Abstract
The cervicovaginal fluid (CVF) coating the vaginal epithelium is an important immunological mediator, providing a barrier to infection. Glycosylation of CVF proteins, such as mucins, IgG and S-IgA, plays a critical role in their immunological functions. Although multiple factors, such as hormones and microflora, may influence glycosylation of the CVF, few studies have examined their impact on this important immunological fluid. Herein we analyzed the glycosylation of cervicovaginal lavage (CVL) samples collected from 165 women under different hormonal conditions including: (1) no contraceptive, post-menopausal, (2) no contraceptive, days 1-14 of the menstrual cycle, (3) no contraceptive, days 15-28 of the menstrual cycle, (4) combined-oral contraceptive pills for at least 6 months, (5) depo-medroxyprogesterone acetate (Depo-Provera) injections for at least 6 months, (6) levonorgestrel IUD for at least 1 month. Glycomic profiling was obtained using our lectin microarray system, a rapid method to analyze carbohydrate composition. Although some small effects were observed due to hormone levels, the major influence on the glycome was the presence of an altered bacterial cohort due to bacterial vaginosis (BV). Compared to normal women, samples from women with BV contained lower levels of sialic acid and high-mannose glycans in their CVL. The change in high mannose levels was unexpected and may be related to the increased risk of HIV-infection observed in women with BV, as high mannose receptors are a viral entry pathway. Changes in the glycome were also observed with hormonal contraceptive use, in a contraceptive-dependent manner. Overall, microflora had a greater impact on the glycome than hormonal levels, and both of these effects should be more closely examined in future studies given the importance of glycans in the innate immune system.
Introduction
The mucosal lining of the female genital tract provides a robust barrier to infection from pathogens such as HIV-1 [1, 2]. Cervical mucus, a natural hydrogel consisting predominantly of water (95%-98%) and large and structurally complex mucin glycoproteins (2%-5%), is secreted into the vagina providing lubrication and a natural barrier to microorganisms and viruses [3–6]. Cervicovaginal fluid (CVF) contains this mucus along with an assortment of other anti-microbial glycoproteins including S-IgA, IgG, cathepsin G, lysozyme and lactoferrin [7–11]. Glycosylation of proteins in the CVF influence their stability, activity and function [12]. For example, mannose structures on S-IgA in vaginal fluid act as an alternative ligand for uropathogenic type-1 Escherichia Coli inhibiting vaginal colonization and subsequent urinary tract infection[13]. Thus, glycosylation plays an important role in the anti-microbial properties of the CVF.
Multiple factors may influence the glycomic composition of the CVF including hormones and vaginal microflora. Oral contraceptives have been shown to regulate the glycosylation of serum glycoproteins such as α1-acid glycoprotein [14]. In one of the only studies on the CVF glycome, changes in sialylation were observed in cervical mucin O-glycans at ovulation. However, few differences were observed at other time points [3]. Microflora may also play a role in CVF glycome composition. Women with bacterial vaginosis (BV), in which the balance between Lactobacillus species and competing anaerobic bacteria shifts towards the anaerobes [15], display high levels of vaginal sialidase, first reported by Briselden et al. [16, 17]. These enzymes cleave the negatively charged sugar sialic acid from terminal glycans of glycoproteins in the CVF, changing the glycan composition of the CVF and increasing proteolysis of innate immune factors such as S-IgA and lactoferrin [18].
To date, no systematic study has examined the effects of exogenous hormones and microflora on the vaginal glycome. Herein, we utilize lectin microarrays [19, 20], our high-throughput glycomic analysis platform, to profile the glycosylation patterns of cervicovaginal lavage (CVL) samples from 169 women. The sample cohort includes women on different hormonal contraceptives and with different microflora. Lectin microarrays, in which carbohydrate binding proteins are arrayed, are a versatile glycomic platform that has been used to analyze the glycosylation of samples from bacteria to human cancer tissues [21–26]. Our lectin microarray data demonstrates that while both exogenous hormones and microflora affect the glycome, the strong impact of microflora on the glycome, confounds assessment of hormonal effects. Decreased high mannose levels were observed in the CVL of women with BV, which may increase susceptibility to other pathogens. This study sets the stage for more detailed analysis of the effects of both hormone and individual microbes on the glycome of vaginal fluids and its function in innate immunity against pathogens.
Materials and Methods
Study Population
Following Institutional Review Board approval by the University of Pittsburgh (#PRO11020218), written informed consent was obtained from subjects enrolled in our study. Women were excluded if: they were breastfeeding or pregnant; presented vaginal symptoms; had a hysterectomy; had been diagnosed with any cervical or vaginal infections or had used any antimicrobials in the past 14 days; had used any vaginal devices or vaginally-applied products (excluding tampons) in the past week. Upon enrollment the women had: an OraQuick advance rapid HIV test; a pregnancy test; their demographic information was recorded; height and weight taken and medical, gynecologic and sexual histories taken. Cervicovaginal lavage (CVL) was collected from 169 women. 4 of 169 samples were excluded.
These women were characterized into six groups: (1) post-menopausal; (2) not contracepting on days 1–14 of the menstrual cycle; (3) not contracepting on days 15–28 of the menstrual cycle; (4) combined oral contraceptive; (5) DMPA (medroxyprogesterone acetate, Depoprovera); and (6) Mirena IUD (levonogerestrol Intrauterine Device) usage. These data were also stratified according to microflora status using the Nugent score [27].
Sample Collection
First a vaginal swab was taken for a Gram Stain evaluation for microflora status using the Nugent method of scoring [27]. Cervicovaginal lavage specimens (CVL) were collected in 10 mL of normal saline (Hospira, Inc. Lake Forest, IL). The saline and a syringe were used to gently wash the cervix and vaginal vault. The CVL was collected and placed in a 15 mL plastic centrifuge tube and stored at 4°C until processing. Within one hour the samples were transported to the laboratory. Upon receipt in the laboratory, CVLs were dispensed into 2 mL cryovials. All samples were stored at -80°C.
Sample labeling
The CVL samples (100 μg protein based on Lowry) were labeled with NHS-Cy5 dye (6 μg GE Healthcare Life Sciences, Piscataway, NJ) in 100 mM NaHCO3 buffer, pH 9.3. A pooled sample of the CVL was labeled with NHS-Cy3 dye (60 μg per mg of protein) for use as the reference. Samples were incubated for 45 min at room temperature with gentle shaking to allow labeling and the free dye was then quenched by addition of 2 M Tris buffer, pH 6.8 (final concentration 250 mM). Samples were then dialyzed overnight against PBS at 4°C using a Microdialyzer (3,000 MW membrane, Spectrum Laboratories). All the steps were performed in the dark. Protein concentrations of samples were obtained after dialysis using the microBCA assay (Pierce).
Lectin Microarray Analysis
Print conditions
Lectin microarrays were printed as previously described [20]. In brief, 92 lectins and antibodies (S1 Table) were kept on ice during preparation and transferred to 384-well microplates for printing. Samples were maintained at 12°C and printed on Nexterion H slides (SCHOTT North America, Elmsford, NY) using a Nanoplotter 2.1 piezoelectric printer (GeSIM, Germany) with a pico-tip (Quantum analytics, Foster City, CA) at 45–50% humidity. Three spots per protein were printed in each of the 24 arrays per slide. After printing, slides were incubated at RT for 2 hr to obtain maximum coupling to the NHS ester surface. The lectins and antibodies, print concentrations and buffers are listed in S1 Table. Lectin microarrays were tested for quality using Cy5-labeled glycoprotein standards (10 μg asialofetuin, fetuin and RNase B).
Microarray hybridization
Printed slides were blocked with 25 mM ethanolamine in 100 mM sodium borate (pH 8.5) for 1 hr at RT in a coplin jar with gentle shaking. Slides were washed 3 × with PBST (PBS with 0.005% Tween 20) and 1 × with PBS for 5 min each and then dried using a slide spinner (Labnet International). An ArrayIt multi-well hybridization cassette (Arrayit Corporation, Sunnyvale, CA) was used to create 24 distinct arrays for hybridization. 2.5 μg of Cy5-labeled CVL sample was incubated with equal amounts of Cy3-labeled reference in 100 μl total volume PBST. Samples were added to each array and incubated for 2 h at RT with gentle agitation. Sample solutions were then aspirated and slide chambers were washed 5 × with PBST (100 μL, 3 min, RT), followed by removal of the hybridization chamber and a final wash with PBS (5 min in a coplin jar). The slide was spun-dry and scanned in the Cy3 (ex/em 532/550–600 nm) and Cy5 (635/655–695 nm) channels with a Genepix 4300A slide scanner (Molecular Devices, Sunnyvale, CA). Data were extracted using Genepix 7 (Molecular Devices) and processed with Microsoft Excel 2011.
Microarray data analysis
The background-subtracted median fluorescence of the three replicate spots per protein was tested for outliers using the Grubbs outlier test with α = 0.05. The average value was determined for the three replicates after excluding the outliers if there were any. The log2 values of the average signals were median-centered over the array in each channel to account for differences in labeling efficiency [28]. The values obtained were log2 ratios for each lectin or antibody. Hierarchical clustering of the processed data (S1 Data) was performed using Pearson correlation coefficient with average linkage analysis by Cluster 3.0 [29] and the data sets were visualized with Java Treeview [30]. We excluded from the analysis probes whose average signal was not higher than the median of the array for at least of 10% of samples as these were considered inactive. This resulted in the exclusion of 22 lectins from further analysis. Lilliefors test [31–33] was used to test the normality for sample distributions. Data was considered normal by this standard. P values were calculated with one-way ANOVA [34]. All the box plots were made with Matlab 2013a.
Results and Discussions
Study Design
Cervicovaginal lavage (CVL) samples, which represent a comprehensive collection of fluid and mucus from the lower reproductive tract [35], were collected from 169 women under different hormonal and reproductive conditions including: (1) post-menopausal, 29 women; (2) days 1–14 of the menstrual cycle, 27 women; (3) days 15–28 of the menstrual cycle, 26 women; (4) combined oral contraceptive for at least 6 months, 27 women; (5) depo-medroxyprogesterone acetate (Depo-Provera) injections for at least 6 months, 28 women; (6) levonorgestrel Intrauterine Device (IUD) usage for at least 1 month, 28 women [36]. Women in groups 1–3 were free of hormonal contraceptives. Four of the 169 samples were excluded from further analysis due to failure to meet the inclusion criterion. One sample was excluded due to Chlamydia trachomatis. The other three samples were excluded because three women participated in the sample collection protocol for two different periods of the same menstrual cycle, i.e. their samples were collected for groups 2 and 3. The vaginal microflora of study participants was evaluated using the Nugent scoring system to determine bacterial vaginosis (BV) status [27]. Normal microflora was defined as a Nugent score of 0–3, intermediate: 4–6 and BV: 7–10. In a recent study, the Nugent score was found to accurately reflect shifts in the microflora composition of the vagina [15]. The Nugent score is validated only for use among women of reproductive age, so it does not apply to postmenopausal women [27]. Consequently postmenopausal women were excluded from our comparative analysis of Normal and BV samples.
Lectin Microarray Analysis of the CVL
In lectin microarray analysis, we label the glycoproteins in our samples with Cy5-dye through lysine coupling to the corresponding NHS-ester. The labeled glycoproteins are then mixed with a Cy3-labeled biological reference and incubated with our microarrays to obtain a semiquantitative dual-color analysis that is accurate for relative glycomic composition (Fig 1A) [37–39]. Cervical vaginal lavage fluid is known to contain large amounts of free sugars, mainly glucose, mannose and glucosamine [7]. As high concentrations of these monosaccharides can inhibit lectins, such as those on our microarrays, we tested whether dialysis of labeled CVL samples would alter the resulting microarray profiles. In initial studies, we observed lower signals for some lectins in undialyzed samples presumably due to free sugar content, thus we dialyzed all CVL samples post-labeling using a low molecular weigh cutoff (3 kD, S1 Fig). For our analysis, we combined 2.5 μg of Cy5-labeled CVL sample with equal amounts of a Cy3-labeled pooled reference created from all samples and analyzed them on our lectin microarrays. Median-centered log2 (sample/reference) ratios were calculated after data extraction. Normalized data was then hierarchically clustered using the Pearson correlation coefficient as the distance metric and average linkage analysis to generate the heatmap shown in Fig 1B.
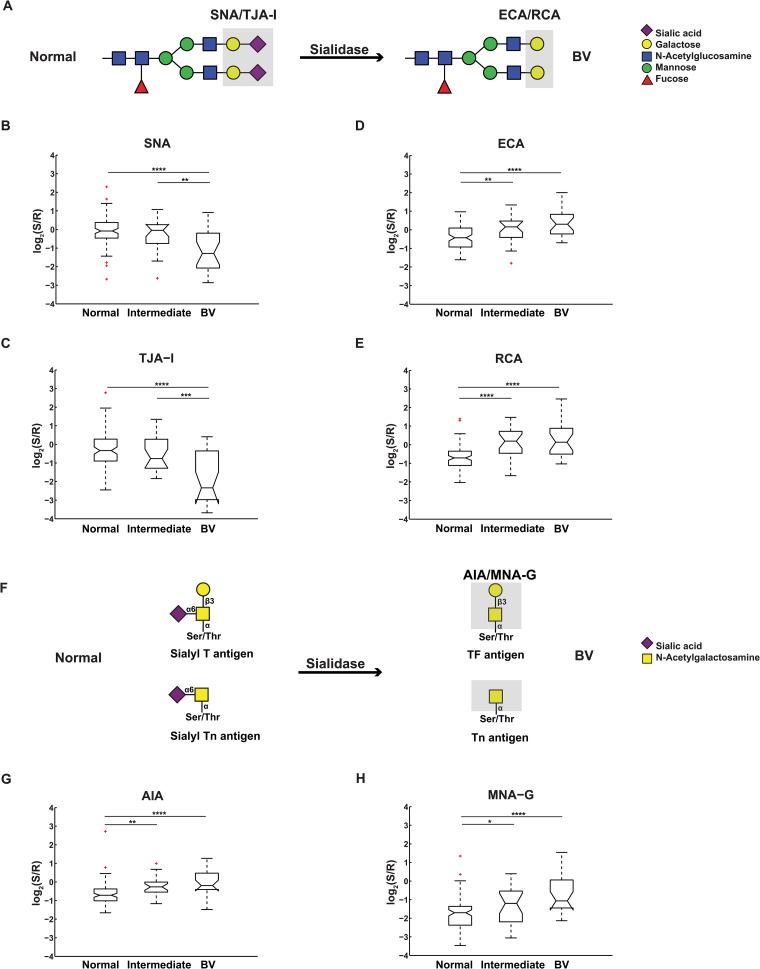
Fig 1. Lectin Microarray Analysis of CVL.
(A) Experimental scheme. Equal amounts of Cy5-CVL sample and Cy3-pooled CVL reference were analyzed on the lectin microarray. (B) Heat map of hierarchical clustering. Median-normalized log2 ratios (Sample (S) /Reference (R)) of 165 CVL samples were hierarchically clustered using Pearson correlation coefficient as the distance metric and average linkage analysis (yellow, log2(S) > log2(R); blue, log2(R) > log2(S)). Samples are color coded by hormonal and microbial status across the top: Postmenopausal (orange), Days 1–14 (black), Days 15–28 (light teal), Oral contraceptive (purple), Depo-Provera (gray), IUD (pink), BV (green), Intermediate (dark teal), Normal (blue). (C) Select sample and lectin clusters showing BV vs. Normal patterns. Pearson correlation coefficient scale is shown.
Initial examination of the heatmap suggested that both microbial status and hormonal levels impact the glycome. Samples most clearly segregated by microbial status (Fig 1C). This pattern was established by three sets of lectins: β-galactose (Gal)/ β-N-acetyl-D-galactosamine (GalNAc) (AIA, ECA, RCA, a list of lectin abbreviations and rough specificities is shown in S1 Table), high mannose (CVN, UDA, SVN, GRFT) and α2, 6-sialic acid binders (TJA-I and SNA). In contrast, a clear pattern was difficult to discern for hormonal regulation, although some clustering was observed for postmenopausal women (PM) and women on Depo-Provera (Depo). Microbial status interfered with our assessment of the role that hormones play in establishing the CVL glycome. We observed two distinct Depo clusters (one with BV and one without) with different glycan profiles in our large dataset (Fig 1C), arguing that microbial status may predominate over hormones in the control of glycosylation. We cannot address the microbial status of the postmenopausal women in this study, so conclusions on the role of hormone and/or microbial status on glycosylation in that cohort are not possible, thus we excluded these samples from further analysis. Based on our findings, we undertook a more detailed statistical analysis of our data to better define the roles of microbial status (BV/normal) and hormones on specific glycosylation signatures in the CVL.
Bacterial Vaginosis has a Strong Effect on Glycosylation in the CVL
To more closely examine the effects of bacterial vaginosis (BV) on CVL glycosylation in this cohort, we performed ANOVA analysis on a combined cohort of BV (n = 23), intermediate (n = 23) and normal (n = 90) samples, disregarding hormonal status, for each lectin on our microarray. In line with our earlier findings, we observed statistically significant decreases in lectins corresponding to α2,6-sialic acid, and high mannose epitopes and an increase in β-Gal, β-GalNAc binding (Figs 2 and 3). We tested whether hormonal differences in the cohorts could account for these changes as we had a predominance of 2 groups within the BV cohort (days 1–14 of the menstrual cycle and Depo-Provera, n = 8 each, 35% each of cohort). Normal samples from the days 1–14 and Depo-Provera groups did not follow the trends observed in BV (S2 Fig). In addition, comparison of normal vs. BV samples within the each group demonstrated similar effects on the glycome due to aberrant microflora seen in the combined cohort (i.e. decreases in α2,6-sialic acid and high mannose) arguing that the microbiome overrides hormonal effects (S3 and S4 Figs).
Fig 2. Effects of vaginal microflora on sialylation.
(A) Representative N-linked glycan before and after sialidase digestion. Lectin binding determinants are shaded in grey with lectin names indicated. (B-E) Notched boxplot representation of binding levels of (B) SNA, (C) TJA-I, (D) ECA, (E) RCA to Normal, Intermediate and BV sample cohorts. (F) Representative O-linked glycans before and after sialidase. (G-H) Notched boxplot representation of binding levels of (G) AIA and (H) MNA-G. For all plots, significance levels between groups indicated by lines are as follows: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
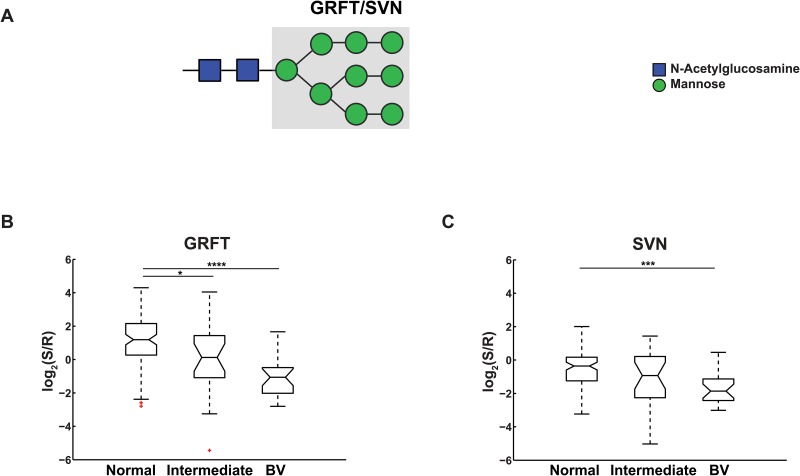
Fig 3. Effects of vaginal microflora on high mannose.
(A) High mannose glycan structure. Lectin binding determinants are shaded in grey. (B-C) Notched boxplot representation of binding levels of (B) GRFT and (C) SVN. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are in marked red.
Several of these changes are consistent with the known biological effects of BV on the glycome of the vagina. In bacterial vaginosis higher levels of sialidase, an enzyme that cleaves sialic acid molecules from underlying β-Gal and β-GalNAc structures, are observed [6, 16]. In a companion paper, Moncla et al. show higher levels of sialidase activity correlated with BV in these CVL samples. This would cause a loss of sialic acids and an increase in exposed terminal β-Gal and β-GalNAc residues (Fig 2A and 2F). In our data we observed the loss of α2, 6-sialic acid residues (p < 0.0001 for both SNA [40] and TJA-I [41], Fig 2B and 2C) and the gain of terminal β-Gal and β-GalNAc structures (β-Gal: ECA [42] and RCA [43], p < 0.0001 for both, Fig 2D and 2E; β-GalNAc: AIA [44] and MNA-G [45], p < 0.0001 for both, Fig 2G and 2H). We also observed an effect of BV on levels of α2, 3-sialic acid as probed by Maackia amuerensis lectin-I (MAL-I) binding but the effect is not statistically significant (p = 0.4). Similar results for SNA and Maackia amuerensis lectin were observed by enzyme-linked lectin assays (see the accompanying paper by Moncla et al., PONE-D-15-01714). This more mild effect on MAL-I binding may be due to the strong binding of MAL-I to sulfated glycans, which are present in CVL but are not affected by sialidase [3, 46, 47] (S5 Fig). We also observed a gain in binding to terminal β-Gal and β-GalNAc residues, consistent with their exposure by sialidase (Fig 2D, 2E, 2G and 2H). This increase is observed in both the N-linked (ECA, RCA) and O-linked (AIA, MNA-G) cohorts and is clear even in intermediate samples where the changes in sialic acid are not readily apparent. Levels of α-GalNAc, however, were unaffected by BV (HPA, S6 Fig).
Our data also shows a loss of high mannose residues on glycoproteins of the CVL from women with BV (Fig 3). High-mannose glycans can contain five to nine mannose residues attached to the chitobiose (GlcNAc2) core and are early products of N-glycan biosynthesis. We observed a significant loss of binding to two algal lectins, Griffithsin (GRFT) and Scytovirin (SVN), which are both specific to Man7-Man9 high mannose structures, in the BV cohort (Fig 3 B and C, p < 0.0001 and p = 0.0002, respectively). This data is supported by work by Moncla et al. (see accompanying paper). Both of these proteins are known anti-viral lectins and are currently being examined for use as microbicides against viruses including HIV-1 and hepatitis-C [48–50]. We do not observe statistically significant differences with other mannose-binding lectins, such as AMA, ASA [51], ConA [52], GNA and NPA [53] (S7 Fig), which can also bind Man5-Man6, suggesting that this loss is restricted to the Man7-Man9 subset.
Previous studies have shown that both HIV infection risk and urinary tract infection risk increase with bacterial vaginosis [54–57]. High mannose residues on S-IgA have been proposed to act as a natural inhibitor of urinary tract infections by inhibiting Escherichia coli expressing FimH, a bacterial lectin specific for high mannose, to vaginal epithelia [58, 59]. High mannose epitopes found on the gp120 coat protein of HIV-1 bind to endogenous lectins DC-SIGN and the macrophage mannose receptor (MMR) and aid in the infection of macrophages and dendritic cells [60, 61]. Binding of vaginal MMR by gp120 on HIV-1 may also play a role in sexual transmission of HIV-1 [62]. Native high mannose residues found on glycoproteins in vaginal fluids may thus act as natural inhibitors of these pathogenic interactions, helping to prevent viral entry and bacterial adhesion in women with normal microflora. The alteration in high mannose levels associated with abnormal microflora may alter the innate immune properties of the vaginal mucosa enabling enhanced pathogenesis in concordance with the increased infection risk.
Exogenous Hormones Influence the Glycome
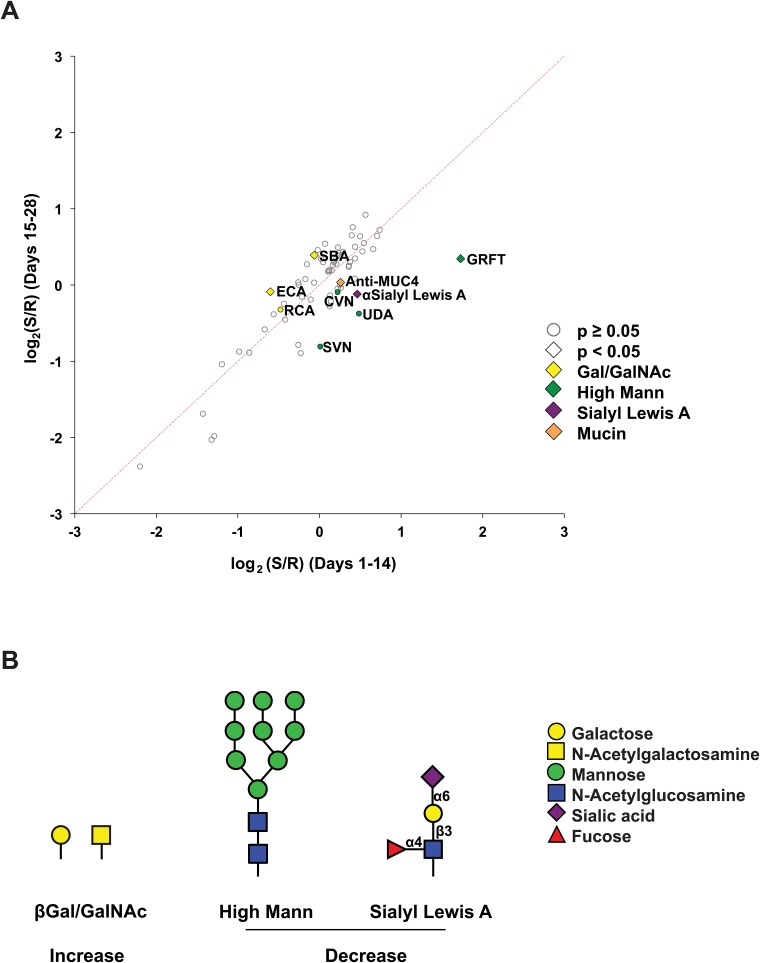
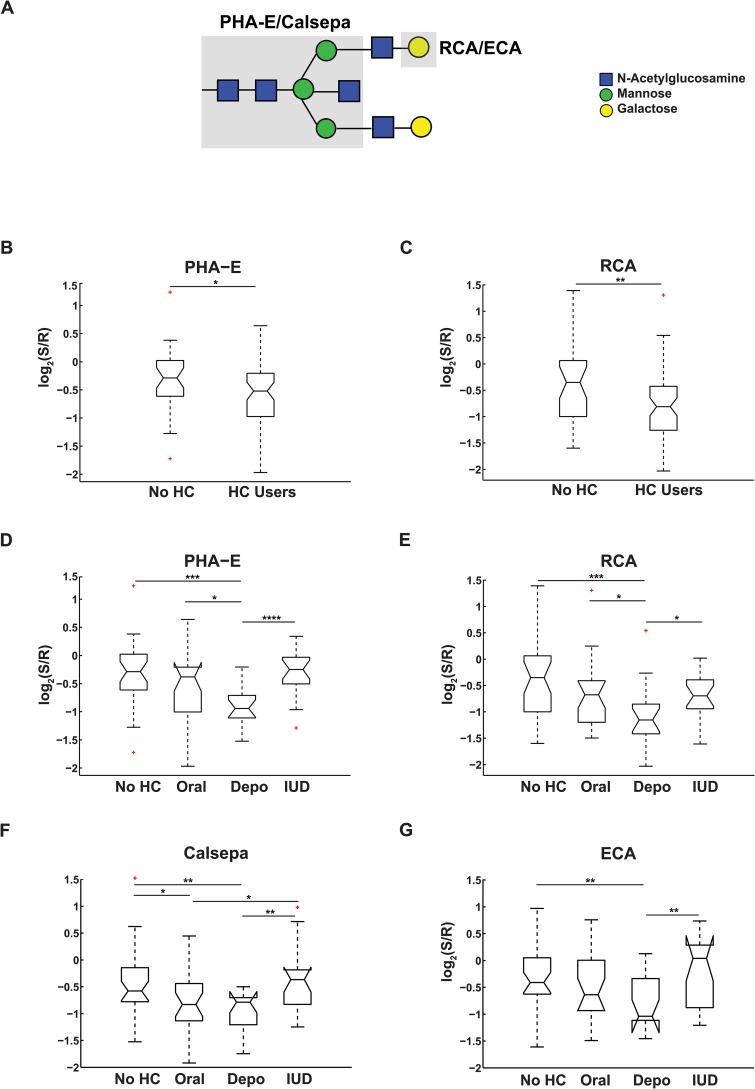
We reanalyzed our lectin microarray data for hormonal effects, examining the data for the 90 women with normal microflora. We excluded postmenopausal women, as we could not evaluate the status of their microbiome. We examined the effects of normal hormonal status on the CVL glycome by comparing women in days 1–14 of their menstrual cycle to women in days 15–28. We observed several changes associated with the menstrual cycle including a loss of GRFT binding in the later stages (p = 0.036, Fig 4), which is consistent with analysis in the companion paper by Moncla et al. To examine the effects of exogenous hormone contraceptives on glycomic composition of the CVL, we combined women in menstrual cycle without contraceptives (No HC, n = 31, Fig 5) and compared them to contraceptive users (HC Users, n = 59). Only two lectins gave statistically significant differences in signal for the cohorts. PHA-E, which binds to N-linked glycans containing bisecting GlcNAc [63], and RCA, which binds terminal galactose, both showed decreased binding to the CVL of contraceptive users (Fig 5A–5C, p = 0.046 and p = 0.002 respectively). We next ran a more detailed analysis, looking at the contribution of individual contraceptive cohorts (Oral, Depo-Provera and IUD) to the differences observed. All three contraceptive methods contain different synthetic hormones; combined oral contraceptives have estrogen and progesterone, Depo-Provera contains depo-medroxyprogesterone acetate, a progesterone derivative, and the IUD contains a different progesterone derivative, levonorgestrel. Depo-Provera users showed significantly lower levels of bisecting GlcNAc (PHA-E, Calsepa [64], Fig 5D and 5F, p = 0.0004 and p = 0.007 respectively) and terminal galactose (RCA, ECA, Fig 5E and 5G, p = 0.0009 and p = 0.004, respectively) than other contraceptive users and were the major contributor to the pattern observed for contraceptive use. In recent studies bisecting GlcNAc epitopes were found to enhance binding of IgG Fc to the FcγIIIa receptor leading to antibody-dependent cellular cytotoxicity [65]. Decrease in this epitope would presumably lower the levels of antibody-dependent immune response. This is consistent with recent work showing an anti-inflammatory effect by Depo-Provera on endocervical cells [66]. Oral contraceptive users also showed some glycomic changes in comparison to non-users, although the effects were less significant (0.01 < p < 0.05) and a clear glycomic pattern did not emerge. IUD users showed no real changes compared to the no-HC group. Our data suggests that both hormonal contraceptive composition and delivery method impact the vaginal glycome in ways that may affect immunity.
Fig 4. Effects of menstrual cycle on glycosylation of CVL.
(A) Bi-plot of lectin microarray data for CVL from women in days 1–14 (x-axis) versus days 15–28 (y-axis). Graph shows average data for each lectin. Lectins and antibodies showing significant differences (p < 0.05) between the two groups are labeled with diamonds. Lectins with similar binding glycans are labeled in the same color (yellow: Gal/GalNAc; green: high mannose; purple: sialyl Lewis A). Lectins above the red dashed line showed increased expression levels during days 15–28 of the menstrual cycle compared to days 1–14. (B) Visual representation of glycans showing significant differences between the two groups.
Fig 5. Effects of exogenous hormones on CVL glycome.
(A) Representative N-linked complex glycan with bisecting GlcNAc. Lectin binding epitopes are shaded in grey. (B-C) Notched boxplot representation of binding levels of (B) PHA-E and (C) RCA for women on no hormonal contraceptives (No HC) or on hormonal contraceptives (HC). (D-G) Notched boxplot representation of detailed analysis of binding of (D) PHA-E, (E) RCA, (F) Calsepa and (G) ECA for women on oral contraceptives (Oral), Depo-Provera (Depo) or IUD in comparison to the No HC cohort. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
Conclusions
The glycome of fluids from the cervico-vaginal tract plays an important role in the innate immune system, preventing pathogenic interactions and modulating immune activation. The study described herein and the accompanying work by Moncla et al (PONE-D-15-01714), which use cervical vaginal lavage samples (CVL) to represent the fluid and mucus from the lower reproductive tract, are the first to examine the role of hormonal contraceptives and microflora on glycosylation in this critical immunological fluid. Our data demonstrates that pathogenic microflora, such as that observed in bacterial vaginosis, have a profound effect on the CVL glycome overriding hormonal effects. These alterations may lower the immunological function of the cervico-vaginal fluids, enhancing the ability of secondary pathogens, such as HIV-1, to infect the host. Hormonal contraceptives also alter this glycome, with currently unknown implications. Both the route of contraceptive administration and the type of synthetic hormone used may play a role in these changes. These differences point out the need for further paired-patient studies examining the direct effects of different hormonal contraception agents on the glycome and on the innate immune protection provided by cervico-vaginal fluids.
Supporting Information
Raw data is available at www.synapse.org (doi:10.7303/syn3243404).
(XLSX)
Lectin microarray analysis of matched dialyzed and undialyzed Cy3-labeled samples. The fluorescence was inhibited by free sugar in the CVL. Sample images are shown.
(TIFF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women on no hormonal contraceptives (days 1–14 and 15–28) and on hormonal contraceptives (oral contraceptives (Oral), Depo-Provera (Depo) or IUD). Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red. For all plots, significance levels between groups indicated by lines are as follows: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red. Differences due to hormone levels do not show the same general glycan patterns as BV vs. non-BV.
(TIF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women at days 1–14 of the menstrual cycle with different flora states. The same effects caused by microflora were observed in these women, matching the trends seen in combined cohorts. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
(TIF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women on Depo with different flora states. The same effects caused by microflora were observed in these women, matching the trends seen in combined cohorts. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
(TIF)
Notched boxplot representation of binding levels of MAL-I to normal, intermediate and BV samples is shown. Outliers are marked red. The observed difference is not statistically significant (p = 0.4).
(TIFF)
Notched boxplot representation of binding levels for HPA, a lectin that binds α-GalNAc, to normal, intermediate and BV samples is shown. None of the differences were statistically significant. Outliers are marked red.
(TIF)
Notched boxplot representation of binding levels for lectins that bind Man5-Man6 to normal, intermediate and BV samples are shown. (A) AMA, (B) ASA, (C) GNA, (D) NPA and (E) ConA. None of the differences were statistically significant. Outliers are marked red.
(TIF)
(DOCX)
Acknowledgments
We would like to acknowledge Boval Biosolutions for lyophilized protease- and IgG-free bovine serum albumi (no. LY-0081) and Dr. B. O’Keefe (NCI, Frederick) for recombinant GRFT and SVN.
Data Availability
All data files are available from www.synapse.org (doi: 10.7303/syn3243404).
Funding Statement
This work was funded by the National Institutes of Health, Grant # U191AI082639. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Current HIV/AIDS reports. 2009;6(1):20–8. . [DOI] [PubMed] [Google Scholar]
- 2. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. International journal of pharmaceutics. 2013;453(1):56–64. 10.1016/j.ijpharm.2013.05.040 . [DOI] [PubMed] [Google Scholar]
- 3. Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC. Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics. 2007;6(4):708–16. Epub 2007/01/16. 10.1074/mcp.M600439-MCP200 . [DOI] [PubMed] [Google Scholar]
- 4. Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophysical journal. 2001;81(4):1930–7. 10.1016/S0006-3495(01)75844-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carlstedt I, Sheehan JK. Structure and macromolecular properties of cervical mucus glycoproteins. Symposia of the Society for Experimental Biology. 1989;43:289–316. . [PubMed] [Google Scholar]
- 6. Olmsted SS, Meyn LA, Rohan LC, Hillier SL. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sexually transmitted diseases. 2003;30(3):257–61. . [DOI] [PubMed] [Google Scholar]
- 7. Rajan N, Cao Q, Anderson BE, Pruden DL, Sensibar J, Duncan JL, et al. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infection and immunity. 1999;67(10):5027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zegels G, Van Raemdonck GA, Coen EP, Tjalma WA, Van Ostade XW. Comprehensive proteomic analysis of human cervical-vaginal fluid using colposcopy samples. Proteome science. 2009;7:17 10.1186/1477-5956-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shafer WM, Katzif S, Bowers S, Fallon M, Hubalek M, Reed MS, et al. Tailoring an antibacterial peptide of human lysosomal cathepsin G to enhance its broad-spectrum action against antibiotic-resistant bacterial pathogens. Current pharmaceutical design. 2002;8(9):695–702. . [DOI] [PubMed] [Google Scholar]
- 10. Bard E, Laibe S, Bettinger D, Riethmuller D, Biichle S, Seilles E, et al. New sensitive method for the measurement of lysozyme and lactoferrin for the assessment of innate mucosal immunity. part I: time-resolved immunofluorometric assay in serum and mucosal secretions. Clinical chemistry and laboratory medicine: CCLM / FESCC. 2003;41(2):127–33. 10.1515/CCLM.2003.021 . [DOI] [PubMed] [Google Scholar]
- 11. Groot F, Geijtenbeek TB, Sanders RW, Baldwin CE, Sanchez-Hernandez M, Floris R, et al. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN—gp120 interaction. Journal of virology. 2005;79(5):3009–15. 10.1128/JVI.79.5.3009-3015.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis AL, Lewis WG. Host sialoglycans and bacterial sialidases: a mucosal perspective. Cellular microbiology. 2012;14(8):1174–82. 10.1111/j.1462-5822.2012.01807.x . [DOI] [PubMed] [Google Scholar]
- 13. Schaeffer AJ, Rajan N, Cao Q, Anderson BE, Pruden DL, Sensibar J, et al. Host pathogenesis in urinary tract infections. Int J Antimicrob Agents. 2001;17(4):245–51. . [DOI] [PubMed] [Google Scholar]
- 14. Brinkman-Van der Linden CM, Havenaar EC, Van Ommen CR, Van Kamp GJ, Gooren LJ, Van Dijk W. Oral estrogen treatment induces a decrease in expression of sialyl Lewis x on alpha 1-acid glycoprotein in females and male-to-female transsexuals. Glycobiology. 1996;6(4):407–12. . [DOI] [PubMed] [Google Scholar]
- 15. Yeoman CJ, Thomas SM, Miller ME, Ulanov AV, Torralba M, Lucas S, et al. A multi-omic systems-based approach reveals metabolic markers of bacterial vaginosis and insight into the disease. PloS one. 2013;8(2):e56111 Epub 2013/02/14. 10.1371/journal.pone.0056111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Briselden AM, Moncla BJ, Stevens CE, Hillier SL. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. Journal of clinical microbiology. 1992;30(3):663–6. Epub 1992/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hillier SL, Holmes KK. Bacterial vaginosis In: Holmes KK, Mardh P, Sparling PF, Wiesner PJ, editors. Sexually transmitted diseases. New York: McGraw-Hill, Health Professions Division; 1999. [Google Scholar]
- 18. Lewis WG, Robinson LS, Perry J, Bick JL, Peipert JF, Allsworth JE, et al. Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J Biol Chem. 2012;287(3):2079–89. 10.1074/jbc.M111.278135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilobello KT, Krishnamoorthy L, Slawek D, Mahal LK. Development of a lectin microarray for the rapid analysis of protein glycopatterns. Chembiochem: a European journal of chemical biology. 2005;6(6):985–9. 10.1002/cbic.200400403 . [DOI] [PubMed] [Google Scholar]
- 20. Pilobello KT, Agrawal P, Rouse R, Mahal LK. Advances in lectin microarray technology: optimized protocols for piezoelectric print conditions. Current protocols in chemical biology. 2013;5(1):1–23. 10.1002/9780470559277.ch120035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, et al. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nature methods. 2005;2(11):851–6. 10.1038/nmeth803 . [DOI] [PubMed] [Google Scholar]
- 22. Kilcoyne M, Twomey ME, Gerlach JQ, Kane M, Moran AP, Joshi L. Campylobacter jejuni strain discrimination and temperature-dependent glycome expression profiling by lectin microarray. Carbohydrate research. 2014;389:123–33. 10.1016/j.carres.2014.02.005 . [DOI] [PubMed] [Google Scholar]
- 23. Bird-Lieberman EL, Neves AA, Lao-Sirieix P, O'Donovan M, Novelli M, Lovat LB, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett's esophagus. Nature medicine. 2012;18(2):315–21. 10.1038/nm.2616 . [DOI] [PubMed] [Google Scholar]
- 24. Gaziel-Sovran A, Segura MF, Di Micco R, Collins MK, Hanniford D, Vega-Saenz de Miera E, et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer cell. 2011;20(1):104–18. 10.1016/j.ccr.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsu KL, Mahal LK. A lectin microarray approach for the rapid analysis of bacterial glycans. Nature protocols. 2006;1(2):543–9. 10.1038/nprot.2006.76 . [DOI] [PubMed] [Google Scholar]
- 26. Inoue K, Wada J, Eguchi J, Nakatsuka A, Teshigawara S, Murakami K, et al. Urinary fetuin-A is a novel marker for diabetic nephropathy in type 2 diabetes identified by lectin microarray. PLoS One. 2013;8(10):e77118 10.1371/journal.pone.0077118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of clinical microbiology. 1991;29(2):297–301. Epub 1991/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002;30(4):e15 Epub 2002/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–8. Epub 1998/12/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–8. Epub 2004/06/08. 10.1093/bioinformatics/bth349 bth349 [pii]. . [DOI] [PubMed] [Google Scholar]
- 31. Conover WJ. Practical Nonparametric Statistics. Hoboken, NJ: John Wiley & Sons, Inc.; 1980. [Google Scholar]
- 32. Lilliefors HW. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. Journal of the American Statistical Association. 1967. citeulike-article-id:163628. 12146215 [Google Scholar]
- 33. Lilliefors HW. On the Kolmogorov-Smirnov Test for the Exponential Distribution with Mean Unknown. Journal of the American Statistical Association. 1969;64(325):387–9. citeulike-article-id:5652186. [Google Scholar]
- 34. Hogg RV, Ledolter J. Engineering Statistics. New York: Macmillan Publ.Comp.; 1987. [Google Scholar]
- 35. Mitchell C, Paul K, Agnew K, Gaussman R, Coombs RW, Hitti J. Estimating volume of cervicovaginal secretions in cervicovaginal lavage fluid collected for measurement of genital HIV-1 RNA levels in women. Journal of clinical microbiology. 2011;49(2):735–6. Epub 2010/11/26. 10.1128/JCM.00991-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, et al. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. American journal of obstetrics and gynecology. 2014;211(3):226 e1–7. Epub 2014/03/26. 10.1016/j.ajog.2014.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu KL, Pilobello K, Krishnamoorthy L, Mahal LK. Ratiometric lectin microarray analysis of the mammalian cell surface glycome. Methods in molecular biology. 2011;671:117–31. 10.1007/978-1-59745-551-0_6 . [DOI] [PubMed] [Google Scholar]
- 38. Pilobello KT, Slawek DE, Mahal LK. A ratiometric lectin microarray approach to analysis of the dynamic mammalian glycome. Proc Natl Acad Sci U S A. 2007;104(28):11534–9. 10.1073/pnas.0704954104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agrawal P, Kurcon T, Pilobello KT, Rakus JF, Koppolu S, Liu Z, et al. Mapping posttranscriptional regulation of the human glycome uncovers microRNA defining the glycocode. Proc Natl Acad Sci U S A. 2014;111(11):4338–43. 10.1073/pnas.1321524111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischer E, Brossmer R. Sialic acid-binding lectins: submolecular specificity and interaction with sialoglycoproteins and tumour cells. Glycoconjugate journal. 1995;12(5):707–13. . [DOI] [PubMed] [Google Scholar]
- 41. Yamashita K, Umetsu K, Suzuki T, Ohkura T. Purification and characterization of a Neu5Ac alpha 2—>6Gal beta 1—>4GlcNAc and HSO3(-)—>6Gal beta 1—>GlcNAc specific lectin in tuberous roots of Trichosanthes japonica. Biochemistry. 1992;31(46):11647–50. . [DOI] [PubMed] [Google Scholar]
- 42. Debray H, Montreuil J, Lis H, Sharon N. Affinity of four immobilized Erythrina lectins toward various N-linked glycopeptides and related oligosaccharides. Carbohydrate research. 1986;151:359–70. . [DOI] [PubMed] [Google Scholar]
- 43. Bhattacharyya L, Haraldsson M, Brewer CF. Precipitation of galactose-specific lectins by complex-type oligosaccharides and glycopeptides: studies with lectins from Ricinus communis (agglutinin I), Erythrina indica, Erythrina arborescens, Abrus precatorius (agglutinin), and Glycine max (soybean). Biochemistry. 1988;27(3):1034–41. . [DOI] [PubMed] [Google Scholar]
- 44. Wu AM, Wu JH, Lin LH, Lin SH, Liu JH. Binding profile of Artocarpus integrifolia agglutinin (Jacalin). Life sciences. 2003;72(20):2285–302. . [DOI] [PubMed] [Google Scholar]
- 45. Singh T, Wu JH, Peumans WJ, Rouge P, Van Damme EJ, Wu AM. Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes. Molecular immunology. 2007;44(4):451–62. 10.1016/j.molimm.2006.02.017 . [DOI] [PubMed] [Google Scholar]
- 46. Bai X, Brown JR, Varki A, Esko JD. Enhanced 3-O-sulfation of galactose in Asn-linked glycans and Maackia amurensis lectin binding in a new Chinese hamster ovary cell line. Glycobiology. 2001;11(8):621–32. . [DOI] [PubMed] [Google Scholar]
- 47. Porter A, Yue T, Heeringa L, Day S, Suh E, Haab BB. A motif-based analysis of glycan array data to determine the specificities of glycan-binding proteins. Glycobiology. 2010;20(3):369–80. 10.1093/glycob/cwp187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, et al. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin. Virology. 2010;402(1):187–96. 10.1016/j.virol.2010.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, et al. Griffithsin has antiviral activity against hepatitis C virus. Antimicrobial agents and chemotherapy. 2011;55(11):5159–67. 10.1128/AAC.00633-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Emau P, Tian B, O'Keefe B R, Mori T, McMahon JB, Palmer KE, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J Med Primatol. 2007;36(4–5):244–53. . [DOI] [PubMed] [Google Scholar]
- 51. Dam TK, Bachhawat K, Rani PG, Surolia A. Garlic (Allium sativum) lectins bind to high mannose oligosaccharide chains. J Biol Chem. 1998;273(10):5528–35. . [DOI] [PubMed] [Google Scholar]
- 52. Wang L, Cummings RD, Smith DF, Huflejt M, Campbell CT, Gildersleeve JC, et al. Cross-platform comparison of glycan microarray formats. Glycobiology. 2014;24(6):507–17. 10.1093/glycob/cwu019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kaku H, Goldstein IJ. Interaction of linear manno-oligosaccharides with three mannose-specific bulb lectins. Comparison with mannose/glucose-binding lectins. Carbohydrate research. 1992;229(2):337–46. . [DOI] [PubMed] [Google Scholar]
- 54. Myer L, Kuhn L, Stein ZA, Wright TC Jr., Denny L. Intravaginal practices, bacterial vaginosis, and women's susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;5(12):786–94. Epub 2005/11/29. S1473-3099(05)70298-X [pii] 10.1016/S1473-3099(05)70298-X . [DOI] [PubMed] [Google Scholar]
- 55. Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire-Mangen F, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350(9077):546–50. Epub 1997/08/23. Doi: S0140673697010635 [pii]. . [DOI] [PubMed] [Google Scholar]
- 56. Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. Aids. 1998;12(13):1699–706. . [DOI] [PubMed] [Google Scholar]
- 57. Royce RA, Thorp J, Granados JL, Savitz DA. Bacterial vaginosis associated with HIV infection in pregnant women from North Carolina. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(4):382–6. . [DOI] [PubMed] [Google Scholar]
- 58. Wold AE, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, et al. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infection and immunity. 1990;58(9):3073–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schoor RA, Anderson B, Klumpp DJ, Schaeffer AJ. Secretory IGA differentially promotes adherence of type 1-piliated Escherichia coli to immortalized vaginal epithelial cell lines. Urology. 2001;57(3):556–61. . [DOI] [PubMed] [Google Scholar]
- 60. Fanibunda SE, Modi DN, Gokral JS, Bandivdekar AH. HIV gp120 binds to mannose receptor on vaginal epithelial cells and induces production of matrix metalloproteinases. PLoS One. 2011;6(11):e28014 10.1371/journal.pone.0028014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Harman AN, Kim M, Nasr N, Sandgren KJ, Cameron PU. Tissue dendritic cells as portals for HIV entry. Reviews in medical virology. 2013;23(5):319–33. 10.1002/rmv.1753 . [DOI] [PubMed] [Google Scholar]
- 62. Jadhav SK, Velhal SM, Deshpande A, Bandivdekar AH. Association of human mannose receptor in sexual transmission of human immunodeficiency virus in serodiscordant couples. AIDS research and human retroviruses. 2013;29(1):156–63. 10.1089/AID.2012.0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kaneda Y, Whittier RF, Yamanaka H, Carredano E, Gotoh M, Sota H, et al. The high specificities of Phaseolus vulgaris erythro- and leukoagglutinating lectins for bisecting GlcNAc or beta 1-6-linked branch structures, respectively, are attributable to loop B. J Biol Chem. 2002;277(19):16928–35. 10.1074/jbc.M112382200 . [DOI] [PubMed] [Google Scholar]
- 64. Nakamura-Tsuruta S, Uchiyama N, Peumans WJ, Van Damme EJ, Totani K, Ito Y, et al. Analysis of the sugar-binding specificity of mannose-binding-type Jacalin-related lectins by frontal affinity chromatography—an approach to functional classification. The FEBS journal. 2008;275(6):1227–39. 10.1111/j.1742-4658.2008.06282.x . [DOI] [PubMed] [Google Scholar]
- 65. Zou G, Ochiai H, Huang W, Yang Q, Li C, Wang LX. Chemoenzymatic synthesis and Fcgamma receptor binding of homogeneous glycoforms of antibody Fc domain. Presence of a bisecting sugar moiety enhances the affinity of Fc to FcgammaIIIa receptor. Journal of the American Chemical Society. 2011;133(46):18975–91. 10.1021/ja208390n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, et al. The Injectable-Only Contraceptive Medroxyprogesterone Acetate, Unlike Norethisterone Acetate and Progesterone, Regulates Inflammatory Genes in Endocervical Cells via the Glucocorticoid Receptor. PLoS One. 2014;9(5):e96497 10.1371/journal.pone.0096497 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data is available at www.synapse.org (doi:10.7303/syn3243404).
(XLSX)
Lectin microarray analysis of matched dialyzed and undialyzed Cy3-labeled samples. The fluorescence was inhibited by free sugar in the CVL. Sample images are shown.
(TIFF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women on no hormonal contraceptives (days 1–14 and 15–28) and on hormonal contraceptives (oral contraceptives (Oral), Depo-Provera (Depo) or IUD). Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red. For all plots, significance levels between groups indicated by lines are as follows: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red. Differences due to hormone levels do not show the same general glycan patterns as BV vs. non-BV.
(TIF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women at days 1–14 of the menstrual cycle with different flora states. The same effects caused by microflora were observed in these women, matching the trends seen in combined cohorts. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
(TIF)
(A-H) Notched boxplot representation of binding levels of (A) SNA, (B) TJA-I, (C) ECA, (D) RCA, (E) AIA, (F) MNA-G, (G) GRFT and (H) SVN for women on Depo with different flora states. The same effects caused by microflora were observed in these women, matching the trends seen in combined cohorts. Significance: *, 0.01< p ≤0.05; **, 0.001< p ≤0.01; ***, 0.0001<p≤0.001; ****, p≤0.0001. Outliers are marked in red.
(TIF)
Notched boxplot representation of binding levels of MAL-I to normal, intermediate and BV samples is shown. Outliers are marked red. The observed difference is not statistically significant (p = 0.4).
(TIFF)
Notched boxplot representation of binding levels for HPA, a lectin that binds α-GalNAc, to normal, intermediate and BV samples is shown. None of the differences were statistically significant. Outliers are marked red.
(TIF)
Notched boxplot representation of binding levels for lectins that bind Man5-Man6 to normal, intermediate and BV samples are shown. (A) AMA, (B) ASA, (C) GNA, (D) NPA and (E) ConA. None of the differences were statistically significant. Outliers are marked red.
(TIF)
(DOCX)
Data Availability Statement
All data files are available from www.synapse.org (doi: 10.7303/syn3243404).