Guo R, Marsh E, Clancy RR, Dlugos DJ. Interobserver reproducibility of electroencephalogram interpretation in critically ill children. J Clin Neurophysiol. 2011;28:15–19. doi: 10.1097/WNP.0b013e3182051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annegers JF, Coan SP. The risks of epilepsy after traumatic brain injury. Seizure. 2000;9:453–457. doi: 10.1053/seiz.2000.0458. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Grabow JD, Groover RV, Laws ER, Jr, Elveback LR, Kurland LT. Seizures after head trauma: a population study. Neurology. 1980;30:683–689. doi: 10.1212/wnl.30.7.683. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Hauser WA, Coan SP, Rocca WA. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 1998;338:20–24. doi: 10.1056/NEJM199801013380104. [DOI] [PubMed] [Google Scholar]
- Barlow KM, Spowart JJ, Minns RA. Early posttraumatic seizures in non-accidental head injury: relation to outcome. Dev Med Child Neurol. 2000;42:591–594. doi: 10.1017/s0012162200001110. [DOI] [PubMed] [Google Scholar]
- Bartoń K. MuMln: Multi-model inference. R package version 1.7.7. 2012 [Google Scholar]
- Bittigau P, Sifringer M, Pohl D, Stadthaus D, Ishimaru M, Shimizu H, Ikeda M, Lang D, Speer A, Olney JW, Ikonomidou C. Apoptotic neurodegeneration following trauma is markedly enhanced in the immature brain. Ann Neurol. 1999;45:724–735. doi: 10.1002/1531-8249(199906)45:6<724::aid-ana6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York: Springer-Verlag; 2010. [Google Scholar]
- Chiaretti A, De Benedictis R, Polidori G, Piastra M, Iannelli A, Di Rocco C. Early post-traumatic seizures in children with head injury. Childs Nerv Syst. 2000;16:862–866. doi: 10.1007/s003810000368. [DOI] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, Wittman J, Connolly ES, Emerson RG, Mayer SA. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–112. doi: 10.1385/NCC:4:2:103. [DOI] [PubMed] [Google Scholar]
- Crouchman M, Rossiter L, Colaco T, Forsyth R. A practical outcome scale for paediatric head injury. Arch Dis Child. 2001;84:120–124. doi: 10.1136/adc.84.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai BT, Whitman S, Coonley-Hoganson R, Coleman TE, Gabriel G, Dell J. Seizures and civilian head injuries. Epilepsia. 1983;24:289–296. doi: 10.1111/j.1528-1157.1983.tb04892.x. [DOI] [PubMed] [Google Scholar]
- Faul MXL, Wald MM, Coronado VG. Centers for Disease Control and Prevention. Atlanta (GA): National Center for Injury Prevention and Control; 2010. Traumatic Brain Injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- Goldstein JL, Leonhardt D, Kmytyuk N, Kim F, Wang D, Wainwright MS. Abnormal neuroimaging is associated with early in-hospital seizures in pediatric abusive head trauma. Neurocrit Care. 2010;15:63–69. doi: 10.1007/s12028-010-9468-5. [DOI] [PubMed] [Google Scholar]
- Hahn CD. Nonconvulsive seizures among critically ill children: look and you shall find. Neurology. 2011;76:1036–1037. doi: 10.1212/WNL.0b013e318211c3dd. [DOI] [PubMed] [Google Scholar]
- Hahn YS, Fuchs S, Flannery AM, Barthel MJ, McLone DG. Factors influencing posttraumatic seizures in children. Neurosurgery. 1988;22:864–867. [PubMed] [Google Scholar]
- Hendrick EB, Harris L. Post-traumatic epilepsy in children. J Trauma. 1968;8:547–556. doi: 10.1097/00005373-196807000-00006. [DOI] [PubMed] [Google Scholar]
- Jennett B. Trauma as a cause of epilepsy in childhood. Dev Med Child Neurol. 1973;15:56–62. doi: 10.1111/j.1469-8749.1973.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Jennett WB, Lewin W. Traumatic epilepsy after closed head injuries. J Neurol Neurosurg Psychiatry. 1960;23:295–301. doi: 10.1136/jnnp.23.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–1755. doi: 10.1001/archneur.63.12.1750. [DOI] [PubMed] [Google Scholar]
- Kaindl AM, Asimiadou S, Manthey D, Hagen MV, Turski L, Ikonomidou C. Antiepileptic drugs and the developing brain. Cell Mol Life Sci. 2006;63:399–413. doi: 10.1007/s00018-005-5348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan HT, Hooper SR, Wetherington CE, Nocera M, Runyan DK. Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics. 2007;119:e616–e623. doi: 10.1542/peds.2006-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Liesemer K, Bratton SL, Zebrack CM, Brockmeyer D, Statler KD. Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma. 2011;28:755–762. doi: 10.1089/neu.2010.1518. [DOI] [PubMed] [Google Scholar]
- Maas AI, Harrison-Felix CL, Menon D, et al. Standardizing data collection in traumatic brain injury. J Neurotrauma. 2011;28(2):177–187. doi: 10.1089/neu.2010.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield RT. Head injuries in children and adults. Crit Care Clin. 1997;13:611–628. doi: 10.1016/s0749-0704(05)70331-6. [DOI] [PubMed] [Google Scholar]
- Olney JW, Young C, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Do pediatric drugs cause developing neurons to commit suicide? Trends Pharmacol Sci. 2004;25:135–139. doi: 10.1016/j.tips.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ong LC, Dhillon MK, Selladurai BM, Maimunah A, Lye MS. Early post-traumatic seizures in children: clinical and radiological aspects of injury. J Paediatr Child Health. 1996;32:173–176. doi: 10.1111/j.1440-1754.1996.tb00917.x. [DOI] [PubMed] [Google Scholar]
- Pagni CA. Posttraumatic epilepsy. Incidence and prophylaxis. Acta Neurochir Suppl (Wien) 1990;50:38–47. doi: 10.1007/978-3-7091-9104-0_8. [DOI] [PubMed] [Google Scholar]
- Ratan SK, Kulshreshtha R, Pandey RM. Predictors of posttraumatic convulsions in head-injured children. Pediatr Neurosurg. 1999;30:127–131. doi: 10.1159/000028779. [DOI] [PubMed] [Google Scholar]
- Ronne-Engstrom E, Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals a high incidence of epileptiform activity. Acta Neurol Scand. 2006;114:47–53. doi: 10.1111/j.1600-0404.2006.00652.x. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengpattrachai M, Sharma R, Hunjan A, Shroff M, Ochi A, Otsubo H, Cortez MA, Carter Snead O., 3rd Nonconvulsive seizures in the pediatric intensive care unit: etiology, EEG, brain imaging findings. Epilepsia. 2006;47:1510–1518. doi: 10.1111/j.1528-1167.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- Shahwan A, Bailey C, Shekerdemian L, Harvey AS. The prevalence of seizures in comatose children in the pediatric intensive care unit: a prospective video-EEG study. Epilepsia. 2010;51:1198–1204. doi: 10.1111/j.1528-1167.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Snoek JW, Minderhoud JM, Wilmink JT. Delayed deterioration following mild head injury in children. Brain. 1984;107(Pt 1):15–36. doi: 10.1093/brain/107.1.15. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Dikmen SS, Wilensky AJ, Keihm J, Chabal S, Winn HR. A randomized, double-blind study of phenytoin for the prevention of post-traumatic seizures. N Engl J Med. 1990;323:497–502. doi: 10.1056/NEJM199008233230801. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur DL, Xu Y, Eliseo M, Etchepare M, Dinov I, Alger J, Glenn TP, Hovda D. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–798. doi: 10.1212/WNL.0b013e3181f07334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, Miller C, McArthur D, Eliseo M, Etchepare M, Hirt D, Glenn TC, Martin N, Hovda D. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007a;35:2830–2836. [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999a;16:1–13. doi: 10.1097/00004691-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Nuwer MR, Nenov V, Ronne-Engstrom E, Hovda DA, Bergsneider M, Kelly DF, Martin NA, Becker DP. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999b;91:750–760. doi: 10.3171/jns.1999.91.5.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, O'Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, Glenn T, Hovda DA. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med. 2007b;35:1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- Wilcox R. Introduction to Robust Estimation and Hypothesis Testing. Amsterdam: Elsevier; 2012. [Google Scholar]
- Williams K, Jarrar R, Buchhalter J. Continuous video-EEG monitoring in pediatric intensive care units. Epilepsia. 2011;52:1130–1136. doi: 10.1111/j.1528-1167.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Hurd RW, Sypert GW. Epileptiform activity initiated by pial iontophoresis of ferrous and ferric chloride on rat cerebral cortex. Brain Res. 1978;152:406–410. doi: 10.1016/0006-8993(78)90273-1. [DOI] [PubMed] [Google Scholar]
- Young KD, Okada PJ, Sokolove PE, Palchak MJ, Panacek EA, Baren JM, Huff KR, McBride DQ, Inkelis SH, Lewis RJ. A randomized, double-blinded, placebo-controlled trial of phenytoin for the prevention of early posttraumatic seizures in children with moderate to severe blunt head injury. Ann Emerg Med. 2004;43:435–446. doi: 10.1016/j.annemergmed.2003.09.016. [DOI] [PubMed] [Google Scholar]