Abstract
Objective
We aimed to review the literature regarding epidemiology of functional abdominal pain disorders in children and to assess its geographic, gender and age distribution including associated risk factors of developing functional abdominal pain.
Methods
The Cochrane Library, MEDLINE, EMBASE, CINAHL and PsychInfo databases were systematically searched up to February 2014. Study selection criteria included: (1) studies of birth cohort, school based or general population samples (2) containing data concerning epidemiology, prevalence or incidence (3) of children aged 4-18 years (4) suffering from functional abdominal pain. Quality of studies was rated by a self-made assessment tool. A random-effect meta-analysis model was used to estimate the prevalence of functional abdominal pain in childhood.
Results
A total of 58 articles, including 196,472 children were included. Worldwide pooled prevalence for functional abdominal pain disorders was 13.5% (95% CI 11.8-15.3), of which irritable bowel syndrome was reported most frequently (8.8%, 95% CI 6.2-11.9). The prevalence across studies ranged widely from 1.6% to 41.2%. Higher pooled prevalence rates were reported in South America (16.8%) and Asia (16.5%) compared to Europe (10.5%). And a higher pooled prevalence was reported when using the Rome III criteria (16.4%, 95% CI 13.5-19.4). Functional abdominal pain disorders are shown to occur significantly more in girls (15.9% vs. 11.5%, pooled OR 1.5) and is associated with the presence of anxiety and depressive disorders, stress and traumatic life events.
Conclusion
Functional abdominal pain disorders are a common problem worldwide with irritable bowel syndrome as most encountered abdominal pain-related functional gastrointestinal disorder. Female gender, psychological disorders, stress and traumatic life events affect prevalence.
Introduction
Chronic abdominal pain is a common problem in childhood, with prevalence rates ranging from 0.3–19% in school-going children in the United States and Europe.[1] In almost 90% of these children, no explanatory organic cause can be identified.[2] Initially this condition was referred to as ‘recurrent abdominal pain’ RAP by Apley and Naish in 1957 and defined as “at least three episodes of abdominal pain, severe enough to affect their activities over a period longer than three months”.[3] In 1999 the pediatric Rome II criteria introduced the term abdominal pain related-functional gastrointestinal disorders (AP-FGIDs); which include functional dyspepsia (FD), irritable bowel syndrome (IBS), abdominal migraine (AM), functional abdominal pain (FAP) and functional abdominal pain syndrome (FAPS).[4] In order to meet these criteria symptoms had to occur weekly, persisting for over three months before diagnosis. With the introduction of the current Rome III in 2006 this criterion was redefined to persisting symptoms two months prior to diagnosis.[5]
Children with AP-FGIDs report significantly lower quality of life (QoL) scores compared to healthy peers and AP-FGIDs are ranked as second in causing school absence[6,7] In 29.1% of patients with chronic abdominal pain, pain persists even for more than 5 years, despite frequent medical attention.[8] Furthermore, functional abdominal pain disorders in childhood have a huge economic burden, as only the diagnostic workup is approximately 6000 dollar per child in the United States.[9]
The pathogenesis underlying AP-FGIDs remains unclear.[10] Altered gut motility, visceral hypersensitivity, abnormal brain-gut interaction, psychosocial disturbance and immune activation have been suggested as possible explanation for the symptoms.[11,12] Moreover, studies conducted in the United States and Europe reported that psychological symptoms, low socio-economic status, parental gastrointestinal complaints and single parent- and immigrant-households are associated with chronic abdominal pain in children.[1,13,14]
It has been commonly believed that functional abdominal pain disorders are a more evident problem in Western populations compared to developing countries. The purpose of the current study is to perform a systematic review and meta-analysis concerning the epidemiology of functional abdominal pain disorders in children worldwide in order to summarize the existing knowledge about its prevalence, geographic, gender and age distribution. In addition, we aim to review factors associated with functional abdominal pain disorders, such as psychosocial factors, quality of life, school absence, life events and socioeconomic factors.
Methods
Search strategy and study selection
The Cochrane Library, MEDLINE, EMBASE, CINAHL and PsychInfo databases were searched, up to February 2014. Studies on functional abdominal pain disorders were identified using the following terms: chronic or functional or recurrent abdominal pain, functional gastrointestinal disorder, stomach ache, abdominal migraine, irritable bowel syndrome or functional dyspepsia (both as medical subject heading (MeSH) and free text terms). These were combined, using the set operator AND, with epidemiology studies, identified with the terms ‘epidemiology, prevalence and incidence’ (MeSH and free text terms). A protocol of the current systematic review, including the full search strategy is provided in the S1 Protocol.
Abstracts were screened for eligibility. Potentially eligible studies were retrieved and read in full text to assess if they fulfilled all of the following inclusion criteria: (1) children aged 4–18 years; (2) with functional abdominal pain according to the ROME I, II, III criteria, Apley and Naish criteria or defined by the presence of nonorganic abdominal pain in children with at least three episodes of abdominal pain and/or weekly episodes of abdominal pain and/or a symptom duration of at least 3 months; (3) epidemiology studies of birth cohort, school based or general population samples and (4) results reported on epidemiology, prevalence or incidence. This screening was done by two reviewers (KD and JK) independently. Disagreement between the two reviewers was resolved by consensus when possible, or by consulting a third reviewer (MT) to made the final decision.
Quality assessment
Because there is currently no gold-standard quality assessment tool for epidemiologic studies,[15] we composed a new assessment tool based on a scale for quantitative studies[16] and on a guideline for evaluating prevalence studies.[17] We screened for the following six criteria: (1) is method of subject selection described and appropriate? (2) Are subject characteristics sufficiently described, i.e. do they match the target population regarding to gender and age? (3) Is functional abdominal pain diagnosed appropriately? (4) Are the survey instruments reliable and valid? (5) Are the analytic methods described/justified and appropriate? And (6) were results reported in sufficient detail? Studies were scored to what extent they met each applicable criterion with: no, partial or yes.
Data extraction
The following information related to data collection and results was extracted and entered into an Excel (Microsoft, Redmond, WA) spreadsheet: location, sampling strategy used to identify participants, sample size, age range, definition of functional abdominal pain disorders and the overall prevalence of functional abdominal pain disorders. If available, the gender, age and geographic distribution of the prevalence, socioeconomic factors, quality of life, psychosocial factors, school absence and life events were also reported.
Statistical analyses
Meta-analysis methods were used to assess the prevalence of functional abdominal pain disorders. Either a fixed-effect model or a random-effect model was adopted to pool data according to heterogeneity. When the heterogeneity was significant, the random-effect model was applied, otherwise the fixed-effect model was used. Heterogeneity was calculated by a Cochrane Q-statistic, and the degree of heterogeneity was quantified by I 2 test.[18] P<0.10 in combination with I 2 >50% indicated significant heterogeneity.[19] Additionally, subgroup analyses were conducted to assess geographical, age and gender distribution of the prevalence. Sensitivity analyses were performed on validation of used criteria and questionnaire and on child or parental report of the functional abdominal pain disorder. Chi-square test was used to analyze age and gender associations, expressed as pooled odds ratio (OR) with 95% confidence intervals (CI). Level of significance was set at p<0.05. Publication bias was assessed by funnel plot and Egger's tests,[20] p<0.05 was considered to be statistically significant. All analyses were conducted using StatsDirect Medical Statistic Software (StatsDirect Ltd, Cheshire, England). Remaining results were reported in a descriptive way.
Results
Study selection and characteristics
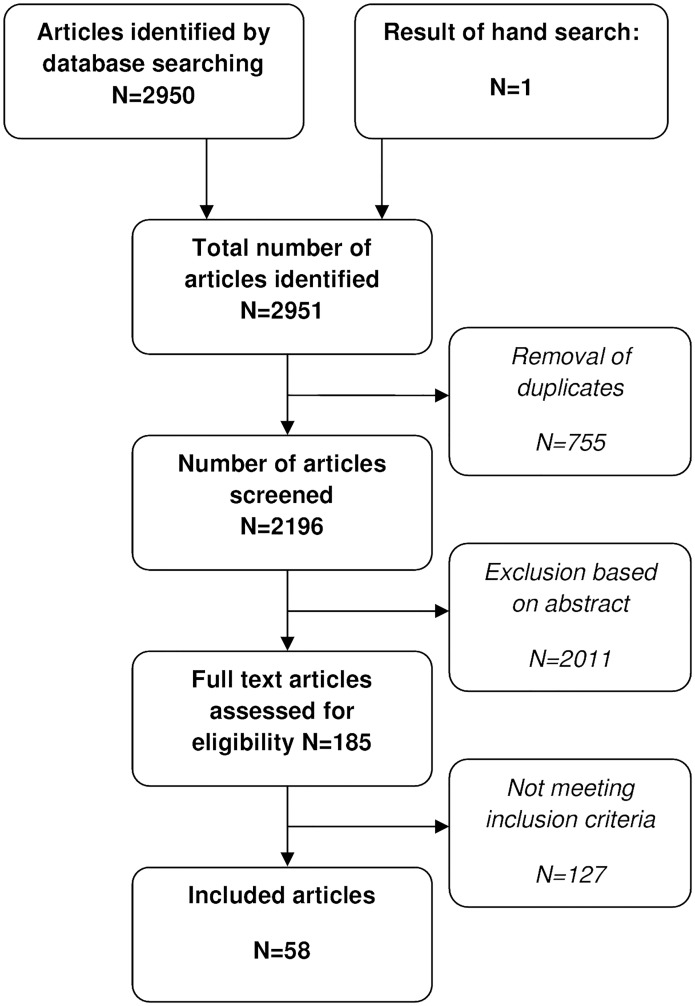
We found a total of 2196 titles and abstracts. After initial evaluation, 185 were judged potentially eligible. Finally, 127 articles did not meet our inclusion criteria. Reasons for exclusion were: adult study population (n = 42), not using appropriate definitions for functional abdominal pain (n = 26), irrelevant outcome measures/subject (n = 14), duplication of data (n = 18) and reviews, retrospective articles, abstracts or letter to the editors with insufficient information (n = 27). 58 articles remained,[3,6,14,21–75] including one systematic review (SR),[34] reviewing three articles[76–78] (Fig 1).
Fig 1. Flowchart showing results of literature search and study inclusion.
A total of 196,472 children with functional abdominal pain disorders were included with sample sizes ranging from 243[68] to more than 65,087.[34] Most children were recruited from school samples or birth cohorts.
Several different case definitions of functional abdominal pain disorders were used, including Apley and Naish (n = 18), ROME II (n = 11), ROME III criteria (n = 12) and self-reported functional abdominal pain disorders (n = 17). Different methodologies of data collection were used among studies. Questionnaires completed by parents and/or children were mostly used to asses functional abdominal pain data (n = 50). Other methods used were face-to-face interview (n = 6), clinical examination (n = 2) and a combination of questionnaire and interview (n = 4). In one single study and in the SR the method of data collection was not clear. Table 1 presents all study characteristics of the included studies.
Table 1. Characteristics of included studies.
| Study, year | Country | Population | Sample size(N) | Age range(years) | Method of data collection | Case definition | Prevalence(%) |
|---|---|---|---|---|---|---|---|
| Europe | |||||||
| Gulewitsch,[22]2013 | Germany | School sample | 1537 | 5–12y | Parental reports of QPGS-III | ROME II | 7.7 |
| Romero,[23] 2013 | Spain | School sample | 2575 | 8–16y | Child questionnaire | >3x AP in last 3 months | 14.4 |
| Luntamo,[25] 2012 | Finland | School sample | 2215 | 13–18y | Child questionnaire | Weekly AP in the last 6 months | 5.6 |
| Helgeland, [37]2010 | Norway | Birth cohort | 456 | 14y | Questionnaire | Apley and Naish | 12.7 |
| Rask,[40] 2009 | Denmark | Birth cohort | 1327 | 5–7y | Parental interview | Apley and Naish | 7.6 |
| Alfven,[42] 2008 | Sweden | General cohort | 2597 | 10–18y | Child and parental interview | AP weekly, for >6 months | 19.8 |
| Brun,[46] 2007 | Sweden | School sample | 1901 | 9–15y | Questionnaire a | last 3 months AP ≥ weekly | 7.4 |
| Ostberg,[47] 2006 | Sweden | Welfare sample | 5380 | 10–18y | Audio questionnaire | last 6 month AP ≥ 1 time a month | 19.3 |
| Bakoula,[74]2006 | Greece | Birth cohort | 7925 | 7y | Parental questionnaire | AP ≥ weekly | 4.1 |
| Dalh,[73] 2005 | Denmark | School sample | 849 | 9–13y | Parental questionnaire | ROME II | 12.2 |
| Tindberg,[49]2005 | Sweden | School sample | 695 | 9–13y | Child/parent questionnaire | Apley and Naish | 12.7 |
| Kokkonen,[69]2004 | Finland | School sample | 404 | 10–11y | Parental questionnaire and clinical examination | Apley and Naish | 15.8 |
| Groholt,[75] 2003 | ScandinaviaIceland | General cohort | 6040 | 7–17y | Child/parent questionnaire | AP weekly or every 2 weeks | 8.3 |
| Petersen,[53]2003 | Sweden | School sample | 1121 | 6–13y | Child/parent questionnaire | AP weekly ≥ 6 months | 19.1 |
| Bode,[51] 2003 | Germany | School sample | 1143 | 5–8y | Parental questionnaire a | Apley and Naish | 2.5 |
| De Giacomo,[55] 2002 | Italy | School sample | 808 | 6–12y | Child/parent questionnaire | ROME II | 8.8 |
| Harma,[54] 2002 | Finland | School sample | 15965 | mean 15y | Questionnaire by students | AP weekly,≥ 6 months | 10.0 |
| Perquin,[59] 2000 | Netherlands | School sample | 4459 | 4–18y | Child/parent questionnaire | > 3 months AP | 2.6 |
| O'Donohoe,[61] 1996 | UK | School sample | 640 | 4–13y | Parental questionnaire | Apley and Naish | 14.2 |
| Abu-Arafeh,[62] 1995 | Scotland | School sample | 1754 | 5–15y | Questionnaire and interview | Symon and Russell | 3.3 |
| Mortimer,[70] 1993 | UK | General cohort | 1083 | 3–11y | Structure interview | Apley and Naish | 8.4 |
| Lundby,[72] 1990 | Denmark | School sample | 648 | 9–12y | Questionnaire | Apley and Naish | 15.4 |
| Faull,[71] 1986 | UK | School sample | 439 | 6y | Parental questionnaire/interview | Apley and Naish | 25.1 |
| Christensen,[63] 1984 | Denmark | School sample | 2530 | 5–16y | Questionnaire | Apley and Naish | 11.4 |
| Apley,[3] 1958 | UK | School sample | 1000 | 3–15y | Mother/child interview | Apley and Naish | 10.8 |
| North America | |||||||
| Stanford,[45] 2008 | Canada | General cohort | 2271 | 12–13y | Child/parent questionnaire | AP weekly in the last 6 months | 19.8 |
| Youssef,[6] 2008 | USA | Longitudinal Study in Adolesc. Health | 20735 | 13–18y | In-home interview children | 2–3 episodes/week the last 12 months | 14.0 |
| Malaty,[67] 2007 | USA | School sample | 925 | 4–15y | Questionnaire | AP >3 months continuous, interfere daily life | 24.0 |
| Uc,[68] 2006 | USA | Annual school physicals | 243 | 4–17y | QPGS-RII a + clinical evaluation | ROME II | 1.6 |
| Hyams,[14] 1996 | USA | School sample | 507 | 12–16y | Bowel disease questionnaire a | Weekly AP in the last year | 15.0 |
| Sharrer,[64] 1991 | USA | School sample | 250 | 8–12y | Questionnaire parents | Apley and Naish | 10.0 |
| South America | |||||||
| Saps,[21] 2014 | Colombia | School sample | 373 | 8–14y | QPGS-RIII a | ROME III | 12.1 |
| Silva,[35] 2011 | Brazil | Birth cohort | 1462 | 7–11y | Questionnaire | RAP for > 3 months, interfering daily life | 21.6 |
| Asia | |||||||
| Sagawa,[24] 2013 | Japan | School sample | 3976 | 10–17y | QPGS-RIII a | ROME III | 12.8 |
| Phavichitr,[26]2012 | Thailand | School sample | 1181 | 12–19y | QPGS-RIII a | ROME III | 24.0 |
| Song,[27] 2012 | Korea | School sample (girls) | 820 | 12–17y | Child/parent questionnaire | ROME II | 12.8 |
| Zheng,[28] 2012 | China | School sample | 668 | mean 14,8y | IBS Inventory a | ROME III | 4.6 |
| Zhou,[29] 2012 | China | School sample | 1362 | 12–18y | Questionnaire | ROME III | 14.8 |
| Devanarayana,[30] 2012 | Sri Lanka | School sample | 1365 | 13–18y | QPGS-RIII a | ROME III | 17.8 |
| Park,[79] 2011 | Korea | School sample | 1877 | 15–18y | IBS Module a | ROME III | 19.0 |
| Liu,[34] 2011 | China | SR | 65087 | ROME II | 4.6–23.4 | ||
| Zhou,[36] 2011 | China | School sample | 3671 | 12–18y | Questionnaire | ROME III | 20.0 |
| Devanarayana,[31] 2011 | Sri Lanka | School sample | 2163 | 10–16y | QPGS-RIII a | ROME III | 12.4 |
| Endo,[33] 2011 | Japan | School sample | 2312 | 14–15y | ROME II modulaire questionnaire, self-reporting IBS questionnaire a | ROME II | 15.4 |
| Devanarayana,[32] 2011 | Sri Lanka | School sample | 428 | 12–16y | QPGS-RIII a | ROME III | 13.7 |
| Zhou,[38] 2010 | China | School sample | 2013 | 10–18y | Questionnaire | ROME III | 20.7 |
| Devanarayana,[43] 2008 | Sri Lanka | School sample | 734 | 5–15y | Parental questionnaire | Apley and Naish | 10.5 |
| Son,[44] 2008 | Korea | School sample, (girls) | 405 | 15–18y | unclear | ROME II | 25.7 |
| Dong,[48] 2005 | China | School sample | 5043 | 6–18y | Questionnaire | ROME II | 14.2 |
| Oh,[50] 2004 | Singapore | School sample | 3590 | 6–17y | Questionnaire | Apley and Naish | 23.4 |
| Boey,[52] 2003 | Malaysia | School sample | 1971 | 12y | Questionnaire and interview by pediatrician | Apley and Naish | 23.1 |
| Boey,[56] 2001 | Malaysia | School sample | 1462 | 9–15y | Interview by pediatrician | Apley and Naish | 11.0 |
| Boey,[57] 2001 | Malaysia | School sample | 1488 | 5–15y | Questionnaire and interview by pediatrician | Apley and Naish | 9.6 |
| Reshetnikov,[58] 2001 | Siberia | School sample | 449 | 14–17y | Bowel disease questionnaire a | ROME II | 20.0 |
| Boey,[60] 1999 | Malaysia | School sample | 148 | 11–12y | Parental questionnaire | ≥ 3 episodes of AP for ≥ 3 months least | 41.2 |
| the Middle East | |||||||
| Demirceken,[39] 2010 | Turkey | Cohort general practitioner | 250 | 5–18y | Questionnaire by child, parent and physician | ROME III | 31.2 |
| Sohrabi,[66] 2010 | Iran | School sample | 1436 | 14–19y | Questionnaire | ROME II | 4.1 |
| Telmesani,[41] 2009 | Saudi Arabia | School sample (boys) | 316 | 12–18y | Questionnaire | Apley and Naish | 17.4 |
QPGS-RII/III; Questionnaire on pediatric gastrointestinal symptoms based on Rome II/III,
aValidated questionnaire
Due to significant heterogeneity a random effect-model was applied for all meta-analyses.
Methodological quality assessment
We assessed the selection of study subjects. In 20 out of 58 studies they were not randomly selected out of a population sample. In 16 studies subjects did not match the target population appropriately, because for example only girls[27] or boys[41] were included, or because age range was limited.[33,37,45,52,69,71,74] In the majority of trials validated instruments were not used (n = 41). A detailed overview of the quality scores of all individual studies is listed in S1 Appendix.
Prevalence
In general, pooled prevalence for functional abdominal pain disorders was 13.5% (95% CI 11.8–15.3). The reported prevalence ranged widely, from 1.6% to 41.2%. The forest plot of these data is shown in S2 Appendix. The funnel plot was symmetric and Egger’s linear regression test was not significant, which gives no indication for publication bias. Nineteen out of 58 studies reported prevalences of subtypes within AP-FGIDs (Table 2). Indication for publication bias was shown for meta-analyses of FD (Egger’s test p = 0.02).
Table 2. Pooled prevalence of functional abdominal pain disorders according to criteria used to define its presence, validation status of questionnaire, child/parental report, subtypes of AP-FDIG and geographical location.
| Number of studies | Number of subjects | Pooled prevalence (%) | 95% CI | Heterogeneity | ||
|---|---|---|---|---|---|---|
| I 2 | P value for I 2 | |||||
| All studies | 58 | 196,472 | 13.5 | 11.8–15.3 | 98.6 | <0.001 |
| Criteria used to define abdominal pain | ||||||
| Self reported criteria | 17 | 77,980 | 13.2 | 10.2–16.6 | 99.4 | <0.001 |
| Apley and Naish | 18 | 20,176 | 12.9 | 9.9–16.2 | 97.7 | <0.001 |
| Rome II | 11 | 78,989 | 12.2 | 9.3–15.5 | 99.0 | <0.001 |
| Rome III | 12 | 19,327 | 16.4 | 13.5–19.4 | 96.6 | <0.001 |
| Validation status of questionnaire | ||||||
| Validated | 17 | 21,809 | 11.9 | 9.0–15.2 | 98.0 | <0.001 |
| Not validated | 41 | 174,663 | 14.1 | 12.1–16.3 | 99.3 | <0.001 |
| Report | ||||||
| Parental report | 12 | 15,639 | 11.0 | 7.4–15.2 | 97.9 | <0.001 |
| Child report | 25 | 77,929 | 13.7 | 11.8–15.7 | 98.2 | <0.001 |
| AP-FGID subtypes | ||||||
| IBS | 16 | 28,399 | 8.8 | 6.2–11.9 | 98.6 | <0.001 |
| FD | 9 | 11,516 | 4.5 | 1.2–9.9 | 99.2 | <0.001 |
| FAP | 7 | 10,085 | 3.5 | 1.8–5.6 | 95.8 | <0.001 |
| AM | 9 | 12,922 | 1.5 | 1.0–2.1 | 83.8 | <0.001 |
| FAPS | 4 | 7,322 | 0.9 | 0.5–1.5 | 76.8 | 0.005 |
| Geographical location | ||||||
| South America | 2 | 1,835 | 16.8 | 8.6–27.0 | N/A | N/A |
| Asia | 22 | 102,213 | 16.5 | 14.6–18.5 | 98.1 | <0.001 |
| The Middle-East | 3 | 2,002 | 15.8 | 2.8–36.4 | 98.7 | <0.001 |
| North America | 6 | 24,931 | 13.4 | 9.4–17.9 | 97.1 | <0.001 |
| Europe | 25 | 65,491 | 10.5 | 8.3–12.8 | 98.8 | <0.001 |
N/A; not applicable, too few studies to assess heterogeneity
Pooled prevalence numbers according to the different criteria used to define its presence, validation status of the questionnaire and the differences between child and parental report are shown in Table 2. Highest prevalence rates were found when using the ROME III criteria. The pooled prevalence of functional abdominal pain disorder was almost identical between studies that used a validated, compared to a non-validated questionnaire, or when functional abdominal pain was reported by children compared to parents (Table 2). The sensitivity analyses for Rome II, self-reported criteria and validated questionnaires were subject to publication bias calculated by Egger’s test, p = 0.04, p = 0.04 and p = 0.02 respectively.
Geographic distribution
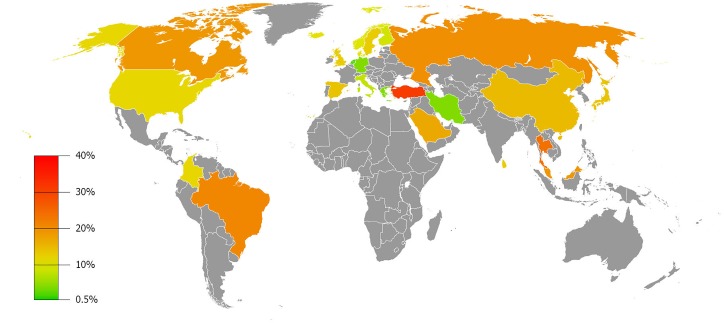
The majority of studies were conducted in Europe and Asia. A few studies were performed in the Middle East, North- and South America and prevalence data for Africa and Australia are currently lacking. The pooled prevalence of functional abdominal pain disorders subdivided for each continent is provided in Table 2. The prevalence rates did not differ extremely, with lowest prevalence occurring in Europe (10.5%) and the highest in South America (16.8%). Publication bias was only shown for meta-analysis of Europe (Egger’s test p<0.01). The prevalence per individual country studied is shown in Fig 2.
Fig 2. Geographic distribution of functional abdominal pain in children, presented in pooled-prevalence rates.
Gender prevalence
Gender prevalence was reported in 24 studies. All, but two studies,[51,70] reported a female predominance. After pooling data, a significant higher proportion of functional abdominal pain disorders among girls compared to boys was seen (15.9% vs. 11.5%, pooled OR 1.5, 95% CI 1.3–1.7, p<0.01). There was no evidence for publication bias by Egger’s test.
Age distribution
Relationship between age and functional abdominal pain prevalence has been evaluated in 36 studies. Because different age groups were used, we were unable to pool data for all single ages separately. Therefore, data were pooled for children <12 years and ≥12 years. No significant difference was found for the prevalence of functional abdominal pain disorders in children younger than 12 years as compared to children ≥12 years old (12.4% vs. 13.8%, pooled OR 0.9, 95% CI 0.5–1.4, p = 0.62). There was no evidence for publication bias (p = 0.26).
Associated factors
Psychological disorders and Quality of life
Several studies reported an association between psychological factors with functional abdominal pain disorders.[22,25,28,36–38,44–46,48,54] Anxiety and depression were reported significantly more frequent among children with functional abdominal pain disorders compared to healthy children.[33,44,48,65] Furthermore one study showed that abdominal pain was a predictor of depression in 14–16 years old adolescents (girls: OR 2.4, 95% CI 2.0–2.9; boys: OR 2.3, 95% CI 1.7–3.1),[54] and vice versa, one study reported that depressive symptoms predict functional abdominal pain (OR 2.4; 95% CI 1.1–5.1).[37]
Two studies used the Strengths and Difficulties Questionnaire to screen for psychological problems, which is a 25 item-questionnaire, divided into five scales: hyperactivity—inattention, emotional symptoms, conduct problem, peer problem, and prosocial behavior scales.[22,25] Compared to children without abdominal pain, AP-FGIDs were associated with conduct problems (OR 4.1, 95% CI 2.8–5.9),[25] which especially concerned IBS patients (p<0.05).[22]
Eighty point five per cent of children with RAP reported school absence, at least one day during the third term of the year, compared to 44.6% of the healthy control group (p<0.01).[43] Furthermore, compared to controls, IBS patients showed significantly lower quality of life (QoL).[33,65] Park et al. investigated QoL with the World Health Organization QOL Scale, a 26-item questionnaire, divided into 5 subscales.[65] On each subscale (ranging between 1–5) children with IBS scored significant lower compared to non-IBS children (p<0.01): physical health (3.12 vs.3.42), mental health (2.94 vs. 3.11), social relationships (3.08 vs. 3.19), environment (3.07 vs. 3.18) and overall aspects (3.06 vs. 3.37).
Stress and Negative life events
Numerous studies showed an increase in prevalence of abdominal pain in children with high stress levels.[26,27,33,44,60,64,65] Measured on a 5-point scale (0 = never, 5 = always), 6.3% girls with mild stress (≤1.7 points) reported IBS, which significantly increased to 20.3% in girls with severe stress (>2.1 points).[27] In addition, mean total stress scores were significantly higher in the IBS group (119.7/200, SD 31.4) compared to healthy controls (95.9/200, SD 34.9, p = 0.03), measured on a 40 items Feel Bad Scale.[60] Similarly, patients with functional abdominal pain disorders reported significantly more traumatic- or negative life events.[30,31,50,80]
Twelve point two per cent of children with AP-FGIDs experienced the death of a close family member compared to 7.7% in the control group (p<0.02).[31] Also children with AP-FGIDs reported more frequent punishment by parents (6.7% vs. 3.8%, p = 0.04), frequent domestic violence (5.6% vs. 2.9%, p = 0.03), parental job loss (5.2% vs. 2.4%, p = 0.01) and hospitalization for another illness (16.3% vs. 9.5%, p<0.01).[31] Furthermore, any form of abuse was associated with an increase in the prevalence of functional abdominal pain. AP-FGIDs were significantly higher in those children exposed to sexual abuse (35.3% vs. 17.3%, p = 0.01), physical abuse (19.7% vs. 12.6%, p<0.01), and emotional abuse (27.4% vs. 16.9%, p<0.01).[30]
Socioeconomic status
Although a lower family income and low-educated families appeared to result in a higher percentage of children experiencing functional abdominal pain disorders, in most studies this trend was not statistically significant.[22,26,27,31,49,60,67,75] Malaty et al. reported the prevalence of RAP in different socioeconomic geographical areas, based on percentage of children receiving free or reduced-price school lunches. Low-income areas did not show a higher prevalence of RAP compared to high-income areas (23% vs. 27%, p = 0.38). On the other hand, a contrary finding was described by Groholt et al., who measured family income as the family's monthly disposable income and divided this into quartiles. RAP was reported in 6.6% in the highest quartile compared to 12.1% in the lowest quartile (p<0.01).[75] In the same study parental education was assessed. The prevalence of RAP was 7.8% among children living in low educated families (< 9 years education) compared to 9.5% in high educated families (> 12 years education), which was not significant.[75]
Discussion
This is the first systematic review focusing on the prevalence of functional abdominal pain disorders in Western populations and developing countries. Our systematic analysis of available studies shows a worldwide prevalence of pediatric functional abdominal pain disorders of 13.5%, with approximately comparable rates across the continents. Irritable bowel syndrome (IBS) was the most often reported subtype of the abdominal pain related functional gastrointestinal disorders (AP-FGID). Higher prevalence rates were seen using the ROME III criteria and associations were shown with female gender, anxiety and depressive disorders, stress and traumatic life events.
Our findings are in line with a previous systematic review of Chitkara et al., which reported a high prevalence of childhood recurrent abdominal pain in Western countries.[1] We found a large variation in prevalence across studies, ranging from 1.6% to even 41.2%. This might be due to the variable age groups studied, the different definitions used to classify functional abdominal pain and different type of questionnaires used. However, sensitivity analyses did not reveal large differences in prevalence numbers (Table 2). The occurrence of abdominal pain reported by children was slightly higher compared to the occurrence reported by their parents. However, this concordance was expected to be less, since agreement between child and parents reporting pain and somatic symptoms is moderate and parents are likely to underestimate their child's pain.[81,82] Furthermore, since the publication of the pediatric Rome criteria for AP-FGIDs in 1999, higher pooled prevalence rates were found regarding studies using these strict AP-FGID criteria, up to a prevalence of 16.4%. A Sri Lankan population study even showed that the pediatric Rome III criteria were able to diagnose FGIDs more comprehensively than Rome II.[32]
The lowest prevalence of 1.6% was reported by Uc et al., though only African American children were included.[68] Other studies conducted in the USA showed a higher prevalence, ranging from 10–24%. The highest prevalence (41.2%) was reported in a small Malaysian study, including 148 children from a rural area. The authors suggested that this was due to the high prevalence of intestinal parasites in rural Malay school children.[83] In developing countries the prevalence of parasitic infections might be higher owing to potentially limited access to clean water, however, a Sri Lankan study identified parasitic infections as organic cause for RAP in only 7.7%.[80] Indeed literature shows that an association between AP-FGIDs and amebiasis is questionable.[84,85]
Prevalence rates range widely between countries. In addition to methodological differences, this may arise from factors such as diverse cultural, dietary, genetic, environmental conditions and different health care systems. Turkey showed the highest prevalence of functional abdominal pain disorders which was based on a small sample of 250 children. A prevalence of FD of 31% was reported, which is considerably higher than the total pooled prevalence of FD (4.5%, Table 2).[39] An explanation for this high prevalence could be that children were not screened for Helicobacter pylori which might result in an overestimation. In 65% of Turkish children presenting with recurrent abdominal pain and dyspepsia an infection with H.pylori can be found. [39]
According to different continents, the pooled prevalence was more stable, though was slightly lower in European studies and generally higher in studies from South-America and Asia. This finding is in line with the observation that the Rome III criteria were able to diagnose FGIDs more comprehensively than Rome II, since most Asian studies were only recently conducted and as a result used these criteria.[32] Moreover South-America and Asia are upcoming economies, with a change in (fast)food habits, a higher expectation from children, particularly towards their school achievements,[44] and consequently higher levels of stress.
In accordance with earlier data a predominance of functional abdominal pain disorders was found in girls.[1] This dominance in girls was reported in all different continents across the world. It has been suggested that levels of sex hormones might play a role, which is supported by observations that premenopausal patients present with exacerbation of their abdominal pain symptoms at time of menses.[86] Ovarian hormones can modulate the process of visceral pain perception and the susceptibility to stress.[87] Although younger children have not reached sexual maturity, this can apply to adolescents as well. However, when analyzing gender distribution at pre-pubertal age (≤10 years), there still was a persisting difference (boys 7.7% vs. girls 9.9%, OR 1.4, 59% CI 1.16, 1.79, p<0.001). Another reason might be the fact that females have a greater willingness to report somatic experiences, such as pain.[88] High pain profiles, indicating higher levels of pain and lower ability to cope with pain, were more often reported among girls.[89] Predominance of girls has been also described in other functional complaints, like functional constipation[90] and headache.[91,92]
In this systematic review, no association was found between age and prevalence of pediatric AP-FGIDs. Chitkara suggested a bimodal peak, between 4 and 6 year and preadolescence, in which the symptoms of abdominal pain are more prevalent.[1] More recent studies, however, showed a peak prevalence at adolescence.[23,24,27] Unfortunately due to great diversity in selected age groups among studies, we were unable to perform meta-analyses on single or narrow age groups and therefore we could not confirm these previous findings.
Epidemiological studies included in this SR showed that children with functional abdominal pain were significantly more often diagnosed with anxiety or depressive disorders compared to healthy children. Mechanisms and routes by which psychological factors affect functional abdominal pain are not fully known. Abdominal pain can cause psychological problems and conversely,[37,54] once developed abdominal pain and depression/anxiety may worsen each other. Moreover, both pain and symptoms of depression and anxiety can be the result of ineffective mechanisms of coping with stress, since low coping strategies are demonstrated in children with chronic abdominal pain.[93] Association of functional abdominal pain with stress and traumatic life events can be explained by unsuccessful coping styles as well. In addition, stressors have shown to be associated with enhanced visceral perception,[94] which is also described in pediatric IBS and RAP.[95,96] Increased responsiveness of central stress and arousal circuits and subsequently increase activity of the sympathetic nervous system can cause visceral hypersensitivity.[97]
Socioeconomic environment of the child has been reported to be a potential contributory factor to RAP.[47,75] Scandinavian studies have demonstrated that children living in low educated, low-income, worker families have higher levels of recurrent abdominal pain.[47,75] Our SR, however, reported that most studies conducted in Europe, Asia and US did not show any significant effect concerning the association between socioeconomic environment and functional abdominal pain. A recent well-conducted SR among adults, covering worldwide data, supports this latter finding.[98]
Strengths of the current study include a comprehensive and contemporaneous literature search that identified sufficient studies to allow pooling of data from almost 200,000 subjects. Because no language restrictions were applied, this is the first study which accomplishes all worldwide publications about the prevalence of pediatric functional abdominal pain. To date, a validated tool to assess the quality of epidemiological studies is lacking. Therefore a possible limitation of our study is the use of a self-made, not validated tool to assess the quality of the different epidemiological studies. A second limitation comes from the inclusion of studies using self-reported criteria for recurrent abdominal pain, since these criteria were not validated and less strict compared to the Apley and Rome criteria this can have distort the prevalence. However, our analyses showed the same prevalence rate in this case compared to the Apley and Rome II criteria. Interpretation of results was hampered by significant heterogeneity of included studies, due to methodological differences. To reduce this effect random effect models were used for meta-analyses. Publication bias was shown in some analyses, which can be eliminated by trim and fill method. However, because this method is known to perform poorly in the presence of substantial between-study heterogeneity, we decided not to correct our data by this method. Another limitation arises from the available studies and the reporting data within them. When calculating a pooled prevalence, there was a notable absence or ‘overrepresentation’ of studies conducted in certain geographical regions making it difficult to accurately estimate true global prevalence. For example, prevalence numbers from Turkey were only reflected by one small sample study. Lastly, information regarding associated factors was limited. Important data from studies comparing associated factors, such as psychosocial and socioeconomic factors, between AP-FGID-patients and controls were missing, because only prevalence studies were included.
In summary, functional abdominal pain occurs commonly worldwide. Female gender, psychological disorders, stress and traumatic life events increase the prevalence, while age and socioeconomic state are not associated. This high prevalence worldwide and its substantial impact on patients’ well-being justifies investment of resources and educational campaigns directed to prevention and optimal treatment, with special attention to psychological disorders and stress reduction.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
Acknowledgments
The authors thank Arnold G. E. Leenders (Medical Library, Academic Medical Center, Amsterdam, the Netherlands) for his assistance with the electronic literature search.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Chitkara DK, Rawat DJ, Talley NJ. The epidemiology of childhood recurrent abdominal pain in Western countries: a systematic review. Am J Gastroenterol. 2005. August;100(8):1868–75. [DOI] [PubMed] [Google Scholar]
- 2. Spee LA, Lisman-Van Leeuwen Y, Benninga MA, Bierma-Zeinstra SMA, Berger MY. Prevalence, characteristics, and management of childhood functional abdominal pain in general practice. Scand J Prim Health Care. 2013. December;31(4):197–202. 10.3109/02813432.2013.844405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child. 1958. April;33(168):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999. September;45 Suppl 2(Suppl II):II60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006. April;130(5):1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Youssef NN, Atienza K, Langseder AL, Strauss RS. Chronic abdominal pain and depressive symptoms: analysis of the national longitudinal study of adolescent health. Clin Gastroenterol Hepatol. 2008. March;6(3):329–32. 10.1016/j.cgh.2007.12.019 [DOI] [PubMed] [Google Scholar]
- 7. Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993. September;38(9):1569–80. [DOI] [PubMed] [Google Scholar]
- 8. Gieteling MJ, Bierma-Zeinstra SM, Passchier J, Berger MY. Prognosis of chronic or recurrent abdominal pain in children. Journal of Pediatric Gastroenterology & Nutrition. 2008;47(3):316–26. [DOI] [PubMed] [Google Scholar]
- 9. Dhroove G, Chogle A, Saps M. A million-dollar work-up for abdominal pain: is it worth it? Journal of Pediatric Gastroenterology & Nutrition. 2010;51(5):579–83. [DOI] [PubMed] [Google Scholar]
- 10. Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, et al. Chronic Abdominal Pain In Children: a Technical Report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. [Review]. Journal of Pediatric Gastroenterology & Nutrition. 2005;40(3):249–61. [DOI] [PubMed] [Google Scholar]
- 11. Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013. January;62(1):159–76. 10.1136/gutjnl-2012-302167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain—gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012. September;61(9):1284–90. 10.1136/gutjnl-2011-300474 [DOI] [PubMed] [Google Scholar]
- 13. Hotopf M, Carr S, Mayou R, Wadsworth M, Wessely S. Why do children have chronic abdominal pain, and what happens to them when they grow up? Population based cohort study. BMJ. 1998. April 18;316(7139):1196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: A community-based study. J Pediatr. 1996. August;129(2):220–6. [DOI] [PubMed] [Google Scholar]
- 15. Sanderson S, Tatt ID, Higgins JPT. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007. June;36(3):666–76. [DOI] [PubMed] [Google Scholar]
- 16. Kmet LM, Lee RC, Cook LS. Standard quality assesment criteria for evaluating primary research papers from a variety of fields. 2004. p. 1–22. [Google Scholar]
- 17. Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Heal. 1998;37–9. [Google Scholar]
- 18. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002. June 15;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. September;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997. September 13;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saps M, Nichols-Vinueza DX, Rosen JM, Velasco-Benítez CA. Prevalence of functional gastrointestinal disorders in colombian school children. J Pediatr. 2014. March;164(3):542–5.e1. 10.1016/j.jpeds.2013.10.088 [DOI] [PubMed] [Google Scholar]
- 22. Gulewitsch MD, Enck P, Schwille-Kiuntke J, Weimer K, Schlarb AA. Rome III criteria in parents’ hands: pain-related functional gastrointestinal disorders in community children and associations with somatic complaints and mental health. Eur J Gastroenterol Hepatol. 2013. October;25(10):1223–9. 10.1097/MEG.0b013e328364b55d [DOI] [PubMed] [Google Scholar]
- 23. Romero-Acosta K, Canals J, Hernández-Martínez C, Penelo E, Zolog TC, Domènech-Llaberia E. Age and gender differences of somatic symptoms in children and adolescents. J Ment Health. 2013. February;22(1):33–41. 10.3109/09638237.2012.734655 [DOI] [PubMed] [Google Scholar]
- 24. Sagawa T, Okamura S, Kakizaki S, Zhang Y, Morita K, Mori M. Functional gastrointestinal disorders in adolescents and quality of school life. J Gastroenterol Hepatol. 2013. February;28(2):285–90. 10.1111/j.1440-1746.2012.07257.x [DOI] [PubMed] [Google Scholar]
- 25. Luntamo T, Sourander A, Rihko M, Aromaa M, Helenius H, Koskelainen M, et al. Psychosocial determinants of headache, abdominal pain, and sleep problems in a community sample of Finnish adolescents. Eur Child Adolesc Psychiatry. 2012. June;21(6):301–13. 10.1007/s00787-012-0261-1 [DOI] [PubMed] [Google Scholar]
- 26. Phavichitr N, Koosiriwichian K, Tantibhaedhyangkul R. Prevalence and risk factors of dyspepsia in Thai schoolchildren. J Med Assoc Thai. 2012. May;95 Suppl 5:S42–7. [PubMed] [Google Scholar]
- 27. Song S-W, Park S-J, Kim S-H, Kang S-G. Relationship between irritable bowel syndrome, worry and stress in adolescent girls. J Korean Med Sci. 2012. November;27(11):1398–404. 10.3346/jkms.2012.27.11.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng S, Fu W, Zhou J, Dong X, Liu Z, Wang Y, et al. Prevalence and related factors of irritable bowel syndrome among middle-school students in areas affected by Wenchuan Earthquake: an epidemiological study. J Clin Gastroenterol. 2012. April;46(4):345–6. 10.1097/MCG.0b013e31824712d0 [DOI] [PubMed] [Google Scholar]
- 29. Zhou H-Q, Yao M, Chen G-Y, Ding X-D, Chen Y-P, Li D-G. Functional gastrointestinal disorders among adolescents with poor sleep: a school-based study in Shanghai, China. Sleep Breath. 2012. December;16(4):1211–8. 10.1007/s11325-011-0635-5 [DOI] [PubMed] [Google Scholar]
- 30. Devanarayana N, Rajindrajith S, Karunanayake A, Nishanthini S, Perera MS, Benninga MA. Abdominal pain predominant functional gastrointestinal diseases: Association with child abuse, traumatic life events and quality of life. Journal of Gastroenterology and Hepatology. 2012. p.383. [Google Scholar]
- 31. Devanarayana NM, Mettananda S, Liyanarachchi C, Nanayakkara N, Mendis N, Perera N, et al. Abdominal pain-predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. J Pediatr Gastroenterol Nutr. 2011. December;53(6):659–65. 10.1097/MPG.0b013e3182296033 [DOI] [PubMed] [Google Scholar]
- 32. Devanarayana NM, Adhikari C, Pannala W, Rajindrajith S. Prevalence of functional gastrointestinal diseases in a cohort of Sri Lankan adolescents: comparison between Rome II and Rome III criteria. J Trop Pediatr. 2011. February;57(1):34–9. 10.1093/tropej/fmq039 [DOI] [PubMed] [Google Scholar]
- 33. Endo Y, Shoji T, Fukudo S, Machida T, Machida T, Noda S, et al. The features of adolescent irritable bowel syndrome in Japan. J Gastroenterol Hepatol. 2011. April;26 Suppl 3:106–9. 10.1111/j.1440-1746.2011.06637.x [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Hou X. A review of the irritable bowel syndrome investigation on epidemiology, pathogenesis and pathophysiology in China. J Gastroenterol Hepatol. 2011. April;26 Suppl 3:88–93. 10.1111/j.1440-1746.2011.06641.x [DOI] [PubMed] [Google Scholar]
- 35. Silva AA, Barbieri MA, Cardoso VC, Batista RF, Simões VM, Vianna EO, et al. Prevalence of non-communicable diseases in Brazilian children: follow-up at school age of two Brazilian birth cohorts of the 1990’s. BMC Public Health. 2011. January;11:486 10.1186/1471-2458-11-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou H, Yao M, Cheng G-Y, Chen Y-P, Li D-G. Prevalence and associated factors of functional gastrointestinal disorders and bowel habits in Chinese adolescents: a school-based study. J Pediatr Gastroenterol Nutr. 2011. August;53(2):168–73. 10.1097/MPG.0b013e3182125388 [DOI] [PubMed] [Google Scholar]
- 37. Helgeland H, Sandvik L, Mathiesen KS, Kristensen H. Childhood predictors of recurrent abdominal pain in adolescence: A 13-year population-based prospective study. J Psychosom Res. 2010. April;68(4):359–67. 10.1016/j.jpsychores.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 38. Zhou H, Li D, Cheng G, Fan J, Lu H. An epidemiologic study of irritable bowel syndrome in adolescents and children in South China: a school-based study. Child Care Health Dev. 2010. November;36(6):781–6. 10.1111/j.1365-2214.2010.01120.x [DOI] [PubMed] [Google Scholar]
- 39. Demirceken FG, Kurt G, Dulkadir R, Alpcan A, Bulbul S. Functional dyspepsia in children: A Turkish prospective survey in kirikkale province. Journal of Pediatric Gastroenterology and Nutrition. 2010. p. E122–3. [Google Scholar]
- 40. Rask CU, Olsen EM, Elberling H, Christensen MF, Ornbøl E, Fink P, et al. Functional somatic symptoms and associated impairment in 5–7-year-old children: the Copenhagen Child Cohort 2000. Eur J Epidemiol. 2009. January;24(10):625–34. 10.1007/s10654-009-9366-3 [DOI] [PubMed] [Google Scholar]
- 41. Telmesani AMA. Helicobacter pylori: prevalence and relationship with abdominal pain in school children in Makkah City, western Saudi Arabia. Saudi J Gastroenterol. Medknow Publications; 2009. April 1;15(2):100–3. 10.4103/1319-3767.45359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alfven G, Ostberg V, Hjern A. Stressor, perceived stress and recurrent pain in Swedish schoolchildren. J Psychosom Res. 2008. October;65(4):381–7. 10.1016/j.jpsychores.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 43. Devanarayana NM, de Silva DGH, de Silva HJ. Recurrent abdominal pain syndrome in a cohort of Sri Lankan children and adolescents. J Trop Pediatr. 2008. June;54(3):178–83. 10.1093/tropej/fmm114 [DOI] [PubMed] [Google Scholar]
- 44. Son Y-J, Jun E-Y, Park JH. Prevalence and risk factors of irritable bowel syndrome in Korean adolescent girls: a school-based study. Int J Nurs Stud. 2009. January;46(1):76–84. 10.1016/j.ijnurstu.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 45. Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: A population-based approach. Pain. 2008;138:11–21. [DOI] [PubMed] [Google Scholar]
- 46. Brun Sundblad GM, Saartok T, Engström LMT. Prevalence and co-occurrence of self-rated pain and perceived health in school-children: Age and gender differences. Eur J Pain. 2007;11:171–80. [DOI] [PubMed] [Google Scholar]
- 47. Ostberg V, Alfven G, Hjern A. Living conditions and psychosomatic complaints in Swedish schoolchildren. Acta Paediatr. 2006. August;95(8):929–34. [DOI] [PubMed] [Google Scholar]
- 48. Dong L, Dingguo L, Xiaoxing X, Hanming L. An epidemiologic study of irritable bowel syndrome in adolescents and children in China: a school-based study. Pediatrics. 2005. September;116(3):e393–6. [DOI] [PubMed] [Google Scholar]
- 49. Tindberg Y, Nyren O, Blennow M, Granstrom M. Helicobacter pylori infection and abdominal symptoms among Swedish school children. Journal of Pediatric Gastroenterology & Nutrition. 2005;41(1):33–8. [DOI] [PubMed] [Google Scholar]
- 50. Oh MC, Aw MM, Chan YH, Tan LZ, Quak SH. Epidemiology of recurrent abdominal pain among Singaporean adolescents. Ann Acad Med Singapore. 2004. September;33(5 Suppl):S10–1. [PubMed] [Google Scholar]
- 51. Bode G, Brenner H, Adler G, Rothenbacher D. Recurrent abdominal pain in children: evidence from a population-based study that social and familial factors play a major role but not Helicobacter pylori infection. Journal of Psychosomatic Research. 2003;54(5):417–21. [DOI] [PubMed] [Google Scholar]
- 52. Boey CCM, Omar A, Arul Phillips J. Correlation among academic performance, recurrent abdominal pain and other factors in Year-6 urban primary-school children in Malaysia. J Paediatr Child Health. 2003;39:352–7. [DOI] [PubMed] [Google Scholar]
- 53. Petersen S, Bergström E, Brulin C. High prevalence of tiredness and pain in young schoolchildren. Scand J Public Health. 2003. January;31(5):367–74. [DOI] [PubMed] [Google Scholar]
- 54. Härmä A-M, Kaltiala-Heino R, Rimpelä M, Rantanen P. Are adolescents with frequent pain symptoms more depressed? Scand J Prim Health Care. 2002. June;20(2):92–6. [PubMed] [Google Scholar]
- 55. De Giacomo C, Valdambrini V, Lizzoli F, Gissi A, Palestra M, Tinelli C, et al. A population-based survey on gastrointestinal tract symptoms and Helicobacter pylori infection in children and adolescents. Helicobacter. 2002. December;7(6):356–63. [DOI] [PubMed] [Google Scholar]
- 56. Boey CCM, Goh KL. Recurrent abdominal pain and consulting behaviour among children in a rural community in Malaysia. Dig Liver Dis. 2001;33:140–4. [DOI] [PubMed] [Google Scholar]
- 57. Boey CCM, Goh KL. Predictors of health-care consultation for recurrent abdominal pain among urban schoolchildren in malaysia. J Gastroenterol Hepatol. 2001;16:154–9. [DOI] [PubMed] [Google Scholar]
- 58. Reshetnikov OV, Kurilovich SA, Denisova DV, Zavyalova LG, Tereshonok IN. Prevalence of dyspepsia and irritable bowel syndrome among adolescents of Novosibirsk, western Siberia. International Journal of Circumpolar Health. 2001;60(2):253–7. [PubMed] [Google Scholar]
- 59. Perquin CW, Hazebroek-Kampschreur AA, Hunfeld JA, Bohnen AM, van Suijlekom-Smit LW, Passchier J, et al. Pain in children and adolescents: a common experience. Pain. 2000. July;87(1):51–8. [DOI] [PubMed] [Google Scholar]
- 60. Boey CC, Yap SB. An epidemiological survey of recurrent abdominal pain in a rural Malay school. J Paediatr Child Health. 1999. June;35(3):303–5. [DOI] [PubMed] [Google Scholar]
- 61. O’Donohoe JM, Sullivan PB, Scott R, Rogers T, Brueton MJ, Barltrop D. Recurrent abdominal pain and Helicobacter pylori in a community-based sample of London children. Acta Paediatr. 1996;85:961–4. [DOI] [PubMed] [Google Scholar]
- 62. Abu-Arafeh I, Russell G. Prevalence and clinical features of abdominal migraine compared with those of migraine headache. Arch Dis Child. 1995. May;72(5):413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Christensen MF, Holm E, Sahlholdt I. [Recurrent abdominal pain in Danish school children. A cross-sectional study]. Ugeskr Laeger. 1984. September 3;146(36):2690–5. [PubMed] [Google Scholar]
- 64. Sharrer VW, Ryan-Wenger NM. Measurements of stress and coping among school-aged children with and without recurrent abdominal pain. J Sch Health. 1991. February;61(2):86–91. [DOI] [PubMed] [Google Scholar]
- 65. Park H, Lim S. Frequency of irritable bowel syndrome, entrance examination-related stress, mental health, and quality of life in high school students. Gastroenterol Nurs. 2011;34(6):450–8. 10.1097/SGA.0b013e318237eb43 [DOI] [PubMed] [Google Scholar]
- 66. Sohrabi S, Nouraie M, Khademi H, Baghizadeh S, Nasseri-Moghaddam S, Malekzadeh R. Epidemiology of uninvestigated gastrointestinal symptoms in adolescents: a population-based study applying the Rome II questionnaire. J Pediatr Gastroenterol Nutr. 2010. July;51(1):41–5. 10.1097/MPG.0b013e3181d1b23e [DOI] [PubMed] [Google Scholar]
- 67. Malaty HM, Abudayyeh S, Fraley K, Graham DY, Gilger MA, Hollier DR. Recurrent abdominal pain in school children: effect of obesity and diet. Acta Paediatr. 2007. April;96(4):572–6. [DOI] [PubMed] [Google Scholar]
- 68. Uc A, Hyman PE, Walker LS. Functional gastrointestinal disorders in African American children in primary care. J Pediatr Gastroenterol Nutr. 2006. March;42(3):270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kokkonen J, Haapalahti M, Tikkanen S, Karttunen R, Savilahti E. Gastrointestinal complaints and diagnosis in children: a population-based study. Acta Paediatr. 2004. July;93(7):880–6. [PubMed] [Google Scholar]
- 70. Mortimer MJ, Kay J, Jaron A. Clinical epidemiology of childhood abdominal migraine in an urban general practice. Dev Med Child Neurol. 1993. March;35(3):243–8. [DOI] [PubMed] [Google Scholar]
- 71. Faull C, Nicol AR. Abdominal pain in six-year-olds: an epidemiological study in a new town. J Child Psychol Psychiatry. 1986. March;27(2):251–60. [PubMed] [Google Scholar]
- 72. Lundby L, Sandbaek A, Juul S. [Recurrent abdominal pain in schoolchildren 9–12 years of age]. Ugeskr Laeger. 1990. September 24;152(39):2851–4. [PubMed] [Google Scholar]
- 73. Dahl-Larsen R, Buhl SB, Husby S, Qvist N. [Recurrent abdominal pain, dyspepsia and constipation in children aged 9–13. A questionnaire investigation]. Ugeskr Laeger. 2005. April 25;167(17):1848–51. [PubMed] [Google Scholar]
- 74. Bakoula C, Kapi A, Veltsista A, Kavadias G, Kolaitis G. Prevalence of recurrent complaints of pain among Greek schoolchildren and associated factors: a population-based study. Acta Paediatr. 2006. August;95(8):947–51. [DOI] [PubMed] [Google Scholar]
- 75. Grøholt E-K, Stigum H, Nordhagen R, Köhler L. Recurrent pain in children, socio-economic factors and accumulation in families. Eur J Epidemiol. 2003. January;18(10):965–75. [DOI] [PubMed] [Google Scholar]
- 76. Li D, Zhou H, Song Y, Zong C, Hu Y, Xu X, et al. [An epidemiologic study of irritable bowel syndrome among adolescents in China]. Zhonghua nei ke za zhi. 2007. February;46(2):99–102. [PubMed] [Google Scholar]
- 77. Wang Y, Zhang D. The prevalence investigation of IBS in Qinhuandao area. Shandong Med. 2008;48:107–9. [Google Scholar]
- 78. Zhou H-Q, Li D-G, Song Y-Y, Zong C-H, Hu Y, Lu H-M. Epidemiologic study of irritable bowel syndrome among adolescents in Western China. Journal of Shanghai Jiaotong University (Medical Science). China; 2009;29:581–3. [Google Scholar]
- 79. Park JW, Cho Y-S, Lee SY, Kim E-S, Cho H, Shin HE, et al. Concomitant functional gastrointestinal symptoms influence psychological status in Korean migraine patients. Gut and Liver. South Korea; 2013;7:668–74. 10.5009/gnl.2013.7.6.668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Devanarayana NM, de Silva DG, de Silva HJ. Aetiology of recurrent abdominal pain in a cohort of Sri Lankan children. Journal of Paediatrics & Child Health. 2008;44(4):195–200. [DOI] [PubMed] [Google Scholar]
- 81. Kröner-Herwig B, Morris L, Heinrich M, Gassmann J, Vath N. Agreement of parents and children on characteristics of pediatric headache, other pains, somatic symptoms, and depressive symptoms in an epidemiologic study. Clin J Pain. 2009;25:58–64. 10.1097/AJP.0b013e31817fc62d [DOI] [PubMed] [Google Scholar]
- 82. Sundblad GMB, Saartok T, Engström L-MT. Child-parent agreement on reports of disease, injury and pain. BMC Public Health. 2006;6:276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sinniah B, Hassan A KR, Sabaridah I, Soe MM, Ibrahim Z, Ali O. Prevalence of intestinal parasitic infections among communities living in different habitats and its comparison with one hundred and one studies conducted over the past 42 years (1970 to 2013) in Malaysia. Trop Biomed. 2014. June;31(2):190–206. [PubMed] [Google Scholar]
- 84. De Jong MJ, Korterink JJ, Benninga MA, Hilbink M, Widdershoven J, Deckers-Kocken JM. Dientamoeba fragilis and chronic abdominal pain in children: a case-control study. Arch Dis Child. 2014;1–5. 10.1136/archdischild-2014-307251 [DOI] [PubMed] [Google Scholar]
- 85. Krogsgaard LR, Engsbro AL, Stensvold CR, Nielsen HV, Bytzer P. The Prevalence of Intestinal Parasites Is Not Greater Among Individuals With Irritable Bowel Syndrome: a Population-Based Case-Control Study. Clin Gastroenterol Hepatol. 2014. September 16; [DOI] [PubMed] [Google Scholar]
- 86. Whitehead WE, Cheskin LJ, Heller BR, Robinson JC, Crowell MD, Benjamin C, et al. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology. 1990. June;98(6):1485–9. [DOI] [PubMed] [Google Scholar]
- 87. Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: Potential mechanisms of sex hormones. World J Gastroenterol. 2014. June 14;20(22):6725–43. 10.3748/wjg.v20.i22.6725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wise EA, Price DD, Myers CD, Heft MW, Robinson ME. Gender role expectations of pain: relationship to experimental pain perception. Pain. 2002. April;96(3):335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012. September;153(9):1798–806. 10.1016/j.pain.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. Elsevier Ltd; 2011. February;25(1):3–18. 10.1016/j.bpg.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 91. Roth-Isigkeit A, Thyen U, Raspe HH, Stöven H, Schmucker P. Reports of pain among German children and adolescents: an epidemiological study. Acta Paediatr. 2004. February;93(2):258–63. [PubMed] [Google Scholar]
- 92. Cuvellier J-C. [Management of chronic daily headache in children and adolescents]. Rev Neurol (Paris). 2009;165(6–7):521–31. [DOI] [PubMed] [Google Scholar]
- 93. Walker LS, Smith CA, Garber J, Claar RL. Appraisal and coping with daily stressors by pediatric patients with chronic abdominal pain. J Pediatr Psychol. 2007. March;32(2):206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayer EA, Bradesi S, Chang L, Spiegel BMR, Bueller JA, Naliboff BD. Functional GI disorders: from animal models to drug development. Gut. 2008. March;57(3):384–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Iovino P, Tremolaterra F, Boccia G, Miele E, Ruju FM, Staiano A. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil. 2009. September;21(9):940–e74. 10.1111/j.1365-2982.2009.01303.x [DOI] [PubMed] [Google Scholar]
- 96. Di Lorenzo C, Youssef NN, Sigurdsson L, Scharff L, Griffiths J, Wald A. Visceral hyperalgesia in children with functional abdominal pain. Journal of Pediatrics. United States; 2001. p. 838–43. [DOI] [PubMed] [Google Scholar]
- 97. Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med. 2011. January;62:381–96. 10.1146/annurev-med-012309-103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhu J-Z, Yan T-L, Yu C-H, Wan X-Y, Wang Y-M, Li Y-M. Is national socioeconomic status related to prevalence of irritable bowel syndrome? J Gastroenterol Hepatol. 2014. May 29;29(8):1595–602. 10.1111/jgh.12609 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.