Abstract
IMPORTANCE
As Alzheimer disease (AD) research moves to intervene in presymptomatic phases of the disease, we must develop outcome measures sensitive to the earliest disease-related changes.
OBJECTIVE
To demonstrate the feasibility of a cognitive composite outcome for clinically normal elderly participants with evidence of AD pathology using the ADCS Preclinical Alzheimer Cognitive Composite (ADCS-PACC). The ADCS-PACC combines tests that assess episodic memory, timed executive function, and global cognition. The ADCS-PACC is the primary outcome measure for the first clinical trial in preclinical AD (ie, the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study).
DESIGN, SETTING, AND PARTICIPANTS
With the ADCS-PACC, we derive pilot estimates of amyloid-related decline using data from 2 observational studies conducted in North America and another conducted in Australia. The participants analyzed had normal cognition and mean ages of 75.81, 71.37, and 79.42 years across the 3 studies.
MAIN OUTCOMES AND MEASURES
For the 2 studies that collected data on Aβ levels (ADNI and AIBL), we estimate decline in a preclinical AD “Aβ-positive” placebo group and compare them with an “Aβ-negative” group. For the study that did not include data on Aβ levels (the ADCS Prevention Instrument [ADCS-PI] study), we grouped participants by the presence of APOE-ɛ4 and by clinical progression.
RESULTS
In ADNI, Aβ-positive participants showed more decline than did Aβ-negative participants with regard to the ADCS-PACC score at 24 months (mean [SE] difference, −1.239 [0.522] [95% CI, −2.263 to −0.215]; P = .02). In AIBL, the mean (SE) difference is significant at both 18 months (−1.009 [0.406] [95% CI, −1.805 to −0.213]; P = .01) and 36 months (−1.404 [0.452] [95% CI, −2.290 to −0.519]; P = .002). In the ADCS-PI study, APOE-ɛ4 allele carriers performed significantly worse on the ADCS-PACC at 24 months (mean [SE] score, −0.742 [0.294] [95% CI, −1.318 to −0.165]; P = .01) and 36 months (−1.531 [0.469] [95% CI, −2.450 to −0.612]; P = .001). In the ADCS-PI study, cognitively normal participants who progress from a global Clinical Dementia Rating score of 0 are significantly worse on the ADCS-PACC than cognitively normal participants who are stable with a global Clinical Dementia Rating score of 0 at months 12, 24, and 36 (mean [SE] ADCS-PACC score, −4.471 [0.702] [95% CI, −5.848 to −3.094]; P < .001). Using pilot estimates of variance and assuming 500 participants per group with 30% attrition and a 5% α level, we project 80% power to detect effects in the range of Δ = 0.467 to 0.733 on the ADCS-PACC.
CONCLUSIONS AND RELEVANCE
Analyses of at-risk cognitively normal populations suggest that we can reliably measure the first signs of cognitive decline with the ADCS-PACC. These analyses also suggest the feasibility of secondary prevention trials.
The field of Alzheimer disease (AD) research has evolved to conceptualize AD as a continuum of disease.1–4 Although, historically, AD was considered to begin with the onset of dementia, a predementia stage, characterized clinically as mild cognitive impairment and, more specifically, using biomarkers, as prodromal AD, has been widely accepted.5–7 Most recently, the preclinical stage of AD has been postulated. This asymptomatic stage, believed to precede mild cognitive impairment by years, is characterized by accumulating amyloid pathology and neurodegeneration accompanied by very subtle cognitive decline detectable with sensitive neuropsychological tests and cognitive complaint measures.1 Individuals with preclinical AD (ie, cognitively normal individuals with biomarker evidence of brain amyloid deposition) represent a group at high risk for decline and an ideal population for a “secondary prevention” trial aimed at delaying the emergence of the clinical syndromes of mild cognitive impairment and dementia.8
Drug development strategies in very early stages of the AD process initially focused on biomarkers that might efficiently demonstrate change-occurring years before the onset of symptoms. Examples of such candidate biomarker outcomes have included volumetric magnetic resonance imaging,9 positron emission tomography (PET) with18 fluorodeoxyglucose,10 amyloid PET imaging,11,12 and cerebrospinal fluid (CSF) markers.13 Although each of these proposed outcomes reflect disease progression, the impact of therapeutic interventions aimed at disease modification has been surprising. For example, antiamyloid immunotherapy may paradoxically accelerate brain atrophy as measured by volumetric magnetic resonance imaging.14 Until a reliable surrogate biomarker is validated, the field must rely on clinical outcome measures that reflect cognitive function.
Studies have shown that cognitive performance, measured using tests ranging from the Mini-Mental State Examination (MMSE) to word list learning tasks, may also show changes many years before the onset of functional decline.2,15,16 Cognitive measures have important advantages over imaging and biochemical biomarkers: they are closely related to the core symptoms of disease progression and, at later stages, are sensitive to treatment effects. The US Food and Drug Administration has recently indicated support for the potential utility of cognitive composite measures as outcome measures in AD trials conducted at the preclinical stage.17
We describe a composite cognitive performance measure, the Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite (ADCS-PACC). The ADCS-PACC is designed to serve as the primary outcome measure for trials conducted at the asymptomatic phase of AD. We describe, in particular, how the ADCS-PACC will be implemented in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s study (hereafter referred to as the A4 study), which is being conducted by the ADCS in partnership with Eli Lilly.18
Methods
The A4 Study Design
The A4 study will be a 168-week placebo-controlled “secondary prevention” trial of an anti-Aβ treatment, aimed at slowing cognitive decline in cognitively normal older individuals who have elevated brain amyloid levels (ie, “Aβ-positive” individuals), based on florbetapir PET amyloid imaging.18 The A4 study will include a natural history arm of “Aβ-negative” cognitively normal individuals followed up with longitudinal cognitive outcome measures collected at the same intervals. There are also 2 embedded substudies: (1) an ethics protocol to investigate the impact of disclosure of Aβ status and (2) a novel outcome instrument development protocol to optimize the detection of early decline over the course of preclinical AD.
Eligible participants will be 65 to 85 years of age at the time of screening, with a global Clinical Dementia Rating (CDR-G) score of 0, an MMSE score of 27 to 30, and a Delayed Recall score on the Logical Memory IIa subtest of 8 to 15 for participants with 13 or more years of education, or with an MMSE score of 25 to 30 and a Delayed Recall score on the Logical Memory IIa subtest of 6 to 13 for participants with 12 or less years of education. A study goal is to include approximately 20% of participants from underrepresented minority groups.
The antiamyloid intervention for the A4 study is solanezumab, a monoclonal antibody targeting the midsequence of monomeric Aβ; this treatment was selected by the consensus of a panel of experts advising the A4 study team. A total of 1000 Aβ-positive participants will be randomly assigned to solanezumab or placebo. Identifying these Aβ-positive participants will require screening approximately 3000 cognitively normal older individuals by use of florbetapir PET amyloid imaging. This screening process will provide an opportunity to collect plasma biomarkers and imaging and neuropsychological data on a large number of Aβ-negative individuals representing a well-characterized “gold standard” cognitively normal control group.
The ADCS-PACC
The primary objective of the A4 study is to test the hypothesis that solanezumab, administered as a 400-mg intravenous infusion every 4 weeks for 168 weeks, will slow cognitive decline compared with placebo in participants with preclinical AD. This objective will be assessed using a mixed model of repeated measures (MMRM) analysis of change in the ADCS-PACC score. The specific hypothesis of the A4 study is that there will be less of a decrease in the ADCS-PACC score at the end of the treatment period for participants treated with solanezumab than for participants treated with placebo.
Based on a review of the literature for cohort studies in “normal controls” who progressed to mild cognitive impairment or Alzheimer dementia, we determined that a composite measure sensitive to change in preclinical AD would likely require assessment of 3 key domains: episodic memory, executive function, and orientation. Previous studies19–21 have reported evidence that both list learning and paragraph recall (measures of episodic memory) tend to decline 7 to 10 years prior to the diagnosis of MCI or Alzheimer dementia. Recent data from amyloid imaging studies25–29 have reported a decline in multiple cognitive domains looking retrospectively at cognitive trajectories over 8 to 10 years prior to PET amyloid imaging22–24 and prospectively over 1- to 3-year longitudinal follow-up.
Based on this review, we propose a composite of 4 measures that are well established as showing sensitivity to decline in prodromal and mild dementia, and with sufficient range to detect early decline in the preclinical stages of the disease. The ADCS-PACC includes:
The Total Recall score from the Free and Cued Selective Reminding Test (FCSRT) (0–48 words),20,30
The Delayed Recall score on the Logical Memory IIa sub-test from the Wechsler Memory Scale (0–25 story units),31
The Digit Symbol Substitution Test score from the Wechsler Adult Intelligence Scale–Revised (0–93 symbols),32 and
The MMSE total score (0–30 points).33
The composite score is determined from its components using an established normalization method.34 Each of the 4 component change scores is divided by the baseline sample standard deviation of that component, to form standardized z scores. These z scores are summed to form the composite. Thus, a change of 1 baseline standard deviation on each component would correspond to a 4-point change on the composite. In the A4 study, the ADCS-PACC will be administered at baseline and at 24, 48, 72, 96, 120, 144, and 168 weeks, alternating between 3 test versions.
Sensitivity of the ADCS-PACC
The ideal outcome measure for the A4 study is one that is sensitive to decline that is specific to the Aβ-positive cognitively normal target population, as opposed to decline that is associated with aging. To estimate the rate of Aβ-mediated decline and inform the sample size justification for the A4 study, we examined several natural history data sets. With each data set, a group similar to the A4 study cognitively normal Aβ-positive target population is identified and compared longitudinally with a reference cognitively normal Aβ-negative population. Estimated group differences provide an upper bound on potential treatment effects in our target population. We also explore group differences between those who maintain a CDR-G score of 0 (“CDR-G stable”) vs those who progress from a CDR-G score of 0 to a worse score (“CDR-G pro-gressor”). These progression group differences provide a sense of the clinical interpretation of the composite.
Data Sets and Measures
AD Neuroimaging
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) has followed up with volunteers who were cognitively normal or who had varying degrees of cognitive impairment since 2005.35 The ADNI battery includes serial neuroimaging, CSF measures, other biomarkers, and clinical and neuropsychological assessments. For the present analysis, we analyze the subset of cognitively normal participants from the initial wave of ADNI with known CSF Aβ42 levels or Pittsburgh compound B (PiB) PET images. We classify these cognitively normal participants as Aβ-positive participants, with a PiB standardized uptake value ratio (SUVR) above 1.5 and a CSF Aβ42 level below 192 pg/mL, or as Aβ-negative participants, with a PiB SUVR below 1.5 and a CSF Aβ42 level above 192 pg/mL. If only 1 of the 2 Aβ measures is known, we use that measure for classification. Data were obtained from the ADNI database on June 7, 2013.
The ADNI battery does not include the FCSRT. In place of the FCRST, we use Delayed Word Recall from the Alzheimer’s Disease Assessment Scale–Cognitive Subscale36 to construct an approximation of the proposed ADCS-PACC. To more closely reflect the inclusion criteria for the A4 study, we exclude ADNI participants with Delayed Recall scores greater than 15 on the Logical Memory IIa subtest.
Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing
The Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing (AIBL) is a longitudinal biomarker cohort study,37 similar to ADNI. We used the same PiB threshold to determine Aβ positivity (PiB SUVR > 1.5). The AIBL battery also does not include the FCSRT, so we use delayed recall from List A of the California Verbal Learning Test38 to construct the composite in the analysis of AIBL data.
ADCS Prevention Instrument Study
The ADCS Prevention Instrument (ADCS-PI)study was a 4-year study of cognitively normal individuals 75 years of age or older to assess potential outcome measures for future prevention studies.16,30 The ADCS-PI study used New York University Paragraphs,39 instead of Logical Memory, and the Modified Mini-Mental State Examination,40 instead of the MMSE. The study data do not include CSF or PET measures of amyloid level. Therefore, as a proxy for Aβ status, we use the presence of at least 1 APOE-ɛ4 allele, although this is less predictive of decline than Aβ markers.26 We also compare participants who were CDR-G stable with those who were CDR-G progressors. This last group definition is based on post baseline progression data and is bound to demonstrate larger group differences than the other analyses based on baseline covariates only. However, this analysis of postbaseline progression puts the scale of the composite in perspective relative to clinically meaningful CDR-G change.
The ADNI, ADCS-PI, and AIBL studies were all approved by the institutional review boards of all of the participating institutions. Informed written consent was obtained from all participants at each site.
Sample Size Justification for the A4 Study
For each of the data sets and group comparisons already described, we apply an MMRM to estimate the key variance and covariance parameters that inform sample size calculations. The model includes effects for baseline ADCS-PACC score and age, which is known to be associated with Aβ accumulation in brain. The MMRM treats time as a categorical variable and estimates group differences at each visit while making no assumptions about the shape of trajectories. We use the Akaike information criterion41 to select the covariance structure between unstructured, compound symmetric, and autoregressive correlations of the order 1. From the final model, we report the difference between the at-risk population and the reference population at the final visit, which is typically the test statistic of primary interest in a clinical trial. We also report P values with an adjustment for simultaneous inference42 and area between the curves using the trapezoid rule.43 Power calculations assume an MMRM to estimate treatment effect at 36 months, 6-month visit intervals, 500 participants per group, 30% attrition, and a 5% α level. We use the formula by Lu et al44 implemented in the R package longpower45 to project the smallest detectable effect. The formula accommodates general attrition patterns. We assume that attrition accumulates linearly to an overall 30% attrition rate at 3 years with 5% worse attrition in the active arm. We report minimum detectable effects on the raw scale (eg, ADCS-PACC units) and as a percentage of the mean decline in the at-risk group (eg, Aβ-positive individuals, APOE-ɛ4 carriers, or CDR-G progressors). All analyses are conducted using R version 3.0.146 and the nlme47 and longpower45 packages. Graphics are produced using the ggplot2 package.48
Optimized Item Weights
We explored optimized reweighting of the ADCS-PACC components (see eAppendix in the Supplement for results). We fit Item Response Theory models49 to a training set composed of ADNI cognitively normal participants with unknown Aβ status to optimize the ADCS-PACC and also search for other items that might improve performance. We also reweighted the ADCS-PACC item z scores based on a logistic regression of AIBL Aβ statusand a Nelder-Mead optimization50 of MMRM power in terms of minimized detectable percentage of Aβ group difference. We also assessed the power of CDR Sum of Boxes and each of the ADCS-PACC items.
Results
Baseline Characteristics
Table 1 and Table 2 summarize baseline characteristics for each of the groups analyzed. In ADNI and AIBL, we see that the Aβ-positive groups are significantly older at baseline and have significantly higher percentages of APOE-ɛ4 carriers compared with the Aβ-negative groups. Not surprisingly in ADNI, the Aβ-positive groups also show significantly lower CSF Aβ42 levels, higher T-tau levels, higher PiB SUVRs, and smaller hippocampi than do the Aβ-negative groups. In AIBL, the Aβ-positive group shows more impairment on Digit Symbol Coding than does the Aβ-negative group. In the ADCS-PI study, the CDR-G progressor group demonstrated greater baseline impairment on the FCSRT and Modified Mini-Mental State Examination than did the CDR-G stable group, and the APOE-ɛ4 carriers were younger than the noncarriers.
Table 1.
Baseline Characteristics of Participants in ADNI and AIBL, by Aβ Statusa
| Characteristic | Participants With Available Data, No. | Aβ-Negative Participants | Aβ-Positive Participants | All | P Valueb |
|---|---|---|---|---|---|
| ADNI | |||||
| Total No. | 60 | 37 | 97 | ||
| Age, y | 97 | 74.80 (5.43) | 77.45 (4.74) | 75.81 (5.31) | .006 |
| Female sex | 97 | 30 (50) | 12 (32) | 42 (43) | .09 |
| Education, y | 97 | 15.17 (2.91) | 15.46 (3.22) | 15.28 (3.02) | .49 |
| APOE-ɛ4 alleles | 97 | ||||
| 0 | 53 (88) | 20 (54) | 73 (75) | <.001 | |
| 1 | 7 (12) | 16 (43) | 23 (24) | ||
| 2 | 0 (0) | 1 (3) | 1 (1) | ||
| Word List Delayed Recall score | 97 | 3.02 (1.65) | 3.24 (1.64) | 3.10 (1.64) | .48 |
| Logical Memory Delayed Recall score | 97 | 11.10 (2.56) | 11.35 (2.68) | 11.20 (2.59) | .62 |
| MMSE score | 97 | 28.83 (1.15) | 29.05 (1.05) | 28.92 (1.11) | .33 |
| Digit Symbol Substitution Test score | 97 | 45.60 (9.27) | 42.30 (8.49) | 44.34 (9.08) | .09 |
| ADAS-Cog score | 97 | 9.96 (3.81) | 11.13 (4.12) | 10.41 (3.95) | .12 |
| CSFAβ42 level, pg/mL | 90 | 244.3 (27.2) | 144.9 (26.3) | 206.8 (55.4) | <.001 |
| CSF T-tau level, pg/mL | 90 | 61.2 (19.8) | 82.2 (35.8) | 69.2 (28.7) | .005 |
| PiB SUVR | 15 | 1.244 (0.101) | 1.900 (0.122) | 1.594 (0.356) | <.001 |
| FDG uptake, average intensity score | 52 | 6.500 (0.607) | 6.327 (0.703) | 6.430 (0.646) | .38 |
| CDR-SB score of 0.5 | 97 | 5 (8) | 1 (3) | 6 (6) | .26 |
| UCSF hippocampi, %ICV ×1000 | 87 | 485.7 (62.1) | 459.4 (50.0) | 476.3 (59.1) | .04 |
| UCSF ventricles, %ICV ×1000 | 87 | 1989 (980) | 2387 (878) | 2131 (959) | .02 |
| AIBL | |||||
| Total No. | 114 | 50 | 164 | ||
| Age, y | 164 | 69.75 (6.83) | 75.06 (6.91) | 71.37 (7.26) | <.001 |
| Female sex | 164 | 61 (54) | 26 (52) | 87 (53) | .86 |
| Education, y | 164 | 12.51 (2.53) | 12.27 (2.70) | 12.44 (2.58) | .49 |
| APOE-ɛ4 alleles | 164 | ||||
| 0 | 77 (68) | 17 (34) | 94 (57) | <.001 | |
| 1 | 34 (30) | 31 (62) | 65 (40) | ||
| 2 | 3 (3) | 2 (4) | 5 (3) | ||
| Word List Delayed Recall score | 164 | 11.95 (2.97) | 11.82 (3.16) | 11.91 (3.02) | .88 |
| Logical Memory Delayed Recall score | 162 | 11.87 (3.75) | 10.88 (4.14) | 11.57 (3.89) | .12 |
| MMSE score | 164 | 28.89 (1.20) | 28.68 (1.17) | 28.83 (1.19) | .20 |
| Digit Symbol Substitution Test score | 164 | 59.7 (13.2) | 55.5 (12.9) | 58.5 (13.2) | .05 |
Abbreviations: ADAS-Cog, Alzheimer’s Disease Assessment Scale–Cognitive Subscale; ADNI, Alzheimer’s Disease Neuroimaging Initiative; AIBL, Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; CDR-SB, Clinical Dementia Rating–Sum of Boxes; CSF, cerebrospinal fluid; FDG,18 fluorodeoxyglucose; MMSE, Mini-Mental State Examination; PiB, Pittsburgh compound B; SUVR, standardized uptake value ratio; UCSF, University of California, San Francisco; %ICV, percentage of intracranial volume.
All values are given as mean (SD) values or number (%) of participants, unless otherwise indicated.
Determined by use of the Wilcoxon test for continuous variables and the Pearson χ2 test for categorical variables.
Table 2.
Baseline Characteristics of Participants in the ADCS-PI Study, by Groupa
| Characteristic | Participants With Available Data, No. | Group of Participants | All (n = 505) |
P Valueb | |
|---|---|---|---|---|---|
| CDR-G Stable (n = 478) |
CDR-G Progressor (n = 27) |
||||
| Age, y | 505 | 79.36 (3.60) | 80.52 (3.82) | 79.42 (3.62) | .08 |
| Female sex | 505 | 286 (60) | 19 (70) | 305 (60) | .28 |
| Education, y | 505 | 15.05 (2.95) | 14.15 (3.46) | 15.00 (2.98) | .18 |
| APOE-ɛ4 alleles | 305 | ||||
| 0 | 223 (77) | 13 (76) | 236 (77) | .93 | |
| 1 | 63 (22) | 4 (24) | 67 (22) | ||
| 2 | 2 (1) | 0 (0) | 2 (1) | ||
| FCSRT Total Free Recall score | 504 | 28.97 (5.53) | 25.37 (5.99) | 28.77 (5.61) | .01 |
| FCSRT Total Recall score | 504 | 47.851 (0.464) | 47.556 (0.847) | 47.835 (0.495) | .005 |
| 3MSE score | 505 | 95.86 (3.37) | 92.04 (4.10) | 95.66 (3.51) | <.001 |
| CDR-SB score of 0.5 | 505 | 90 (19) | 8 (30) | 98 (19) | .17 |
| Digit Symbol Substitution Test score | 495 | 42.1 (11.8) | 34.2 (10.5) | 41.7 (11.9) | <.001 |
| NYU Paragraph Recall score | 497 | 7.45 (2.80) | 5.44 (2.39) | 7.34 (2.81) | <.001 |
|
APOE-ɛ4 Noncarrier (n = 310) |
APOE-ɛ4 Carrier (n = 103) |
All (n = 413) |
|||
| Age, y | 413 | 79.73 (3.63) | 78.58 (3.15) | 79.44 (3.55) | .002 |
| Female sex | 413 | 170 (55) | 59 (57) | 229 (55) | .67 |
| Education, y | 413 | 14.94 (3.33) | 15.38 (2.76) | 15.05 (3.20) | .43 |
| APOE-ɛ4 alleles | 413 | ||||
| 0 | 310 (100%) | 0 (0%) | 310 (75%) | <.001 | |
| 1 | 0 (0) | 99 (96) | 99 (24) | ||
| 2 | 0 (0) | 4 (4) | 4 (1) | ||
| FCSRT Total Free Recall score | 412 | 27.99 (6.01) | 28.92 (5.71) | 28.22 (5.94) | .06 |
| FCSRT Total Recall score | 412 | 47.803 (0.554) | 47.853 (0.515) | 47.816 (0.545) | .24 |
| CDR-SB score | 413 | 0.332 (0.507) | 0.403 (0.560) | 0.350 (0.521) | .28 |
| 3MSE score | 413 | 95.20 (3.88) | 95.85 (3.15) | 95.37 (3.72) | .27 |
| Digit Symbol Substitution Test score | 411 | 40.7 (12.1) | 40.4 (11.9) | 40.6 (12.0) | .79 |
| NYU Paragraph Recall score | 413 | 7.14 (2.82) | 7.23 (2.72) | 7.16 (2.80) | .65 |
Abbreviations: ADCS-PI, Alzheimer’s Disease Cooperative Study Prevention Instrument; CDR-G progressor, global Clinical Dementia Rating score from 0 to worse score; CDR-G stable, global CDR score of 0; CDR-SB, Clinical Dementia Rating–Sum of Boxes; FCSRT, Free and Cued Selective Reminding Test; NYU, New York University; 3MSE, Modified Mini-Mental State Examination.
All values are given as mean (SD) values or number (%) of participants, unless otherwise indicated.
Determined by use of the Wilcoxon test for continuous variables and the Pearson χ2 test for categorical variables.
Longitudinal Analysis of the ADCS-PACC
The Figure, Table 3, and Table 4 summarize the change in the ADCS-PACC scores over time as estimated by the MMRM, controlling for baseline ADCS-PACC score and age. The Akaike information criterion selected the compound symmetric correlation over the other correlation structures considered. In ADNI, there was significant separation of the Aβ groups at 24 months but a reconvergence of the trajectories at 36 months. The mean (SE) area between the curves is −26.4 (13.6) (P = .05). In AIBL, we see consistent significant separation at both month 18 and month 36 and area between curves. In the ADCS-PI study CDR-G stable vs progressor analysis, we see highly significant (P < .001) separation at months 12, 24, and 36 and area between curves. In the ADCS-PI study APOE-ɛ4 carriers vs noncarriers analysis, we see significant separation at months 24 and 36 and significant area between the curves.
Figure. MMRM Estimates of Composite Change From Baseline in the ADCS-PACC.
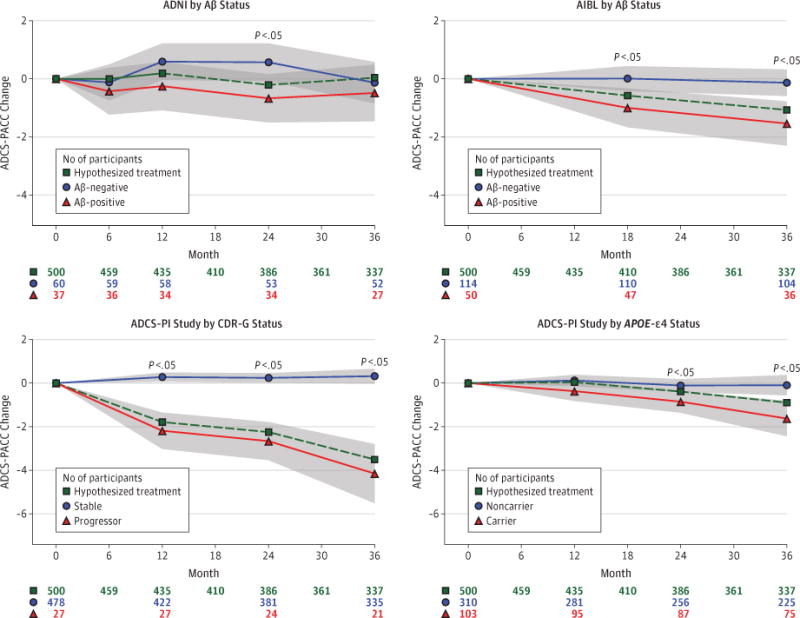
The models assume heterogeneous compound symmetric covariance structure, which allows for a different variance per visit and for a single correlation parameter. Age and composite score at baseline are included as covariates. The dashed line indicates the hypothesized minimum treatment benefit that can be detected with 80% power, a 5% α level, and the indicated sample size and attrition. The shaded regions depict 95% CIs. Group differences are significant at P < .05. ADCS-PACC indicates Alzheimer’s Disease Cooperative Study Preclinical Alzheimer Cognitive Composite; AIBL, Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; CDR-G, global Clinical Dementia Rating; MMRM, mixed model of repeated measures; and PI, Prevention Instrument.
Table 3.
MMRM Estimates of Composite Change From Baseline, by Studya
| Month | Group | Participants, No. | Estimate (SE) | P Value | Adjusted P Valueb | 95% CI | σ Valuec | ρ Valued |
|---|---|---|---|---|---|---|---|---|
| ADNI Aβ+ (n = 36)vs Aβ− (n = 59) | ||||||||
| 6 | Aβ− | 59 | −0.133 (0.310) | .67 | −0.740 to 0.473 | |||
| Aβ+ | 36 | −0.439 (0.401) | .28 | −1.225 to 0.348 | ||||
| Difference | −0.306 (0.503) | .54 | .94 | −1.291 to 0.679 | 2.327 | |||
| 12 | Aβ− | 58 | 0.581 (0.315) | .07 | −0.037 to 1.199 | |||
| Aβ+ | 34 | −0.263 (0.414) | .53 | −1.075 to 0.549 | ||||
| Difference | −0.844 (0.516) | .10 | .30 | −1.857 to 0.168 | 2.358 | |||
| 24 | Aβ− | 53 | 0.558 (0.325) | .09 | −0.080 to 1.196 | |||
| Aβ+ | 34 | −0.681 (0.415) | .10 | −1.494 to 0.132 | ||||
| Difference | −1.239 (0.522) | .02 | .06 | −2.263 to −0.215 | 2.361 | |||
| 36 | Aβ− | 52 | −0.145 (0.356) | .68 | −0.843 to 0.553 | |||
| Aβ+ | 27 | −0.497 (0.487) | .31 | −1.451 to 0.457 | ||||
| Difference | −0.352 (0.599) | .56 | .94 | −1.527 to 0.823 | 2.578 | 0.459 | ||
| Area between curves | −26.4 (13.6) | .05 | ||||||
| AIBL Aβ+ (n = 47) vs Aβ− (n = 110) | ||||||||
| 18 | Aβ− | 110 | 0.009 (0.215) | .97 | −0.412 to 0.429 | |||
| Aβ+ | 47 | −1.000 (0.334) | .003 | −1.655 to −0.345 | ||||
| Difference | −1.009 (0.406) | .01 | .02 | −1.805 to −0.213 | 2.213 | |||
| 36 | Aβ− | 104 | −0.134 (0.229) | .56 | −0.583 to 0.315 | |||
| Aβ+ | 36 | −1.538 (0.381) | <.001 | −2.285 to −0.791 | ||||
| Difference | −1.404 (0.452) | .002 | .004 | −2.290 to −0.519 | 2.315 | 0.520 | ||
| Area between curves | −30.8 (10.1) | .002 | ||||||
Abbreviations: ADNI, Alzheimer’s Disease Neuroimaging Initiative; AIBL, Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; MMRM, mixed model of repeated measures;
, positive;
, negative.
The models assume heterogeneous compound symmetric covariance structure, which allows different variance parameters (σ) per visit, and a single correlation parameter (ρ).
Adjusted for model-based simultaneous inference.
Residual standard deviation estimate at each visit.
Estimated correlation between visits.
Table 4.
MMRM Estimates of Composite Change From Baseline, by ADCS-PI Study Groupa
| Month | Group | Participants, No. | Estimate (SE) | P Value | Adjusted P Valueb | 95% CI | σ Valuec | ρ Valued |
|---|---|---|---|---|---|---|---|---|
| CDR-G Progressor (n = 27) vs Stable(n = 422) | ||||||||
| 12 | Progressor | 27 | −2.187 (0.418) | <.001 | −3.006 to −1.367 | |||
| Stable | 422 | 0.276 (0.105) | .009 | 0.070–0.482 | ||||
| Difference | −2.463 (0.435) | <.001 | <.001 | −3.316 to −1.610 | 2.121 | |||
| 24 | Progressor | 24 | −2.661 (0.438) | <.001 | −3.519 to −1.804 | |||
| Stable | 381 | 0.238 (0.109) | .03 | 0.024–0.453 | ||||
| Difference | −2.899 (0.455) | <.001 | <.001 | −3.791 to −2.008 | 3.208 | |||
| 36 | Progressor | 21 | −4.153 (0.679) | <.001 | −5.484 to −2.823 | |||
| Stable | 335 | 0.318 (0.170) | .06 | −0.015 to 0.651 | ||||
| Difference | −4.471 (0.702) | <.001 | <.001 | −5.848 to −3.094 | 2.133 | 0.542 | ||
| Area between curves | −91.2 (12.4) | <.001 | ||||||
| APOE-ɛ4 Carrier (n = 95) vs Noncarrier (n = 281) | ||||||||
| 12 | Carrier | 95 | −0.370 (0.227) | .10 | −0.815 to 0.075 | |||
| Noncarrier | 281 | 0.117 (0.131) | .37 | −0.140 to 0.374 | ||||
| Difference | −0.487 (0.263) | .06 | .16 | −1.003 to 0.028 | 2.197 | |||
| 24 | Carrier | 87 | −0.854 (0.254) | .001 | −1.352 to −0.356 | |||
| Noncarrier | 256 | −0.112 (0.147) | .45 | −0.400 to 0.176 | ||||
| Difference | −0.742 (0.294) | .01 | .03 | −1.318 to −0.165 | 3.646 | |||
| 36 | Carrier | 75 | −1.628 (0.406) | <.001 | −2.423 to −0.833 | |||
| Noncarrier | 225 | −0.098 (0.234) | .68 | −0.557 to 0.362 | ||||
| Difference | −1.531 (0.469) | .001 | .003 | −2.450 to −0.612 | 2.388 | 0.547 | ||
| Area between curves | −23.9 (7.84) | .002 | ||||||
Abbreviations: ADCS-PI, Alzheimer’s Disease Cooperative Study Prevention Instrument; CDR-G progressor, global Clinical Dementia Rating score from 0 to worse score; CDR-G stable, global CDR score of 0; MMRM, mixed model of repeated measures.
The models assume heterogeneous compound symmetric covariance structure, which allows different variance parameters (σ) per visit, and a single correlation parameter (ρ).
Adjusted for model-based simultaneous inference.
Residual standard deviation estimate at each visit.
Estimated correlation between visits.
Minimum Detectable Treatment Effect on the ADCS-PACC
Based on the variance and correlation estimates in Tables 3 and 4, we can estimate the minimum treatment effect that can be found by assuming 80% to 90% power, a 5% α level (2-sided), 500 participants in each group, and 30% attrition. The Figure depicts the minimum detectable treatment effect for 80% power.
Using ADNI pilot estimates of variance and correlation (Table 3), we project a minimum treatment difference of Δ = 0.525 to 0.607 units for 80% to 90% power. This is larger than the observed Aβ group difference in ADNI at month 36 but is 0.525/1.239 = 42.4% to 0.607/1.239 = 49.0% of that difference at month 24. Similarly, using the AIBL pilot estimates (Table 3), we project Δ = 0.467 to 0.540 units, or 0.467/1.404 = 33.3% to 0.540/1.404 = 38.5% of the Aβ group difference at month 36. Based on estimates from the analysis of ADCS-PI study CDR-G stable vs progressor groups (Table 4), we project Δ = 0.654 to 0.746 units, or 0.654/4.471 = 14.6% to 0.746/4.471 = 16.7% of the group difference at month 36. Based on the analysis of ADCS-PI study APOE-ɛ4 carriers vs noncarriers (Table 4), we project Δ = 0.733 to 0.847 units, or 0.733/1.531 = 47.9% to 0.847/1.531 = 55.3% of the month 36 group difference. Again, the Figure graphically represents these smallest detectable treatment effects.
Discussion
Our analyses demonstrate consistent evidence that Aβ-positive cognitively normal participants demonstrate greater cognitive decline than do Aβ-negative participants on a composite of verbal list learning, paragraph recall, timed executive function, and global cognition. Moreover, we found that decline on this composite was robust across cohorts, regardless of the exact measures used;however, in ADNI, we did not see significant changes from baseline, and the amyloid group difference was only significant at month 24. The inconsistencies between the various studies used in our retrospective analysis also present some limitations. The particular tests that comprised each study’s entire battery, and their order of presentation, varied from study to study. In addition, none of the studies analyzed were treatment trials. Owing to these factors, the ADCS-PACC may behave differently in the A4 study.
These limitations not with standing, we project that the A4 study has about 80% power to detect a treatment benefit of 0.5 ADCS-PACC units over 3 years. A quarter standard deviation change in each component of the ADCS-PACC equates to a1-point change in the ADCS-PACC total score. The ADCS-PACC is standardized according to the baseline distribution of 4 instruments with established face validity in more impaired populations. We believe 0.5 ADCS-PACC units is small enough to be a realistically attainable, yet large enough to suggest benefit to patients, including a reduction in later clinical deterioration.
The Item Response Theory approach applied to ADCS-PACC items did not improve power in ADNI, although a model with 16 items did achieve more consistent decline and Aβ group separation in ADNI (eFigure and eTable in the Supplement). The logistic regression approach decreased the smallest detectable effect (percentage of Aβ group difference) at 80% power by 6.5% when applied to same AIBL data that were used to obtain the weights. The weighting favored list and paragraph recall over MMSE and Digit Symbol Substitution. However, when these weights were applied to the other studies, it performed poorly. The smallest possible effect size was only 1.5% smaller than the logistic regression weights, and this required weighting Digit Symbol Substitution in the wrong direction. We have concerns about the validity of optimized weighting, particularly given that there is no information about treatment response for these items in the target population. It is conceivable, for example, that we would down-weight a particular item that would respond to treatment, but we have no information with which to assess this risk. At this point, we do not find strong evidence to support unequal weighting of the ADCS-PACC items.
Ideally, the A4 study would be powered to detect a clinically meaningful effect. The term clinically meaningful effect is somewhat nebulous but presumably indicates an effect on symptoms of importance to the treated individual. In a 3-year study in the clinically normal target population for the A4 study, we will not necessarily observe the emergence of functional impairment seen in late mild cognitive impairment and dementia. However, because a composite measure of memory, orientation, and executive function has face validity as an indicator of AD-related clinical progression, the recent US Food and Drug Administration draft guidance17,51 suggests that such a measure may serve as a primary outcome measure for the purpose of accelerated approval, with clinical meaningfulness supported by postmarketing study.
The A4 study will include a number of secondary and exploratory measures to inform interpretation of the treatment effect on the primary measure. These include molecular, structural, and functional neuroimaging measures, CSF biochemical markers, and patient- and informant-reported measures of perceived global and specific cognitive function. Experience with such measures in longitudinal studies in the preclinical AD population is limited, and their sensitivity to treatment effects is unknown. However, they may clarify not only the pathophysiological impact of the antiamyloid intervention but also the implications of the cognitive effects.
Conclusions
The concept of preclinical AD, a stage of amyloid-mediated neurodegeneration before the emergence of clinical symptoms,1,8 represents an attractive target for disease-modifying intervention in AD. The relationship of longitudinal change in the ADCS-PACC to the presence of amyloid plaques in the brains of asymptomatic older individuals supports the notion that this measure may be useful in establishing favorable treatment effects. While much remains to be learned about preclinical AD, the enormity of the need for effective therapy requires the rapid initiation of trials. Presumably, the A4 study and other very early interventional studies will further elucidate the trajectory of cognitive decline during the preclinical stages of AD and facilitate the successful development of disease-modifying treatments.
Supplementary Material
Acknowledgments
Funding/Support: Dr Donohue was supported by National Institute on Aging grant U01-AG10483 (principal investigator) funded by the National Institutes of Health. Dr Donohue was funded by grant 1KL2RR031978 from the National Institutes of Health and was supported by the generous contributions from Abbott; AC Immune; the Alzheimer’s Association; the Alzheimer’s Diseases Research Center; the University of California, San Diego; the Alzheimer’s Drug Discovery Foundation; Amorfix Life Sciences; Araclon; AstraZeneca; Avid Radiopharmaceuticals; Bayer HealthCare; BioClinica; Biogen Idec; Bristol-Myers Squibb; Eisai; Elan; Eli Lilly; F. Hoffmann–La Roche and its affiliated company Genentech; GE Healthcare; Innogenetics NV; IXICO; Janssen Alzheimer Immunotherapy Research and Development, LLC; Johnson & Johnson Pharmaceutical Research and Development LLC; Kenes, International; Medpace; Merck; Meso Scale Diagnostics, LLC; National Brain Research Center, India, for Johns Hopkins Medicine; New York Academy of Sciences; Novartis; Pfizer; sanofi-aventis; Servier; Smartfish AS; Synarc; Takeda; and the University of Californa, Los Angeles.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Group Information: A list of the researchers of the Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing (AIBL) are provided at http://aibl.csiro.au/. A complete listing of the investigators of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. A list of the investigators of the Alzheimer’s Disease Cooperative Study (ADCS) are provided at http://adcs.org/.
Author Contributions: Dr Donohue had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Donohue, Sperling, Salmon, Raman, Aisen.
Acquisition, analysis, or interpretation of data: Donohue, Sperling, Rentz, Thomas, Weiner, Aisen.
Drafting of the manuscript: Donohue, Sperling.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Donohue, Aisen.
Obtained funding: Weiner, Aisen.
Administrative, technical, or material support: Salmon, Thomas, Aisen.
Study supervision: Sperling, Rentz, Weiner, Aisen.
Conflict of Interest Disclosures: Drs Donohue and Salmon are consultants for Bristol-Myers Squibb. Dr Aisen serves on a scientific advisory board for NeuroPhage; has served as a consultant to Elan, Wyeth, Eisai, Schering-Plough, Bristol-Myers Squibb, Eli Lilly, NeuroPhage, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis, Bayer, Astellas, Dainippon, Biomarin, Solvay, Otsuka, Daiichi, AstraZeneca, Janssen, Medivation, Ichor, Toyama, Lundbeck, Biogen Idec, iPerian, Probiodrug, Somaxon, Biotie, Cardeus, Anavex, Kyowa Hakko Kirin Pharma, and Medtronic; and receives research support from Eli Lilly. Dr Sperling is funded by Boehringer-Ingelheim, Bristol-Myers Squibb, Eisai, Genentech, Janssen, Lundbeck, Merck, and Roche.
Additional Information: Data used in the preparation of this article were obtained from AIBL and were made available at the ADNI database. The AIBL researchers contributed data but did not participate in the analysis or writing of this report. The investigators within ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the ADCS at the University of California, San Diego. The Laboratory for Neuroimaging at the University of Southern California disseminates ADNI data (http://www.loni.usc.edu). The data collection and sharing for this project were funded by ADNI. The Canadian Institutes of Health Research are providing funds to support ADNI clinical sites in Canada. For AIBL, the design and conduct of the study and the collection and management of the data are funded by the Commonwealth Scientific and Industrial Research Organisation. For the ADCS-PI study, the design and conduct of study; the collection and management of the data; the analysis and interpretation of the data; and the preparation, review, and approval of the manuscript were supported by the National Institute on Aging/National Institutes.
Additional Contributions: We would like to acknowledge the invaluable contributions of our ADCS and ADNI collaborators, coinvestigators, volunteers, and their families, none of whom were financially compensated for their work.
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman RJ, Xiong C, Benzinger TL, et al. Dominantly Inherited Alzheimer Network Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, Burnham S, Bourgeat P, et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 4.Donohue MC, Jacqmin-Gadda H, Le Goff M, et al. Alzheimer’s Disease Neuroimaging Initiative Estimating long-term multivariate progression from short-term data [published online March 24, 2014] Alzheimers Dement. doi: 10.1016/j.jalz.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 6.Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology. 2011;76(3):280–286. doi: 10.1212/WNL.0b013e318207b1b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3(111):111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Barkhof F, Bernstein MA, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement. 2011;7(4):474–485e4. doi: 10.1016/j.jalz.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiman EM, Alzheimer’s Disease Biomarkers Working Group for the Alliance for Aging Research Fluorodeoxyglucose positron emission tomography: emerging roles in the evaluation of putative Alzheimer’s disease-modifying treatments. Neurobiol Aging. 2011;32(suppl 1):S44–S47. doi: 10.1016/j.neurobiolaging.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9(4):363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 12.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69(2):198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JL. Biomarkers in Alzheimer’s disease drug development. Alzheimers Dement. 2011;7(3):e13–e44. doi: 10.1016/j.jalz.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Fox NC, Black RS, Gilman S, et al. AN1792(QS-21)-201 Study Effects of Aβ immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64(9):1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 15.Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64(5):492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 16.Salmon DP, Ferris SH, Thomas RG, et al. Age and apolipoprotein E genotype influence rate of cognitive decline in nondemented elderly. Neuropsychology. 2013;27(4):391–401. doi: 10.1037/a0032707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Dept of Health and Human Services; US Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for industry: Alzheimer’s disease: developing drugs for the treatment of early stage disease (draft guidance) http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338287.pdf. Published February 2013. Accessed April 28, 2014.
- 18.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: Stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):28fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of Alzheimer disease: A 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57(6):808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- 20.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derby CA, Burns LC, Wang C, et al. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80(14):1307–1314. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74(10):807–815. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landau SM, Mintun MA, Joshi AD, et al. Alzheimer’s Disease Neuroimaging Initiative Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72(4):578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with β-amyloid deposition in the oldest-old without dementia. Neurology. 2013;80(15):1378–1384. doi: 10.1212/WNL.0b013e31828c2fc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim YY, Ellis KA, Pietrzak RH, et al. AIBL Research Group Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79(16):1645–1652. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]
- 27.Knopman DS, Jack CR, Jr, Wiste HJ, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–1582. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doraiswamy PM, Sperling RA, Coleman RE, et al. AV45-A11 Study Group Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study. Neurology. 2012;79(16):1636–1644. doi: 10.1212/WNL.0b013e3182661f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawas CH, Greenia DE, Bullain SS, et al. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimers Dement. 2013;9(2):199–203. doi: 10.1016/j.jalz.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris SH, Aisen PS, Cummings J, et al. Alzheimer’s Disease Cooperative Study Group ADCS Prevention Instrument Project: overview and initial results. Alzheimer Dis Assoc Disord. 2006;20(4 suppl 3):S109–S123. doi: 10.1097/01.wad.0000213870.40300.21. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. WMS-R: Wechsler Memory Scale–Revised: Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 32.Wechsler D. Wechsler Adult Intelligence Scale–Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 34.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope: the Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 37.Ellis KA, Bush AI, Darby D, et al. AIBL Research Group The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr. 2009;21(4):672–687. doi: 10.1017/S1041610209009405. [DOI] [PubMed] [Google Scholar]
- 38.Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 39.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12(4):168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 40.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 41.Akaike H. Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Second International Symposium on Information Theory. Budapest, Hungary: Akadémiai Kiadó; 1973. pp. 267–281. [Google Scholar]
- 42.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50(3):346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 43.Wilding GE, Chandrasekhar R, Hutson AD. A new linear model-based approach for inferences about the mean area under the curve. Stat Med. 2012;31(28):3563–3578. doi: 10.1002/sim.5387. [DOI] [PubMed] [Google Scholar]
- 44.Lu K, Luo X, Chen PY. Sample size estimation for repeated measures analysis in randomized clinical trials with missing data. Int J Biostat. 2008;4(1):9. doi: 10.2202/1557-4679.1098. [DOI] [PubMed] [Google Scholar]
- 45.Donohue MC, Gamst AC, Edland SD. longpower: sample size calculation for longitudinal data. http://www.cran.r-project.org/web/packages/longpower/index.html. Published September 21, 2013. Accessed May 1, 2014.
- 46.The Comprehensive R Archive Network. http://www.cran.r-project.org/. Accessed May 1, 2014.
- 47.Pinheiro J, Bates D, DebRoy S, Sarkar D, editors. nlme: linear and nonlinear mixed effects models. http://www.cran.r-project.org/web/packages/nlme/index.html. Published March 31, 2014. Accessed May 1, 2014.
- 48.Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York, NY: Springer; 2009. [Google Scholar]
- 49.Rizopoulos D. ltm: an R package for latent variable modeling and item response analyses. J Stat Softw. 2006;17(5):1–25. http://www.jstatsoft.org/v17/i05/. Accessed May 1, 2014. [Google Scholar]
- 50.Nelder JA, Mead R. A simplex method for function minimization. Comput J. 1965;7(4):308–313. doi: 10.1093/comjnl/7.4.308. [DOI] [Google Scholar]
- 51.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. N Engl J Med. 2013;368(13):1169–1171. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


