Abstract
Qualitative Case Studies Were conducted at seven international sites conducting HIV prevention research in Africa, Asia, and the Americas to identify strategies for ensuring that health needs of research participants identified in the course of research are adequately addressed. Key factors were identified that contribute to the balance between direct care and healthcare referrals at a research site, as well as the overall quality of the healthcare made available to research participants. The case studies exemplify the concept of “moral negotiation” in research (Weijer & LeBlanc, 2006), that is, a process where researchers and sponsors negotiate with increasingly empowered local communities and host countries to achieve meaningful and substantive benefits from biomedical research for all stakeholders.
Keywords: HIV prevention, clinical trials, ancillary care, health care, partnership
Every Day More Than 7400 People are infected with HIV (UNAIDS, 2008). The factors that contribute to this infection rate are complex and include behavioral, social, political, and economic dimensions. Research to identify effective interventions and to translate them into effective programs is necessary but also expensive and time-consuming. This is especially true for biomedical interventions including circumcision, vaccines, microbicides, and the use of antiretrovirals pre- and post-exposure to prevent transmission via perinatal, sexual, and parenteral means. Each year tens of thousands of people participate in such research globally.
HIV travels with a broad array of other health threats, a phenomenon that has been described as a syndemic (Singer & Clair, 2003; Milstein, 2008; Stall et al., 2003). As a result, HIV prevention research tends to identify a wide range of health needs among study participants, including family planning, pregnancy, sexually transmitted infections (STIs), alcohol and drug abuse, and domestic violence as well as HIV infection. Since poverty is also often a part of the global HIV syndemic, many HIV research participants tend to have a wide range of other chronic and acute health needs that are not addressed because they lack the personal resources needed to access appropriate care and treatment.
There are two ways that researchers can address health needs identified in the course of research: through direct provision of care or through referrals to hospitals, clinics, or other organizations. In general, HIV prevention research includes some combination of direct care and referrals. For example, in trials evaluating interventions to prevent mother-to-child transmission, the research clinicians may have advanced training in the treatment of HIV infection but they will nonetheless make referrals for conditions outside their expertise such as cervical cancer. They may also lack the resources for laboratory tests and medications necessary for effective treatment of HIV, regardless of their expertise. In a microbicide trial enrolling uninfected and largely healthy women, clinical expertise is likely to be limited to a set of easily treated ailments such as bacterial STIs and malaria while referrals will be needed for HIV infection as well as other specialized health needs.
Direct provision of healthcare to research participants in prevention research is generally a viable option when the need is acute rather than chronic, the per-participant cost associated with a specific health need is small, and the care is not highly specialized. For example, treatment for bacterial STIs can be easily addressed. However, for chronic health problems and for more complex acute health needs, the medical resources needed, the challenges of sustainability, and the cumulative costs of direct treatment can quickly become prohibitive. There may be hidden societal costs as well. Direct care provision requires the hiring and training of skilled medical personnel, often in settings where severe staffing shortages exist in community health facilities. Increasing the capacity of the research staff to provide medical care may contribute to the “brain drain” of critically needed staff from the public health sector in such situations (Marchal & Kegels, 2003; Kupfer et al., 2004).
The alternative to direct care is referral to local health services. But in the countries hardest hit by HIV, where most HIV prevention trials take place, participants may be referred to a poor and overburdened health infrastructure that is not equipped to address their needs (London, 2005).
The challenges associated with both direct provision of care and referral to local health services are brought into sharp relief by HIV infection, which is the primary outcome measure for biomedical HIV prevention trials. The health needs of people living with HIV extend well beyond the life of a clinical trial—potentially decades beyond. Logically, sustainability and continuity of care are best ensured if participants can be linked to appropriate healthcare in their local communities. Yet despite efforts to expand global access to antiretroviral treatment for HIV, in 2006 only one-fifth of those in clinical need of the drugs in low- and middle-income countries were able to receive them (Merson, 2006). Consensus now exists that HIV treatment arrangements need to be defined during protocol design for HIV prevention trials (UNAIDS, 2006; UNAIDS-WHO, 2007) but many researchers and other stakeholders confront difficult challenges in meeting participants’ needs without undermining local health systems, jeopardizing the conduct of trials, or detracting from the goal of identifying critically needed new HIV prevention technologies.
The experience of established research sites is a valuable but largely untapped resource for addressing these challenges of meeting the health needs of research participants in resource-poor settings. The Partnering for Care Project was undertaken to document the variety of healthcare partnerships in the HIV Prevention Trials Network (HPTN) research sites and the strategies developed to meet the health needs of research participants. HPTN is a worldwide collaborative clinical trials network that develops and tests the safety and efficacy of primarily non-vaccine interventions designed to prevent the transmission of HIV. Established in 1999 and refunded in 2006 by the Division of AIDS (DAIDS) of the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), the HPTN carries out its mission through a network of expert scientists and investigators from more than two dozen international sites partnered with a leadership group comprised of three U.S.-based institutions.
Methods
This study was reviewed by Family Health International’s institutional review board and determined to be exempt under 45 CFR 46.
A brief e-mail survey was sent to principal investigators and study coordinators at all HPTN sites in summer 2004 (33 sites). Respondents were asked, “Do you have any partnerships, collaborations, agreements, or other efforts in place at your site to facilitate the ability of HPTN research participants to access health care?” (Yes or No) and, if their answer was yes, who to contact for further information about such partnerships at the site. At least one follow-up e-mail was sent to non-responders. Twenty-five responses were received, providing information on 22 research sites. Of these, 20 sites noted one or more partnerships. No survey was returned for 11 sites.
In June 2005, a second e-mail survey (Survey 2) was sent to investigators and study coordinators at 17 eligible sites, defined as those who responded to Survey 1 and with an active or pending HPTN protocol between May 2004 and May 2005. “No” responders to Survey 1 were asked to complete an abbreviated survey focusing on healthcare referral options and regulatory requirements or policies regarding provision of care. Survey 1 “Yes” responders were asked the same questions plus others describing their partnerships. Follow-up phone calls and e-mails were conducted in order to encourage survey completion. During follow-up, one site became ineligible when it was dropped from the HPTN. As of December 2005, completed surveys were obtained from each of the 16 remaining eligible research sites (Table 1). Four criteria were then established for selecting a subset of sites for in-depth case study analysis:
TABLE 1.
HPTN Respondents to Survey 2.
| Research Site | Place of Central Coordination | Performance Sites |
|---|---|---|
| Kampala, Uganda | MU/JHU Research House, Mulago Hospital/Makerere University | MU/JHU Research House, Mulago Hospital |
| Chiang Mai, Thailand | Research Institute for Health Sciences (RIHES) | Research Institute for Health Sciences (RIHES) |
| Rio de Janeiro, Brazil | FIOCRUZ | Fiocruz; Nova Iguacu General Hospital; Servidores do Estado Hospital |
| Porto Alegre, Brazil | FIOCRUZ | Hospital Nossa Senhora da Conceicao |
| Lusaka, Zambia | CIDRZ | George, Matero, Kamwala, and Chilenje Clinics |
| Hlabisa, South Africa | Medical Research Council | Medical Research Council |
| Durban, South Africa | Medical Reseach Council | RK Khan Hospital, Chatsworth |
| Philadelphia, PA, USA | University of Pennsylvania HIV Prevention Research Unit | Market St. Office and RAP office |
| Dar es Salaam, Tanzania | Harvard-MUCHS Collaborative Research Projects Building | Muhimbili Medical Center (Makuti clinic) and Temeke District Hospital |
| Lilongwe, Malawi | UNC Project | Kamuzu Central Hospital multiple performance sites |
| Pune, India | NARI | |
| Chennai, India | YRGCare | YRGCare |
| Miraflores-Lima, Peru | Asociacion Civil Impacta Salud y Educacion | Asociacion Civil Impacta Salud y Educacion |
| Pucallpa, Peru | Asociacion Civil Impacta Salud y Educacion | Asociacion Civil Cayetano heredia (Centro Medico Cayetano) |
| Blantyre, Malawi | JH/Malawi COM Project | Queen Elizabeth Central Hospital |
| Harare, Zimbabwe | UZ/UCSF Collaborative Research Programme | 7 performance sites |
Unique aspects regarding referral systems, referral follow-up, and capacity building
Geographic diversity with at least one site each from Africa, Asia, Latin America, and the U.S.
Adequacy of detail provided in the survey and in follow-up e-mails and phone calls
Willingness on the part of the site research team to be part of a case study
Based on these criteria and in consultation with investigators responding to Survey 2, seven case study sites were selected (Table 2). Between March and May 2006, visits were made to the selected research sites. Through on-site observations and interviews with study site staff, referral site staff, and community advisory board (CAB) members, the assessment team chronicled the efforts of each site in providing direct care to participants and developing referral systems with healthcare partners, explored how partnerships between the study and referral sites were maintained, and described the strengths and challenges of developing and maintaining healthcare partnerships to benefit research participants. A minimum of five days was spent at each site, and additional information was sought as needed at HPTN meetings and through follow-up phone calls and e-mails.
TABLE 2.
Characteristics of HPTN Sites Participating as Case Studies.
| Site | Length of Time Site Has Been Active | Focus | Study Population of HPTN Studies | Type of Facility | Clinic Facilities |
|---|---|---|---|---|---|
| MU-JHU Research House, Kampala, Uganda | 18 years | Research and care programs | HIV positive mothers and HIV negative infants | University-based | Study clinics on-site; most care provided on-site or at nearby hospital |
| University of Pennsylvania, Philadelphia, USA | 17 years | Research | Women at risk for HIV; IDUs and members of their sex and drug networks | University-based | One study clinic on-site; one study clinic off-site; no care provided on-site |
| MRC, Durban, South Africa | 37 years (since 1969) | Research | Women at risk for HIV | Government-sponsored but autonomous | Study clinic off-site; only FP counseling provided on-site |
| FIOCRUZ, Rio de Janiero, Brazil | Unknown | Research and care programs | Serodiscordant couples | Government-sponsored | 1 study clinic on-site (at FIOCRUZ) and 2 off-site (within hospitals); most care provided on-site or within hospitals in which clinics located |
| UZ-UCSF Collaborative Research Programme, Harare, Zimbabwe | Unknown | Research | High risk HIV-negative, HSV-2 positive women and men who have sex with men; women at risk for HIV; serodiscordant couples; HIV positive mothers and HIV negative infants | University-based | 1 study clinic on-site and other clinics off-site |
| NARI, Pune, India | 14 years | Research and care programs | Serodiscordant couples | Government-sponsored | 1 study clinic on-site and the others off-site; some care provided on-site or within hospitals in which clinics are located |
| UNC Project, Tidziwe Centre, Lilongwe, Malawi | 7 years | Research and care programs | Women at risk for HIV; serodiscordant couples | University-based | Study clinics on-site; most care provided on-site or at nearby hospital |
Results
E-mail Survey
Considerable variability was seen in the types of policies and requirements in place at the 16 sites responding to Survey 2 as of December 2005 (Table 3). The implications of a policy or requirement were also likely to vary from site to site. For example, more than a third of the sites reported regulatory requirements to provide the local government standard of care to research participants. Those standards, in turn, could result in different types of care for participants at different sites.
TABLE 3.
Policies and Other Requirements Regarding Provision of Health Care for Research Participants Reported by Survey 2 Respondents (n = 16 sites).
| Type of Policy or Requirement | Number of Sites Reporting Policy or Requirement | Examples of Policies or Requirements |
|---|---|---|
| Regulatory requirements | 9 | Researchers required to provide the government standard of care to research participants (Chiang Mai, Porto Alegre, Rio de Janeiro, Lilongwe, Blantyre, Dar es Salaam) Researchers are required to include information about care for research participants in the informed consent (Chiang Mai, Rio de Janeiro) Informed consent must include information on types of indemnity to cover possible injury resulting from the research (Rio de Janeiro) |
| Institutional Review Board or Ethics Committee policies | 12 | Care and treatment of participants with HIV required (Dar es Salaam) Researchers must provide information about the availability of referrals for participants who are screened out, seroconverters, and participants who become pregnant during a trial (Durban) Researchers encouraged to provide healthcare as part of their studies (Lusaka) Issues related to care and support are discussed with the lead investigators of each protocol reviewed (Pune) |
| Institutional and other policies | 9 | Provide support for care and treatment not available through the research site via networking and partnering with other government facilities (Pune) Standard operating procedures established for clinical management of study participants (Pune) Institutional policies developed in alignment with regulatory and/or IRB/EC policies (Rio de Janeiro, Blantyre, Dar es Salaam) Formal Care Plan developed for research participants (Durban) |
The sites also varied with regard to partnerships, collaborations, agreements, and other efforts aimed at facilitating the ability of HPTN research participants to access healthcare. Fourteen sites reported partnerships, with most established to facilitate participant referrals for care that the research site did not or could not provide. A few partnerships were made for technical or laboratory support. Some sites also reported the development of partnerships in order to recruit participants at the partner’s site.
Most of the partnerships stemmed from long-standing relationships between the research site and other organizations, some dating back to the 1980s. Personal contacts between research staff and providers in the community also were important for partnership development. A few sites described a formal process of establishing partnerships when the need arose. For example, a clinic or hospital may be identified as a potential partner and then a study staff member would go to the organization to make a presentation and ask for a formal partnership to be formed.
In a few sites, a staff member was hired specifically to build and sustain partnerships. This could be a Community Liaison Officer hired to facilitate the partnerships and visit referral organizations to see if the care that was agreed upon was being provided to participants. Or it could entail multiple employees such as community educators, health outreach workers, community coordinators, and health visitors who worked in the community or directly with the partner organizations. More commonly, however, the building and sustaining of partnerships was incorporated into existing research staff work. For example, project leadership may be responsible for maintaining partnerships with hospitals or clinical research staff may be expected to contribute time and effort for patient care at local hospitals or clinics.
Nine of the 14 sites reporting partnerships stated that they contributed resources to their partner organizations including staff time (n = 8), infrastructure or supplies (6), training (5), and funding (2). The amount of staff time provided varied from occasional help to an across-the-board requirement that clinical staff devote 20% of their time to partner hospitals and clinics. Examples of infrastructure and supplies provided included renovations to clinics, provision of lab equipment, supplying drugs when needed, installing a generator, providing a garden hose, and printing or copying materials for partners when needed. Examples of training topics included ethics, HIV voluntary counseling and testing, study procedures, and biohazard waste disposal. Two sites reported that they provided direct funding to support partner organizations, including funds for pap readings and Western blot kit readings, services provided by referral hospitals, honorariums for staff partners, and expenses for staff attending meetings.
Thirteen sites reported using referral sites, with most referral sites also listed as partner organizations. Problems reported with regard to referrals were few and included waiting lists and overcrowding (3 sites), lack of required medications (3 sites), unaccompanied participants getting lost in the system or not showing up (3 sites), and difficulty in locating clinic records (1 site). Several sites were located within a complex, center, or compound that housed multiple healthcare facilities where participants were referred. Others reported at least one referral site within walking distance, and several had partnerships with clinics throughout the cities where they were located which helped participants to access care close to where they lived.
The number of referral sites reported ranged from 1 to 7, with a total of 40 referral sites described. Of these, 23 were open during business hours 4 to 5 days a week; 8 were open 24 hours a day, 7 days a week; and 4 had emergency and in-patient care available at any time combined with outpatient care during business hours 4 to 5 days a week. Of sites without 24/7 accessibility, 10 had weekend and/or evening hours at least one day a week. Staff at all 13 sites using referrals reported they did some form of follow-up. Two sites reported that staff accompanied participants to a referral site. A number of sites reported following up with participants at their next study visit to find out if they had gone to the referral and what their experience was like. Two sites reported that follow-up was facilitated by research staff who also worked in the referral site, as well as by close proximity of the research and care facilities. Two sites reported using referral slips to track follow-up but with mixed success. For almost all referral sites, someone from the research staff had made a visit in the six months preceding the survey. Six sites reported at least one community advisory board member who was affiliated with a referral site.
Case Studies
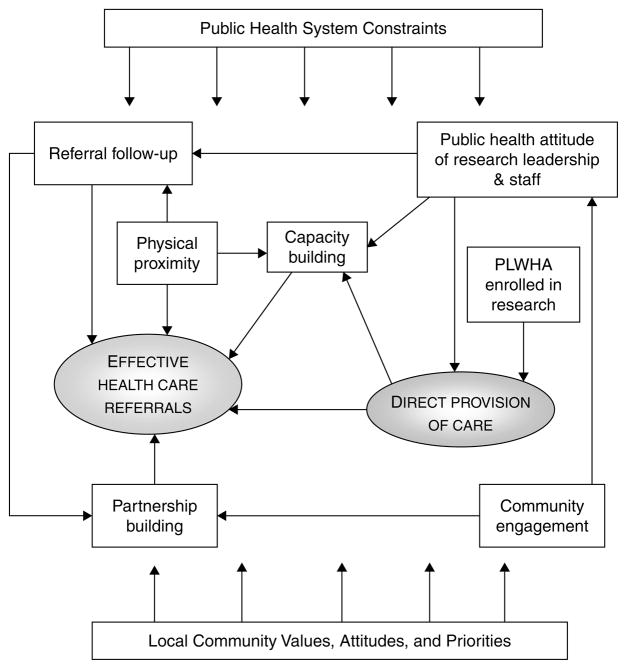
Characteristics of the seven sites participating in the case study component are summarized in Table 2.1 A cross-site analysis of the case studies identified a number of factors that contributed to the balance between direct care and healthcare referrals at each research site (Figure 1). The factors are synergistic, that is, they are mutually reinforcing in their effects. Each factor is briefly described below, followed by several vignettes that illustrate the types of synergies that emerged from the interplay among factors.
FIG. 1.
Factors that Contribute to Improved Healthcare for Research Participants.
PUBLIC HEALTH SYSTEM CONSTRAINTS
Public health system constraints were a pervasive influence at all sites. The syndemic nature of HIV meant that people who participated in HPTN research often had multiple treatment, care, and service needs. Simultaneously, clinics serving the neediest members of society had long lines, waiting lists, and restrictive rules about who could access what care, when, and where. Attempts to improve healthcare for research participants needed to be made with consideration of these larger health system constraints.
For example, a valued benefit of research participation at a number of sites was the ability to “jump the queue.” To be effective, this needed to be more than helping research participants “cut in line.” It was most likely to succeed when the research team included medical staff who conducted initial screenings and evaluations that were then forwarded to the referral clinic with the research participant. The medical information saved time and money at the clinic, which could then be devoted to other clients in the queue who needed screening and evaluation prior to accessing treatment. The factors that came together to make it possible to “jump the queue” were often complex, especially in contexts where healthcare systems were severely strained as in Malawi, Zimbabwe, and Uganda. Importantly, the advantage gained from research-related screenings and evaluations could be lost if other barriers were allowed to intervene, such as lack of transportation or stigma.
PUBLIC HEALTH ATTITUDE
[W]hy are we doing this? Is it solely for the research so we can write papers and say this is effective? Or are we also looking at things, on a more micro level, and saying these are actually human beings, and how can I benefit them? (MU-JHU study coordinator)
One of the most important factors to emerge from the case studies was a public health attitude on the part of research leadership and staff that fostered pro-active efforts to address healthcare challenges. Elements of this attitude included recognition that HIV prevention research is conducted in a larger context of healthcare delivery and public health policy, that the research team may identify healthcare needs that exceed local response capacity, and that researchers must therefore be prepared to respond to health needs that go beyond what is necessary to meet scientific goals. A public health attitude also meant that research teams understood the limits of what they could accomplish on their own and hence the importance of partnering with other public health stakeholders as they sought to meet healthcare needs identified in the course of research.
Staff attitude was important in overcoming obstacles, creating solutions for difficult problems, and building effective partnerships with providers and service organizations in the community. At several sites, a “can-do” attitude grew from a stated sense of moral responsibility for the well-being of research participants, which in turn created a willingness to invest personally in building relationships, identifying resources, and creating solutions.
PLWHA ENROLLMENT IN RESEARCH
Sites that conducted studies where people living with HIV/AIDS were enrolled in research, for example, those focused on the prevention of mother-to-child transmission, tended to have in-house capacity to provide treatment and care directly to participants. Conversely, those organizations that enrolled primarily or only uninfected persons tended to have much more limited clinical capacity and were therefore more reliant on meeting participant needs through referrals. This in turn affected the type of capacity building that a research organization could do. Obviously, if the research staff included several clinicians who were highly trained in HIV treatment and care, they could potentially transfer those skills and even staff time to help treat others in the community. They sometimes provided drugs, laboratory tests, and supplies such as gloves. The organizations employing clinicians with specialized skills were in a position to apply for programmatic treatment funding, either on their own or in partnership with other organizations in the community.
As previously noted, however, there was also a risk that research sites providing substantial healthcare directly to participants would draw staff away from already-stressed healthcare facilities, thus inadvertently undermining local capacity. Here, a public health attitude went a long way toward minimizing unintended negative consequences.
CAPACITY BUILDING
The type and extent of capacity-building efforts reflected the range of local needs as well as the type of research, the nature of the research organization, and the resources available. At times, both community stakeholders and research staff needed to address false assumptions and misunderstandings about each other’s strengths and weaknesses before effective capacity-building plans could be put in place. Capacity building as part of a strategy for meeting research participant health needs required willingness on the part of researchers and community stakeholders to engage in a long-term mutual learning process.
Community stakeholders tended to view researchers as having a wealth of resources—which was sometimes true, relative to the resources otherwise available to care and service organizations. Researchers, however, were keenly aware that the funding they received for research had strict rules governing how it could be used. They also knew that they needed to meet the same standards established for wealthy nations in the implementation of research protocols funded by those nations; if not, the research would be shut down and resources withdrawn. Thus the relative “wealth” of research funding was subject to restrictions and stringent accountability.
Nonetheless, opportunities existed for clinical research funding to serve as a mechanism to enhance the existing healthcare system. For example, capacity building can be as simple as providing a hose pipe that can enable an impoverished referral site to function better. Or it may entail looking for more complex synergies such as using study funds to develop enhanced laboratory capacity to offer quick confirmatory HIV testing results to affiliated voluntary counseling and testing centers that then facilitate timely referral of potential participants for study protocols.
PHYSICAL PROXIMITY
Not surprisingly, research sites in close proximity to clinics, hospitals, and service providers were generally better able to address referral challenges. When research participants were referred to a clinic down the hall from the research site or in a building on the same grounds, it reduced the impact of barriers such as poor transportation and time spent traveling. It also made it easier for study staff to follow up on referrals, either by accompanying participants or by subsequently meeting with healthcare staff. At research sites that were situated either in hospitals or on the same campus, medical research staff were able to follow up directly in the care of participants who were admitted to their partner hospitals. Physical proximity also facilitated capacity building as research teams were more likely to know what kinds of resources their partners needed and could provide support on an ad hoc basis.
REFERRAL FOLLOW-UP
Referral follow-up procedures were important for identifying barriers to care, including lack of transportation, program enrollment fees or other costs, long queues, understaffing, and drug stock-outs. Once barriers were identified, steps could often be taken to address them. For example, the research team might be able to provide transportation directly to the referral site, provide medical documentation that reduced referral site burden, maintain basic medicines in the research pharmacy to cover stock-out problems, or generate funding to cover program fees. Considerable effort and resources were often needed to address the identified problems.
Follow-up procedures that were burdensome for research participants or for the referral site created rather than ameliorated obstacles. Development of an effective follow-up strategy generally required in-person visits by a member of the research team to the referral site to observe what happens when a client enters the system. Options for documentation and follow-up that placed a minimal burden on the referral site, research team, and participant could then be jointly developed.
COMMUNITY ENGAGEMENT
As members of the research team interacted with community members, they enriched and deepened their understanding of the lives of research participants. They gained a clearer sense of the role of their research in assuring the conditions of health for the community as a whole. They also became aware of opportunities where they could substantively contribute to improving those conditions, including empowering the community to raise funds and advocate for people living with HIV/AIDS (PLWHA). All of the sites had active community advisory boards (CABs) that provided a mechanism for ongoing engagement and collaboration with community stakeholders. Many also conducted formative research including qualitative assessments such as focus groups and in-depth interviews with community, civil society, and public health stakeholders to better understand local perspectives on research-related topics.
PARTNERSHIP BUILDING
The process of seeking out partners among healthcare institutions and organizations is an obvious step in improving referrals. Partnership building is also an outcome of effective community engagement. For the partnerships to work, researchers often needed to show a willingness to provide services to the community as well as engage in research.
Partnership-building efforts undertaken by research teams were crucial for facilitating healthcare referrals. Some partnerships grew organically from personal contacts among research staff and word-of-mouth among community-based professionals working in similar areas. Others emerged as specific needs were identified among research participants or related research groups such as CABs. Sometimes a fortuitous event brought people together to fill a need, such as that described in Vignette 1 below.
LOCAL COMMUNITY VALUES, ATTITUDES, AND PRIORITIES
Local community values, attitudes, and priorities, such as public health system constraints, were a pervasive influence on site responses. Since the beginning of the epidemic, HIV has highlighted the importance of cultural sensitivity and respect for community in the way public health responds. This is no less true for the context of HIV prevention research than for the provision of prevention services, treatment, and care more broadly. One example illustrates how unique historical, cultural, and political factors come together to influence the context of research in a given community.
In the early 1980s, HIV emerged in Brazil alongside political uprisings against dictatorship. Solidarity of the people for the freedom to meet and express themselves supported the emergence of voices from the communities most impacted by HIV. Alongside emerging nongovernmental organizations and a committed professional community, these voices were heard by the government. As a result of this history, HIV prevention research in Rio de Janeiro exists in a context of successful activism and community empowerment. The HPTN Rio site approached the issue of establishing a standard of care for their participants based on the belief of Brazilian AIDS activist Herbert de Souza (“Betinho”) that “AIDS has to be viewed as a social issue and not an individual problem.” There is a strong commitment within the Rio clinical sites to have teams that are interdisciplinary and jointly available for on-site management, screening, and provision of care for participants. The end result—an array of advanced counseling and psychological management—is an important supplement to the government-supported treatment access program.
Vignettes
The following vignettes are examples of how specific partnerships, referral procedures, and care scenarios evolved at HPTN sites. They were selected to illustrate the important role of creativity and synergy among the factors described above.
VIGNETTE 1: MU-JHU PSYCHOSOCIAL SUPPORT GROUP, KAMPALA, UGANDA
Factors at work:
Public health attitude of research leadership and staff
PLWHA enrolled in research
Community engagement
Partnership building
Capacity building
Referral follow-up
The psychosocial support group at MU-JHU was started in 2003 by a health visitor and several participants of the MTCT Plus Program. Health visitors are a team of nurses and midwives who conduct follow-up with study participants from the time they enroll to study termination. The MTCT Plus Program was a multi-country, family-centered AIDS treatment program for developing countries. Some of the HIV-positive participants in the MTCT Plus Program in Kampala were asked to meet with an outside visitor. The participants enjoyed getting together to share their stories with the outside visitor and the health visitor realized that there was a need for a support group for positive participants. She went to MU-JHU leadership with her idea and they supported development of a group for this purpose. Within a year, the group grew from 5 couples to 200 members. After a few years, MU-JHU leadership created a new position (Psychosocial Coordinator) so that the health visitor who started the program could officially coordinate the group full-time.
The group helped its members in various ways. For example, through a Loan Scheme program, a widow with children was able to buy charcoal to resell. With the earnings. she was then able to send her children to school. An HIV-positive woman who was chased away by her husband was helped by the group to rent a house, paying her rent for three months. Subsequently the woman began selling tomatoes and was able to pay her rent herself. A woman who was a participant in the MTCT Plus program had a husband who became abusive when she disclosed to him that she was HIV-infected. He blamed her for infecting him. The woman and her husband joined the psychosocial support group and after hearing other members’ stories, her husband was able to accept the situation and stopped abusing her.
The growth and success of the psychosocial group was made possible by the study staff and the leadership at the site who were very supportive of the group. Many staff members volunteered to help with group activities. Some staff also helped the group obtain funding from the Doris Duke Foundation to purchase a prefabricated building. The psychosocial support group planned to expand into the community, by starting a community outreach subgroup. Group members wanted to raise awareness, promote VCT, and share their own stories so that others could learn from them.
VIGNETTE 2: NARI COMMUNITY PARTNERSHIP, PUNE, INDIA
Factors at work:
Community engagement
Public health attitude of research leadership and staff
Partnership building
Capacity building
NARI leadership recognized that community members who may not be eligible for current research studies could become eligible for studies in the future. They also understood that people in the community can influence the decision of others to join or stay involved in research studies. NARI therefore proactively sought to educate the entire community and not just those who were eligible for participation.
NARI allotted significant human resources and time to community involvement. The HPTN principal investigator and the community program supervisor each spent 30% and clinic staff approximately 20% of their time on community involvement activities. NARI also had a Community Outreach Office with 15 full-time staff to work on developing and fostering the partnership between NARI and community organizations. As a result, community partners felt that the NARI leadership respected their work and that they were part of a team effort.
Securing funds for community-wide care and training was challenging, however. In the past, NARI found it difficult to convince research funding agencies to pay for diagnostics and treatment of opportunistic infections and antiretroviral therapy. NARI addressed this challenge by forming partnerships with public hospitals that could provide services at lower costs that were affordable for the study participants. Over time, they found that funding agencies were more receptive to the idea of providing care for clinical trial participants. NARI’s community work budget eventually included funds for a community advisory board, community involvement, and support for healthcare. The size of the budget needed to provide care in this setting was relatively small. However, how to continue providing care and support to participants long after the clinical trials end remains a challenge.
VIGNETTE 3: UNC PROJECT RESOURCE SHARING, LILONGWE, MALAWI
Factors at work:
Public health attitude of research leadership and staff
Referral follow-up
Capacity building
Partnership building
At the government-funded and government-run Kamuzu Central Hospital (KCH) in Lilongwe, drugs to treat patients had to be ordered and sent from the main hospital pharmacy because there was no drug supply kept in the medical ward itself. This system created a challenge for efficient treatment of patients in an under-staffed environment. In addition, pharmaceuticals could only be obtained during normal working hours, including medications needed for emergency admissions during the night. Even during the day, when a medication was needed to treat a given patient, the medical ward staff had to walk the patient’s file to the pharmacy, get the order filled, and then return to the ward with the medication. This could take up to half a day or sometimes be delayed until the next morning.
To address this problem, the UNC Project and the KCH medical ward developed a collaborative arrangement where the UNC Project pharmacy supplied a small stock of drugs for the KCH male and female medical wards. The stock was kept in a small, 24-hour “pharmacy” in the medical ward itself rather than in the UNC-Project pharmacy or the KCH central pharmacy. The initial stock was based on need estimates made by the ward clinicians who collaborated on the project, with quantities to be re-evaluated after three months. The medical ward would then place an order with the UNC-Project every three months according to a fixed budget, with UNC continuously financing the effort.
A similar challenge emerged with referrals to the STI clinic. The clinic was part of KCH and therefore government-subsidized and government-run. However, UNC was staffing the clinic. Although the government was theoretically responsible for supplying all STI drugs to be dispensed there, the drug supply was erratic. UNC Project began supplementing the clinic with drugs and equipment, such as speculums. On a weekly basis, the STI unit ordered the drugs it needed from the dispensary at the hospital. However, the hospital dispensary was usually out of stock on numerous items. The clinic then advised the UNC Project of any deficiencies and UNC provided the remainder of the drugs and supplies as needed.
Discussion
HIV prevention researchers have received considerable criticism in the past decade for not providing comprehensive healthcare benefits to participants, especially those with HIV infection (WHO-UNAIDS, 2004; Belsky & Richardson, 2004; Crouch & Arras, 1998). The results presented here describe the many challenges faced by researchers who seek to provide enhanced benefits under difficult conditions. Establishing baseline obligations, as called for by many advocates, is unlikely to end the controversy because unmet participant needs too often reflect the failure of others to meet their obligations including governments, local healthcare systems, and bilateral and multilateral programs. Researchers cannot fill such extensive gaps on their own, no matter how strongly motivated they are.
These case studies indicate that HIV prevention researchers can be important partners in efforts to improve healthcare in the communities where they work. But there are significant barriers to partnership, the most basic being the separate funding silos for HIV programs and HIV research. Such silos make it difficult for recipients to work together to solve shared problems. Funding is needed to bring stakeholders together in mutually beneficial ways.
There is no single solution to the challenge of addressing the healthcare needs of participants in HIV prevention research around the globe. Rather, there are fundamental questions that must be asked of each context and each research endeavor. What are the healthcare needs of the research population? What are the resources available to meet them? What healthcare services can be effectively incorporated into the research? What can be done to make those services sustainable? How can the research be leveraged to improve services? How can services be leveraged to improve research? How do we ensure that neither services nor research are undermined? How do we ensure the best possible outcome for research participants and their communities?
Conclusion
HIV prevention research takes place within the context of local communities. Those communities, in turn, exist within a larger social, political, and economic context that can constrain or enrich healthcare options. The research sites that participated in this project represent a broad range of HIV prevention research contexts and demonstrate the many factors that impinge on the way they meet the healthcare needs of research participants. Neither problems nor solutions are simple. Each site must continuously evaluate how far down the list of health needs the research team can go without depleting the time, resources, and energy needed to do the research. The case studies exemplify what Weijer and LeBlanc (2006) have called “moral negotiation,” that is, a process where researchers and sponsors negotiate with increasingly empowered local communities and host countries to achieve meaningful and substantive benefits from biomedical research for all stakeholders. It is only through a process of open dialogue at both the global and the local level that a just and fair way forward will be found. The case studies reported here provide information that can be used to help establish benchmarks for negotiating and achieving fair benefits for research participants and their communities (Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries, 2002; Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries, 2004).
Best Practices
HIV prevention research can be structured to help develop access to healthcare if sponsors fund and researchers design prevention trials to function as integral components of public health systems rather than stand-alone endeavors. Consider the current trend toward establishing an obligation to guarantee access to antiretroviral treatment for 5 or 10 years for participants who become HIV-infected during a trial (Fitzgerald et al., 2003; Tucker & Slack, 2003). Such a guarantee requires pre-existing treatment programs and funding set-asides for future use. It provides a superficially easy solution in that it requires minimal effort on the part of researchers and sponsors to understand the public health and host community context. However, there is very little experience with implementation of this approach and, to our knowledge at least, no coordinated efforts at tracking the success of the approach.
The case study analysis presented here suggests that wider, more immediate, and more sustainable benefits could be achieved by restructuring HIV prevention research as an explicit component of a broader public health endeavor. As part of trial site development and in parallel with the implementation of a trial, funds could be provided to assess referral capacity for critical health-care needs of trial participants, to develop public-private partnerships to fill critical gaps, and to build targeted capacity. This is, in fact, the strategy used by many HIV prevention researchers but they are hindered by structural barriers that separate research from public health practice and foster competition for resources rather than facilitating synergy.
The importance of a public health attitude for overcoming these structural barriers suggests a key element in distinguishing a praiseworthy collaboration between resource-rich and resource-poor partners from a potentially exploitative biomedical research endeavor. Do sponsors view HIV prevention research as a public health partnership or do they focus narrowly on cost and time efficiencies in achieving research outcomes? Do project leaders actively seek ways to support innovative efforts made by study staff to address difficult healthcare challenges? Do they advocate with funders and sponsors to change restrictive policies? Do they seek alternative sources of support when research funds are restricted or insufficient to support effective referrals? Are research projects sufficiently funded to support capacity building activities by the research team in the local community? Such efforts are likely to have a broader, more sustainable impact on improving community health than funding set-asides for HIV treatment for a small number of research participants in settings where treatment access is already established.
Research Agenda
An important distinction between prevention research that targets those who are infected versus those who are at risk of infection is seen in the impact of the enrollment of people infected with HIV on other factors such as capacity building and resources to provide direct care. This is an area of research ethics that has received little attention. How do we establish realistic expectations for prevention research with healthy populations? Should they differ from expectations for research with people already infected? If so, how should they differ and why? Should prevention trials require geographic proximity to healthcare services? If so, should the nature of those services be primarily determined by the health profile of the uninfected participants or by the needs of those identified as infected at screening or after enrollment? Should prevention research require joint funding to build treatment capacity if such capacity does not already exist? What are the obligations of host country governments and public health institutions in building and sustaining capacity?
There is also a need for greater consideration of the ethical implications of providing enhanced health benefits in resource-poor settings. Empirical research is needed to assess the impact of such health benefits on people’s decision to participate in research. To date, the ethical debates on this topic center on rationalistic arguments as to whether health benefits may be coercive or constitute undue inducement (Grant & Sugarman, 2004; Emanuel, Currie, & Herman, 2005). What is needed is a practical understanding of what fully informed, autonomous consent means to the participants in these difficult circumstances.
Effective strategies are needed to foster the development of researcher skills related to partnership building, community engagement, capacity building, and collaboration without detracting from the already-demanding set of skills needed to conduct clinical trials. The e-mail survey and case study results reported here also point to the need for cost analyses and realistic budgeting guidelines to support the staff effort and other resources needed to address participant healthcare needs.
Insufficient attention has been given to the direct and indirect costs of participant healthcare gaps, including the possibility that critical HIV prevention trials may be delayed or stopped if expectations for care are not met. The controversy over the tenofovir pre-exposure prophylaxis (PrEP) trials is perhaps the most well-known example of how differing viewpoints on what constitutes adequate healthcare for participants can bring critical research to a halt (Page-Shafer et al., 2005; Grant et al., 2005; Mills et al., 2005; UNAIDS, 2006). Local and global advocates questioned the ethics of several PrEP trials, in particular the adequacy of healthcare planning for participants after the trials ended. While reports of demands were somewhat variable, they included calls for up to 30 years post-trial healthcare for sex workers participating in a proposed trial in Cambodia and long-term care and treatment for Cameroonian women who seroconverted while enrolled in a separate trial in West Africa. The controversies ultimately stopped the implementation of the Cambodia trial and led to the early closure of the research site in Cameroon. There were also indirect impacts of the controversy on HIV prevention research more broadly, as sponsors and researchers increasingly responded to heightened demands for better healthcare access by limiting trials to sites where access to sustainable HIV treatment already exists (Berkley, 2003; Forbes, 2006; Slack et al., 2005; Tucker & Slack, 2003). This diminishes the potential for HIV prevention research to be a catalyst for access in other settings.
Our analysis represents an initial exploration of a topic that warrants a more comprehensive empirical assessment. Although the data are derived from a broad range of sites in Africa, Asia, and the Americas, they nonetheless represent a time-limited assessment of the experience of seven sites within a single research network. While we identified many important challenges to meeting the healthcare needs of research participants, the period of observation at each site was brief and therefore unlikely to identify the full complexity of issues, in particular, the dynamic way in which research teams and local community context develop independently and in response to each other over time.
Educational Implications
In order to effectively address the challenges outlined here, there are several areas where educational training and capacity building are needed. First, ethicists, policy makers, funding organizations, and treatment advocates need better information on both the challenges faced and the successes achieved by HIV prevention researchers. Unrealistic expectations—and especially unrealistic timelines—regarding what a single research project can accomplish creates an environment where researchers may be maligned for what are in fact notable efforts and achievements. Conversely, researcher resistance to reasonable requirements is less likely to be sustained if the effort, resources, and time needed to meet them are understood, acknowledged, and addressed.
Second, IRB and ethics committee members need to improve their understanding of the way in which individual research projects are situated within larger public health and community contexts. Guidance is needed on the kinds of questions they should ask about healthcare needs, what constitutes reasonable expectations for meeting those needs within the more limited context of a single research protocol, and how to evaluate the adequacy of research plans to meet participant health needs.
Third, outreach to public health providers, NGOs, and others in research communities may be needed to improve understanding of the critical importance of HIV prevention research for local and global responses to the epidemic. Research teams, in turn, may need to be better educated about the intersection between HIV prevention research and public health practice. A good place to begin would be to add a general component on this intersection to Good Clinical Practice (GCP) training and tailored, locally-specific components to protocol-specific training.
Acknowledgments
This study was supported by the HIV Prevention Trials Network (HPTN) and sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, U.S. Department of Health and Human Services (Cooperative Agreement U01-AI-46749 to Family Health International). Financial support was also provided in part by the United States Agency for International Development through Cooperative Agreement No. GPO-A-00-05-00022-00. The content of this report does not necessarily reflect the views or policies of NIAID, HPTN, or USAID, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Biographies
Kathleen M. MacQueen is a senior social scientist and coordinator of interdisciplinary research ethics. She conducts empirical research and consults on a variety of research ethics topics, including ancillary care in HIV prevention trials, community-based participatory research models, reducing social harms, and informed consent. Her publications also include methodological works on the conduct of team-based qualitative research. As lead investigator for this project, she contributed to study design, analysis, and writing.
Kerry McLoughlin is a full-time instructor of sociology and teaches courses on introductory sociology, the family, and social problems. She has experience with qualitative data collection and analysis in both domestic and international settings. She contributed to data collection, data management, study design, analysis, and writing. She is currently with the Department of Social Sciences, Wake Technical Community College, Raleigh, North Carolina.
Patty Alleman is a senior research associate with experience conducing and directing qualitative research related to HIV. She manages multi-site projects and provides technical expertise in socio-behavioral research topics, including protocol development and qualitative data methodology. Her projects include sites in Africa, Latin America, Asia, and North America. She contributed to data collection, analysis, and writing.
Natasha Mack is a linguistic and cultural anthropologist who has conducted research in the Caribbean, Latin America, and Africa. Her research interests include the intersection of political economy, gender norms, and risk of HIV infection. On the horizon is development of a tool to help researchers elicit culturally relevant explanations for concepts essential to obtaining truly informed consent in clinical trials, and research to inform post-trial planning for the potential roll-out of HIV pre-exposure prophylaxis. She contributed to data collection, analysis, and writing.
Holly Burke has coordinated and led the analysis of three large multi-site survey studies related to HIV and sexual health, spanning five countries on multiple continents. Her experience includes survey development and analysis, as well as integrated quantitative/qualitative studies on the subject of reproductive health among adolescents and men. She has led various quantitative data analysis and management training initiatives in developing countries. She contributed to data collection, analysis, and writing.
Footnotes
Detailed case study descriptions are available for each site at the HPTN website (www.hptn.org/researchethics/partneringforcare.htm).
References
- Belsky L, Richardson HS. Medical researchers’ ancillary clinical care responsibilities. British Medical Journal. 2004;328(7454):1494–1496. doi: 10.1136/bmj.328.7454.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley S. Thorny issues in the ethics of AIDS vaccine trials. The Lancet. 2003;362(9388):992. doi: 10.1016/S0140-6736(03)14371-1. [DOI] [PubMed] [Google Scholar]
- Crouch RA, Arras JD. AZT trials and tribulations. Hastings Center Report. 1998;28(6):26–34. [PubMed] [Google Scholar]
- Emanuel EJ, Currie XE, Herman A on behalf of Project Phidisa. Undue inducement in clinical research in developing countries: Is it a worry? The Lancet. 2005;366(9482):336–340. doi: 10.1016/S0140-6736(05)66992-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DW, Pape JW, Wasserheit JN, Counts GW, Corey L. Provision of treatment in HIV-1 vaccine trials in developing countries. The Lancet. 2003;362(9388):993–994. doi: 10.1016/S0140-6736(03)14372-3. [DOI] [PubMed] [Google Scholar]
- Forbes A. Moving toward assured access to treatment in microbicide trials. PLoS Medicine. 2006;3(7):e153. doi: 10.1371/journal.pmed.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant RW, Sugarman J. Ethics in human subjects research: Do incentives matter? Journal of Medicine and Philosophy. 2004;29(6):717–738. doi: 10.1080/03605310490883046. [DOI] [PubMed] [Google Scholar]
- Grant RM, Buchbinder S, Cates W, Jr, Clarke E, Coates T, Cohen MS, Delaney M, Flores G, Goicochea P, Gonsalves G, Harrington M, Lama JR, MacQueen KM, Moore JP, Peterson L, Sanchez J, Thompson M, Wainberg MA. Promote HIV chemoprophylaxis research, don’t prevent it. Science. 2005;309(5744):2170–2071. doi: 10.1126/science.1116204. [DOI] [PubMed] [Google Scholar]
- Kupfer L, Hofman K, Jarawan R, McDermott J, Bridbord K. Strategies to discourage brain drain. Bulletin of the World Health Organization. 2004;82(8):616–619. [PMC free article] [PubMed] [Google Scholar]
- London AJ. Justice and the human development approach to international research. The Hastings Center Report. 2005;35:24–37. [PubMed] [Google Scholar]
- Marchal B, Kegels G. Health workforce imbalances in times of globalization: brain drain or professional mobility? International Journal of Health Planning and Management. 2003;18(Supplement 1):S89–101. doi: 10.1002/hpm.720. [DOI] [PubMed] [Google Scholar]
- Merson MH. The HIV-AIDS pandemic at 25—the global response. New England Journal of Medicine. 2006;354(23):2414–2417. doi: 10.1056/NEJMp068074. [DOI] [PubMed] [Google Scholar]
- Mills E, Rachlis B, Wu P, Wong E, Wilson K, Singh S. Media reporting of tenofovir trials in Cambodia and Cameroon. BMC International Health and Human Rights. 2005;5:6. doi: 10.1186/1472-698X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein B. Hygeia’s Constellation: Navigating Health Futures in a Dynamic and Democratic World. Atlanta, GA: Syndemics Prevention Network, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- Page-Shafer K, Saphonn V, Sun LP, Vun MC, Cooper DA, Kaldor JM. HIV prevention research in a resource-limited setting: the experience of planning a trial in Cambodia. The Lancet. 2005;366(9495):1499–1503. doi: 10.1016/S0140-6736(05)67146-2. [DOI] [PubMed] [Google Scholar]
- Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries. Fair benefits for research in developing countries. Science. 2002;298(5601):2133–2134. doi: 10.1126/science.1076899. [DOI] [PubMed] [Google Scholar]
- Participants in the 2001 Conference on Ethical Aspects of Research in Developing Countries. Moral standards for research in developing countries: from “reasonable availability” to “fair benefits. Hastings Center Report. 2004;34(3):17–27. [PubMed] [Google Scholar]
- Singer M, Clair S. Syndemics and public health: Reconceptualizing disease in bio-social context. Medical Anthropology Quarterly. 2003;17(4):423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- Slack C, Stobie M, Milford C, Lindegger G, Wassenaar D, Strode A, IJsselmuiden C. Provision of HIV treatment in HIV preventive vaccine trials: a developing country perspective. Social Science & Medicine. 2005;60(6):1197–1208. doi: 10.1016/j.socscimed.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Stall R, Mills TC, Williamson J, Hart T, Greenwood G, Paul J, Pollack L, Binson D, Osmond D, Catania JA. Association of co-occurring psychosocial health problems and increased vulnerability to HIV/AIDS among urban men who have sex with men. American Journal of Public Health. 2003;93(6):939–942. doi: 10.2105/ajph.93.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker T, Slack C. Not if but how? Caring for HIV-1 vaccine trial participants in South Africa. The Lancet. 2003;362(9388):995. doi: 10.1016/S0140-6736(03)14374-7. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Creating effective partnerships for HIV prevention trials: report of a UNAIDS Consultation, Geneva, 20–21 June 2005. AIDS. 2006;20(6):W1–W11. doi: 10.1097/01.aids.0000218573.29930.67. [DOI] [PubMed] [Google Scholar]
- UNAIDS. 2008 report on the global epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2008. [Google Scholar]
- UNAIDS-WHO. Ethical considerations in biomedical HIV prevention trials. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2007. [Google Scholar]
- Weijer C, LeBlanc G. The balm of Gilead: Is the provision of treatment to those who seroconvert in HIV prevention trials a matter of moral obligation or moral negotiation? Journal of Law, Medicine, & Ethics. 2006;34(4):793–808. doi: 10.1111/j.1748-720X.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- WHO-UNAIDS. Treating people with intercurrent infection in HIV prevention trials. AIDS; Report from a WHO/UNAIDS consultation; Geneva. 17–18 July 2003; 2004. pp. W1–12. [PubMed] [Google Scholar]